Borosilicate Glass Utilization in Chemical Synthesis Pathways
JUL 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Borosilicate Evolution
Borosilicate glass has undergone significant evolution since its inception in the late 19th century. Initially developed by Otto Schott in 1893, this revolutionary material quickly gained prominence in chemical synthesis pathways due to its exceptional thermal and chemical resistance properties. The evolution of borosilicate glass can be traced through several key stages, each marked by advancements in composition, manufacturing techniques, and applications.
In the early 20th century, the focus was primarily on refining the glass composition to enhance its thermal shock resistance and chemical durability. This period saw the introduction of various formulations, with the most notable being the Pyrex brand, developed by Corning Glass Works in 1915. The improved thermal properties of Pyrex borosilicate glass made it ideal for laboratory glassware and chemical processing equipment.
The mid-20th century witnessed a surge in the use of borosilicate glass in industrial applications. Manufacturers developed specialized techniques for producing large-scale borosilicate glass components, such as reaction vessels and distillation columns. This era also saw the introduction of borosilicate glass pipelines for corrosive chemical transport, revolutionizing chemical manufacturing processes.
The late 20th century brought about significant advancements in borosilicate glass manufacturing technologies. Computer-controlled furnaces and precision molding techniques enabled the production of complex shapes with tighter tolerances. This period also saw the development of borosilicate glass with enhanced optical properties, expanding its use in spectroscopy and other analytical techniques crucial for chemical synthesis.
In recent decades, the evolution of borosilicate glass has focused on tailoring its properties for specific applications in chemical synthesis. Researchers have developed specialized coatings and surface treatments to improve the glass's resistance to specific chemicals and enhance its catalytic properties. Additionally, the integration of borosilicate glass with other materials, such as metals and ceramics, has led to the creation of hybrid systems that combine the benefits of multiple materials.
The latest frontier in borosilicate glass evolution involves nanotechnology and advanced materials science. Researchers are exploring ways to incorporate nanoparticles and nanostructures into borosilicate glass matrices, creating materials with unique properties such as enhanced catalytic activity or selective permeability. These innovations promise to open up new possibilities for chemical synthesis pathways, potentially enabling more efficient and sustainable processes.
Throughout its evolution, borosilicate glass has consistently demonstrated its versatility and adaptability to the changing needs of chemical synthesis. From basic laboratory glassware to advanced reactor systems, its journey reflects the ongoing interplay between materials science and chemical engineering, driving progress in both fields.
In the early 20th century, the focus was primarily on refining the glass composition to enhance its thermal shock resistance and chemical durability. This period saw the introduction of various formulations, with the most notable being the Pyrex brand, developed by Corning Glass Works in 1915. The improved thermal properties of Pyrex borosilicate glass made it ideal for laboratory glassware and chemical processing equipment.
The mid-20th century witnessed a surge in the use of borosilicate glass in industrial applications. Manufacturers developed specialized techniques for producing large-scale borosilicate glass components, such as reaction vessels and distillation columns. This era also saw the introduction of borosilicate glass pipelines for corrosive chemical transport, revolutionizing chemical manufacturing processes.
The late 20th century brought about significant advancements in borosilicate glass manufacturing technologies. Computer-controlled furnaces and precision molding techniques enabled the production of complex shapes with tighter tolerances. This period also saw the development of borosilicate glass with enhanced optical properties, expanding its use in spectroscopy and other analytical techniques crucial for chemical synthesis.
In recent decades, the evolution of borosilicate glass has focused on tailoring its properties for specific applications in chemical synthesis. Researchers have developed specialized coatings and surface treatments to improve the glass's resistance to specific chemicals and enhance its catalytic properties. Additionally, the integration of borosilicate glass with other materials, such as metals and ceramics, has led to the creation of hybrid systems that combine the benefits of multiple materials.
The latest frontier in borosilicate glass evolution involves nanotechnology and advanced materials science. Researchers are exploring ways to incorporate nanoparticles and nanostructures into borosilicate glass matrices, creating materials with unique properties such as enhanced catalytic activity or selective permeability. These innovations promise to open up new possibilities for chemical synthesis pathways, potentially enabling more efficient and sustainable processes.
Throughout its evolution, borosilicate glass has consistently demonstrated its versatility and adaptability to the changing needs of chemical synthesis. From basic laboratory glassware to advanced reactor systems, its journey reflects the ongoing interplay between materials science and chemical engineering, driving progress in both fields.
Market Demand Analysis
The market demand for borosilicate glass in chemical synthesis pathways has been steadily increasing due to its unique properties and versatile applications. This specialized glass is highly valued in the chemical industry for its exceptional resistance to thermal shock, chemical corrosion, and mechanical stress. As a result, it has become an indispensable material in various laboratory and industrial processes.
The pharmaceutical sector represents a significant portion of the market demand for borosilicate glass in chemical synthesis. The glass's inert nature and resistance to chemical reactions make it ideal for drug development and production processes. As the global pharmaceutical industry continues to expand, driven by factors such as aging populations and increased healthcare spending, the demand for borosilicate glass in this sector is expected to grow correspondingly.
In the petrochemical industry, borosilicate glass finds extensive use in catalytic processes and reaction vessels. The material's ability to withstand high temperatures and pressures, coupled with its transparency, allows for efficient monitoring and control of chemical reactions. With the ongoing growth in petrochemical production, particularly in emerging economies, the demand for borosilicate glass in this sector is projected to rise.
The environmental and energy sectors also contribute significantly to the market demand. Borosilicate glass is crucial in the development and production of advanced materials for renewable energy technologies, such as solar panels and fuel cells. As global efforts to combat climate change intensify, the demand for these technologies is expected to surge, driving the need for borosilicate glass in chemical synthesis pathways.
Academic and research institutions form another key market segment. The glass's properties make it essential for conducting experiments and developing new chemical compounds. As research funding increases and new areas of scientific inquiry emerge, the demand for borosilicate glass in laboratory settings is likely to expand.
The electronics industry, particularly in the production of semiconductors and advanced materials, relies heavily on borosilicate glass for various chemical processes. With the rapid growth of the electronics sector, driven by innovations in areas such as 5G technology and the Internet of Things, the demand for borosilicate glass in related chemical synthesis applications is anticipated to grow substantially.
Market analysts predict that the global demand for borosilicate glass in chemical synthesis pathways will continue to grow at a steady rate. This growth is attributed to ongoing technological advancements, increasing industrial applications, and the material's critical role in emerging fields such as nanotechnology and materials science. As industries strive for more efficient and sustainable processes, the unique properties of borosilicate glass position it as a key enabler in the evolution of chemical synthesis techniques.
The pharmaceutical sector represents a significant portion of the market demand for borosilicate glass in chemical synthesis. The glass's inert nature and resistance to chemical reactions make it ideal for drug development and production processes. As the global pharmaceutical industry continues to expand, driven by factors such as aging populations and increased healthcare spending, the demand for borosilicate glass in this sector is expected to grow correspondingly.
In the petrochemical industry, borosilicate glass finds extensive use in catalytic processes and reaction vessels. The material's ability to withstand high temperatures and pressures, coupled with its transparency, allows for efficient monitoring and control of chemical reactions. With the ongoing growth in petrochemical production, particularly in emerging economies, the demand for borosilicate glass in this sector is projected to rise.
The environmental and energy sectors also contribute significantly to the market demand. Borosilicate glass is crucial in the development and production of advanced materials for renewable energy technologies, such as solar panels and fuel cells. As global efforts to combat climate change intensify, the demand for these technologies is expected to surge, driving the need for borosilicate glass in chemical synthesis pathways.
Academic and research institutions form another key market segment. The glass's properties make it essential for conducting experiments and developing new chemical compounds. As research funding increases and new areas of scientific inquiry emerge, the demand for borosilicate glass in laboratory settings is likely to expand.
The electronics industry, particularly in the production of semiconductors and advanced materials, relies heavily on borosilicate glass for various chemical processes. With the rapid growth of the electronics sector, driven by innovations in areas such as 5G technology and the Internet of Things, the demand for borosilicate glass in related chemical synthesis applications is anticipated to grow substantially.
Market analysts predict that the global demand for borosilicate glass in chemical synthesis pathways will continue to grow at a steady rate. This growth is attributed to ongoing technological advancements, increasing industrial applications, and the material's critical role in emerging fields such as nanotechnology and materials science. As industries strive for more efficient and sustainable processes, the unique properties of borosilicate glass position it as a key enabler in the evolution of chemical synthesis techniques.
Technical Challenges
The utilization of borosilicate glass in chemical synthesis pathways faces several significant technical challenges that hinder its widespread adoption and optimal performance. One of the primary obstacles is the difficulty in achieving precise temperature control during reactions. Borosilicate glass, while known for its thermal resistance, can still experience thermal shock when subjected to rapid temperature changes, potentially leading to cracks or breakage.
Another challenge lies in the chemical compatibility of borosilicate glass with certain reagents and solvents. Although it exhibits excellent resistance to many chemicals, some highly alkaline solutions or hydrofluoric acid can etch or degrade the glass surface over time, compromising its integrity and potentially contaminating the reaction mixture.
The scaling up of chemical processes from laboratory to industrial scale presents additional hurdles. Larger borosilicate glass vessels are more prone to stress and strain, making them susceptible to failure under the increased pressures and volumes associated with industrial-scale synthesis. This limitation often necessitates the use of alternative materials for large-scale production, creating discrepancies between research and manufacturing processes.
Surface modifications of borosilicate glass to enhance its properties or functionalities pose another technical challenge. While various coating techniques exist, achieving uniform and durable surface treatments that do not interfere with the glass's inherent properties or the chemical reactions taking place within remains a complex task.
The detection and prevention of micro-cracks in borosilicate glass apparatus is crucial for maintaining safety and integrity during chemical synthesis. However, developing reliable and cost-effective methods for real-time monitoring of glass condition in diverse reaction environments continues to be a significant challenge for researchers and manufacturers alike.
Recycling and disposal of borosilicate glass used in chemical synthesis also present environmental and economic challenges. The presence of chemical residues and potential contamination make it difficult to recycle the glass through conventional means, necessitating the development of specialized recycling processes or safe disposal methods.
Lastly, the integration of borosilicate glass components with other materials and equipment in complex synthesis setups poses compatibility issues. Ensuring seamless connections between glass and non-glass components, such as metal fittings or polymer seals, while maintaining chemical resistance and leak-free operation, remains an ongoing challenge in the design of advanced synthesis apparatus.
Another challenge lies in the chemical compatibility of borosilicate glass with certain reagents and solvents. Although it exhibits excellent resistance to many chemicals, some highly alkaline solutions or hydrofluoric acid can etch or degrade the glass surface over time, compromising its integrity and potentially contaminating the reaction mixture.
The scaling up of chemical processes from laboratory to industrial scale presents additional hurdles. Larger borosilicate glass vessels are more prone to stress and strain, making them susceptible to failure under the increased pressures and volumes associated with industrial-scale synthesis. This limitation often necessitates the use of alternative materials for large-scale production, creating discrepancies between research and manufacturing processes.
Surface modifications of borosilicate glass to enhance its properties or functionalities pose another technical challenge. While various coating techniques exist, achieving uniform and durable surface treatments that do not interfere with the glass's inherent properties or the chemical reactions taking place within remains a complex task.
The detection and prevention of micro-cracks in borosilicate glass apparatus is crucial for maintaining safety and integrity during chemical synthesis. However, developing reliable and cost-effective methods for real-time monitoring of glass condition in diverse reaction environments continues to be a significant challenge for researchers and manufacturers alike.
Recycling and disposal of borosilicate glass used in chemical synthesis also present environmental and economic challenges. The presence of chemical residues and potential contamination make it difficult to recycle the glass through conventional means, necessitating the development of specialized recycling processes or safe disposal methods.
Lastly, the integration of borosilicate glass components with other materials and equipment in complex synthesis setups poses compatibility issues. Ensuring seamless connections between glass and non-glass components, such as metal fittings or polymer seals, while maintaining chemical resistance and leak-free operation, remains an ongoing challenge in the design of advanced synthesis apparatus.
Current Applications
01 Composition and properties of borosilicate glass
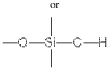
Borosilicate glass is a type of glass with silica and boron trioxide as the main glass-forming constituents. It is known for its low thermal expansion coefficient, high chemical resistance, and excellent thermal shock resistance. These properties make it suitable for various applications in laboratory equipment, cookware, and industrial uses.- Composition and properties of borosilicate glass: Borosilicate glass is a type of glass with silica and boron trioxide as the main glass-forming constituents. It is known for its low thermal expansion coefficient, high chemical resistance, and excellent thermal shock resistance. These properties make it suitable for various applications in laboratory equipment, cookware, and industrial uses.
- Manufacturing processes for borosilicate glass: Various manufacturing processes are employed to produce borosilicate glass, including melting, forming, and annealing. Advanced techniques such as float glass production and precision molding are used to create different forms of borosilicate glass products. The manufacturing process can be optimized to enhance specific properties of the glass.
- Applications of borosilicate glass in laboratory and industrial settings: Borosilicate glass is widely used in laboratory glassware, such as beakers, test tubes, and pipettes, due to its chemical resistance and thermal stability. It is also utilized in industrial applications, including chemical processing equipment, sight glasses, and high-temperature windows for furnaces and kilns.
- Borosilicate glass in consumer products: The unique properties of borosilicate glass make it suitable for various consumer products. It is commonly used in cookware, bakeware, and food storage containers due to its thermal shock resistance and non-porous nature. Additionally, borosilicate glass is utilized in lighting fixtures, solar panels, and electronic display screens.
- Innovations and advancements in borosilicate glass technology: Ongoing research and development in borosilicate glass technology focus on improving its properties and expanding its applications. This includes developing new compositions with enhanced characteristics, exploring novel manufacturing techniques, and creating specialized coatings or treatments to further improve the glass performance in specific applications.
02 Manufacturing processes for borosilicate glass
Various manufacturing processes are employed to produce borosilicate glass, including melting, forming, and annealing. Advanced techniques such as float glass production and precision molding are used to create different forms of borosilicate glass products. The manufacturing process often involves careful control of temperature and composition to achieve desired properties.Expand Specific Solutions03 Applications of borosilicate glass in laboratory and industrial settings
Borosilicate glass is widely used in laboratory glassware, such as beakers, test tubes, and pipettes, due to its chemical resistance and thermal stability. It is also employed in industrial applications, including sight glasses, process vessels, and heat exchangers. The material's durability and transparency make it ideal for these demanding environments.Expand Specific Solutions04 Borosilicate glass in consumer products
Borosilicate glass has found its way into various consumer products, particularly in kitchenware and home appliances. It is commonly used in oven-safe dishes, storage containers, and coffee makers due to its ability to withstand temperature changes without cracking. The glass's clarity and durability also make it popular for high-end drinkware and decorative items.Expand Specific Solutions05 Innovations and modifications in borosilicate glass
Ongoing research and development in borosilicate glass focus on improving its properties and expanding its applications. This includes developing new compositions with enhanced characteristics, such as increased strength or improved optical properties. Innovations also involve surface treatments, coatings, and the incorporation of additional elements to tailor the glass for specific uses in areas like optics, electronics, and renewable energy.Expand Specific Solutions
Industry Leaders
The borosilicate glass utilization in chemical synthesis pathways market is in a growth phase, driven by increasing demand in pharmaceutical and laboratory applications. The global market size is estimated to be over $2 billion, with steady annual growth. Technologically, the field is mature but continues to evolve, with companies like SCHOTT AG, Corning, and Nippon Electric Glass leading innovation. These firms are developing advanced formulations and manufacturing processes to enhance glass properties for specialized applications. Emerging players like SiO2 Medical Products and Hunan Kibing are also making strides in niche segments, indicating a competitive and dynamic landscape poised for further advancements in material science and production techniques.
SCHOTT AG
Technical Solution: SCHOTT AG has developed advanced borosilicate glass compositions specifically tailored for chemical synthesis applications. Their DURAN® borosilicate glass offers exceptional thermal shock resistance and chemical durability, making it ideal for laboratory glassware and industrial process equipment[1]. SCHOTT has also innovated in the field of microfluidic devices, creating borosilicate glass chips with precisely etched microchannels for controlled chemical reactions and analysis[2]. Additionally, they have developed specialized coatings for borosilicate glass to enhance its performance in specific chemical environments, such as improving acid resistance or reducing surface adsorption[3].
Strengths: High chemical resistance, thermal stability, and optical clarity. Weaknesses: Higher cost compared to soda-lime glass, limited flexibility in complex shapes.
SiO2 Medical Products, Inc.
Technical Solution: SiO2 Medical Products has developed a unique hybrid technology that combines the benefits of plastic and glass for chemical synthesis applications. Their proprietary process involves depositing a thin layer of pure silicon dioxide (a key component of borosilicate glass) onto plastic substrates, creating containers with the chemical resistance of borosilicate glass and the break resistance of plastic[10]. This technology has been applied to create vials and syringes for pharmaceutical use, offering superior chemical stability for drug formulations. The company has also explored the application of this technology in microfluidic devices for chemical synthesis, potentially offering a more cost-effective alternative to traditional borosilicate glass chips[11].
Strengths: Innovative hybrid technology combining glass and plastic properties, potential for cost-effective production. Weaknesses: Limited track record in pure borosilicate glass applications, potential concerns about long-term stability in extreme chemical environments.
Key Innovations
Polymer vials with substantially flat bottoms and injection stretch blow molding methods for making the same
PatentWO2020102434A2
Innovation
- The development of polymer vials with substantially flat bottoms produced using injection stretch blow molding, featuring a PECVD water barrier coating and a tri-layer coating set, which enhances thermal efficiency and barrier properties, and a novel mold design that ensures consistent side wall thickness and improved heat transfer.
Method of manufacturing porous glass
PatentWO2012074080A1
Innovation
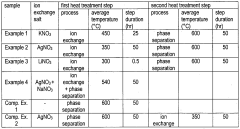
- A method involving ion exchange with silver, potassium, or lithium ions with borosilicate glass to form an ion concentration gradient, followed by phase separation and etching, resulting in a porous glass structure with varying porosity from the surface to depth.
Safety Regulations
The utilization of borosilicate glass in chemical synthesis pathways necessitates strict adherence to safety regulations to mitigate potential risks and ensure the well-being of laboratory personnel. These regulations encompass various aspects of handling, storage, and disposal of borosilicate glass equipment and materials.
Proper personal protective equipment (PPE) is paramount when working with borosilicate glass. Laboratory workers must wear safety goggles, heat-resistant gloves, and appropriate clothing to protect against potential breakage, thermal shock, or chemical splashes. Face shields may be required for high-risk procedures involving pressurized or heated borosilicate glass apparatus.
Handling and storage protocols for borosilicate glass equipment are crucial. Regulations mandate regular inspections for cracks, chips, or other damage that could compromise structural integrity. Proper cleaning and decontamination procedures must be followed to prevent cross-contamination and ensure the longevity of the glassware.
Thermal shock prevention is a key safety consideration. Regulations stipulate gradual heating and cooling of borosilicate glass apparatus to avoid sudden temperature changes that could lead to breakage. Specific guidelines for maximum temperature differentials and heating rates are typically provided based on the glass thickness and composition.
Pressure-related safety measures are essential when using borosilicate glass in pressurized systems. Regulations often specify maximum operating pressures, require the use of pressure relief valves, and mandate regular pressure testing of glass vessels. Shatterproof coatings or enclosures may be required for high-pressure applications.
Disposal of broken or contaminated borosilicate glass is subject to strict regulations. Proper segregation, containment, and labeling of glass waste are mandatory. Specialized disposal containers and procedures are often required to prevent injuries to laboratory and waste management personnel.
Chemical compatibility is a critical safety aspect. Regulations require thorough assessment of the compatibility between borosilicate glass and the chemicals used in synthesis pathways. This includes considerations of potential etching, leaching, or other interactions that could compromise the glass integrity or contaminate the synthesis products.
Emergency response protocols specific to borosilicate glass incidents are typically mandated. These include procedures for handling breakages, spills, and potential exposure to hazardous materials contained within glass apparatus. Proper training and regular drills are often required to ensure preparedness for such incidents.
Regulatory compliance in the use of borosilicate glass extends to documentation and record-keeping. Detailed logs of equipment usage, maintenance, and incident reports are typically required to ensure traceability and facilitate continuous improvement of safety practices.
Proper personal protective equipment (PPE) is paramount when working with borosilicate glass. Laboratory workers must wear safety goggles, heat-resistant gloves, and appropriate clothing to protect against potential breakage, thermal shock, or chemical splashes. Face shields may be required for high-risk procedures involving pressurized or heated borosilicate glass apparatus.
Handling and storage protocols for borosilicate glass equipment are crucial. Regulations mandate regular inspections for cracks, chips, or other damage that could compromise structural integrity. Proper cleaning and decontamination procedures must be followed to prevent cross-contamination and ensure the longevity of the glassware.
Thermal shock prevention is a key safety consideration. Regulations stipulate gradual heating and cooling of borosilicate glass apparatus to avoid sudden temperature changes that could lead to breakage. Specific guidelines for maximum temperature differentials and heating rates are typically provided based on the glass thickness and composition.
Pressure-related safety measures are essential when using borosilicate glass in pressurized systems. Regulations often specify maximum operating pressures, require the use of pressure relief valves, and mandate regular pressure testing of glass vessels. Shatterproof coatings or enclosures may be required for high-pressure applications.
Disposal of broken or contaminated borosilicate glass is subject to strict regulations. Proper segregation, containment, and labeling of glass waste are mandatory. Specialized disposal containers and procedures are often required to prevent injuries to laboratory and waste management personnel.
Chemical compatibility is a critical safety aspect. Regulations require thorough assessment of the compatibility between borosilicate glass and the chemicals used in synthesis pathways. This includes considerations of potential etching, leaching, or other interactions that could compromise the glass integrity or contaminate the synthesis products.
Emergency response protocols specific to borosilicate glass incidents are typically mandated. These include procedures for handling breakages, spills, and potential exposure to hazardous materials contained within glass apparatus. Proper training and regular drills are often required to ensure preparedness for such incidents.
Regulatory compliance in the use of borosilicate glass extends to documentation and record-keeping. Detailed logs of equipment usage, maintenance, and incident reports are typically required to ensure traceability and facilitate continuous improvement of safety practices.
Sustainability Impact
The utilization of borosilicate glass in chemical synthesis pathways has significant implications for sustainability in the chemical industry. This material's unique properties, including high thermal resistance and chemical inertness, contribute to more efficient and environmentally friendly processes. By enabling reactions to occur at higher temperatures and pressures, borosilicate glass vessels can increase reaction rates and yields, potentially reducing energy consumption and waste generation.
The durability of borosilicate glass also plays a crucial role in sustainability. Its resistance to thermal shock and chemical corrosion extends the lifespan of laboratory equipment, reducing the need for frequent replacements. This longevity translates to fewer resources consumed in manufacturing and disposing of glassware, contributing to a reduction in the overall environmental footprint of chemical research and production facilities.
Furthermore, the transparency of borosilicate glass allows for real-time monitoring of reactions, potentially leading to more precise control and optimization of processes. This capability can result in improved resource efficiency and reduced waste generation, aligning with sustainable chemistry principles. The ability to visually inspect reactions also enhances safety, potentially reducing the risk of accidents and associated environmental impacts.
In the context of green chemistry, borosilicate glass supports the development of more sustainable synthesis methods. Its compatibility with a wide range of solvents, including water and ionic liquids, facilitates the exploration of greener reaction media. This versatility enables researchers to move away from traditional organic solvents, which often pose environmental and health risks, towards more benign alternatives.
The recyclability of borosilicate glass further enhances its sustainability profile. Unlike some specialized materials used in chemical synthesis, borosilicate glass can be recycled without significant loss of quality. This characteristic supports circular economy principles, reducing the demand for raw materials and energy associated with producing new glassware.
Lastly, the use of borosilicate glass in microreactor technology represents a promising avenue for sustainable chemical synthesis. These miniaturized reaction systems, often fabricated from borosilicate glass, offer improved heat and mass transfer, leading to more efficient reactions with reduced solvent and energy requirements. The precise control afforded by microreactors can also minimize side reactions and improve product selectivity, potentially reducing waste and simplifying purification processes.
The durability of borosilicate glass also plays a crucial role in sustainability. Its resistance to thermal shock and chemical corrosion extends the lifespan of laboratory equipment, reducing the need for frequent replacements. This longevity translates to fewer resources consumed in manufacturing and disposing of glassware, contributing to a reduction in the overall environmental footprint of chemical research and production facilities.
Furthermore, the transparency of borosilicate glass allows for real-time monitoring of reactions, potentially leading to more precise control and optimization of processes. This capability can result in improved resource efficiency and reduced waste generation, aligning with sustainable chemistry principles. The ability to visually inspect reactions also enhances safety, potentially reducing the risk of accidents and associated environmental impacts.
In the context of green chemistry, borosilicate glass supports the development of more sustainable synthesis methods. Its compatibility with a wide range of solvents, including water and ionic liquids, facilitates the exploration of greener reaction media. This versatility enables researchers to move away from traditional organic solvents, which often pose environmental and health risks, towards more benign alternatives.
The recyclability of borosilicate glass further enhances its sustainability profile. Unlike some specialized materials used in chemical synthesis, borosilicate glass can be recycled without significant loss of quality. This characteristic supports circular economy principles, reducing the demand for raw materials and energy associated with producing new glassware.
Lastly, the use of borosilicate glass in microreactor technology represents a promising avenue for sustainable chemical synthesis. These miniaturized reaction systems, often fabricated from borosilicate glass, offer improved heat and mass transfer, leading to more efficient reactions with reduced solvent and energy requirements. The precise control afforded by microreactors can also minimize side reactions and improve product selectivity, potentially reducing waste and simplifying purification processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!