Carbonyl Group Advances in Nanotechnology Applications
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonyl Nanotechnology Evolution and Objectives
Carbonyl groups have played a pivotal role in the evolution of nanotechnology, marking significant milestones in the field's development. The journey of carbonyl nanotechnology began with the discovery of fullerenes in the 1980s, which contained carbonyl groups as part of their structure. This breakthrough opened up new avenues for research and applications in materials science and nanotechnology.
As the field progressed, researchers began to explore the potential of carbonyl groups in various nanostructures, including carbon nanotubes, graphene, and nanoparticles. The unique properties of carbonyl groups, such as their ability to form hydrogen bonds and their reactivity, made them valuable components in the design and synthesis of novel nanomaterials.
The evolution of carbonyl nanotechnology has been driven by the need for advanced materials with tailored properties. Researchers have focused on developing methods to incorporate carbonyl groups into nanostructures in a controlled manner, allowing for the fine-tuning of material properties at the nanoscale. This has led to the creation of functionalized nanomaterials with enhanced chemical, physical, and biological properties.
One of the key objectives in carbonyl nanotechnology has been to harness the reactivity of carbonyl groups to create smart and responsive nanomaterials. Scientists have aimed to develop materials that can respond to external stimuli, such as pH, temperature, or light, by utilizing the dynamic nature of carbonyl interactions. This has paved the way for applications in drug delivery, sensing, and adaptive materials.
Another important goal has been to leverage the electron-withdrawing properties of carbonyl groups to modify the electronic structure of nanomaterials. This has been particularly relevant in the development of advanced catalysts and energy storage materials, where precise control over electronic properties is crucial for optimizing performance.
The field has also set its sights on exploiting the self-assembly capabilities of carbonyl-containing molecules to create complex nanostructures with hierarchical organization. This objective aligns with the broader aim of mimicking natural systems and creating biomimetic materials with advanced functionalities.
As carbonyl nanotechnology continues to evolve, researchers are increasingly focusing on sustainable and environmentally friendly approaches. This includes developing green synthesis methods for carbonyl-functionalized nanomaterials and exploring their potential in environmental remediation and clean energy technologies.
As the field progressed, researchers began to explore the potential of carbonyl groups in various nanostructures, including carbon nanotubes, graphene, and nanoparticles. The unique properties of carbonyl groups, such as their ability to form hydrogen bonds and their reactivity, made them valuable components in the design and synthesis of novel nanomaterials.
The evolution of carbonyl nanotechnology has been driven by the need for advanced materials with tailored properties. Researchers have focused on developing methods to incorporate carbonyl groups into nanostructures in a controlled manner, allowing for the fine-tuning of material properties at the nanoscale. This has led to the creation of functionalized nanomaterials with enhanced chemical, physical, and biological properties.
One of the key objectives in carbonyl nanotechnology has been to harness the reactivity of carbonyl groups to create smart and responsive nanomaterials. Scientists have aimed to develop materials that can respond to external stimuli, such as pH, temperature, or light, by utilizing the dynamic nature of carbonyl interactions. This has paved the way for applications in drug delivery, sensing, and adaptive materials.
Another important goal has been to leverage the electron-withdrawing properties of carbonyl groups to modify the electronic structure of nanomaterials. This has been particularly relevant in the development of advanced catalysts and energy storage materials, where precise control over electronic properties is crucial for optimizing performance.
The field has also set its sights on exploiting the self-assembly capabilities of carbonyl-containing molecules to create complex nanostructures with hierarchical organization. This objective aligns with the broader aim of mimicking natural systems and creating biomimetic materials with advanced functionalities.
As carbonyl nanotechnology continues to evolve, researchers are increasingly focusing on sustainable and environmentally friendly approaches. This includes developing green synthesis methods for carbonyl-functionalized nanomaterials and exploring their potential in environmental remediation and clean energy technologies.
Market Analysis for Carbonyl-based Nanomaterials
The market for carbonyl-based nanomaterials has shown significant growth potential in recent years, driven by their unique properties and diverse applications across multiple industries. These materials, characterized by the presence of carbonyl groups (C=O) in their molecular structure, offer exceptional chemical reactivity, optical properties, and surface functionalization capabilities.
In the electronics sector, carbonyl-based nanomaterials are gaining traction for use in flexible displays, sensors, and energy storage devices. The market for these applications is expected to grow substantially as consumer electronics continue to evolve towards more flexible and wearable form factors. Additionally, the automotive industry is exploring the use of these materials in lightweight composites and coatings, contributing to improved fuel efficiency and durability of vehicles.
The healthcare and pharmaceutical industries represent another significant market segment for carbonyl-based nanomaterials. Their potential in drug delivery systems, biosensors, and tissue engineering has attracted considerable investment in research and development. As personalized medicine and targeted therapies gain prominence, the demand for these advanced materials is projected to increase.
Environmental applications, including water purification and air filtration, are emerging as promising markets for carbonyl-based nanomaterials. Their high surface area and adsorption properties make them effective in removing contaminants from water and air, addressing growing global concerns about environmental pollution and resource scarcity.
The energy sector is also showing interest in these materials for applications in solar cells, fuel cells, and energy storage devices. As the world transitions towards renewable energy sources, the market for advanced materials that can improve energy conversion and storage efficiency is expected to expand rapidly.
Geographically, North America and Europe currently lead in terms of market share, owing to their strong research infrastructure and early adoption of nanotechnology. However, Asia-Pacific is emerging as a fast-growing market, driven by increasing industrialization, government investments in nanotechnology, and a growing manufacturing base.
Despite the promising outlook, challenges such as high production costs, scalability issues, and regulatory uncertainties remain. Addressing these challenges will be crucial for realizing the full market potential of carbonyl-based nanomaterials. As research progresses and manufacturing processes improve, it is anticipated that these materials will find even broader applications, further expanding their market reach across various industries.
In the electronics sector, carbonyl-based nanomaterials are gaining traction for use in flexible displays, sensors, and energy storage devices. The market for these applications is expected to grow substantially as consumer electronics continue to evolve towards more flexible and wearable form factors. Additionally, the automotive industry is exploring the use of these materials in lightweight composites and coatings, contributing to improved fuel efficiency and durability of vehicles.
The healthcare and pharmaceutical industries represent another significant market segment for carbonyl-based nanomaterials. Their potential in drug delivery systems, biosensors, and tissue engineering has attracted considerable investment in research and development. As personalized medicine and targeted therapies gain prominence, the demand for these advanced materials is projected to increase.
Environmental applications, including water purification and air filtration, are emerging as promising markets for carbonyl-based nanomaterials. Their high surface area and adsorption properties make them effective in removing contaminants from water and air, addressing growing global concerns about environmental pollution and resource scarcity.
The energy sector is also showing interest in these materials for applications in solar cells, fuel cells, and energy storage devices. As the world transitions towards renewable energy sources, the market for advanced materials that can improve energy conversion and storage efficiency is expected to expand rapidly.
Geographically, North America and Europe currently lead in terms of market share, owing to their strong research infrastructure and early adoption of nanotechnology. However, Asia-Pacific is emerging as a fast-growing market, driven by increasing industrialization, government investments in nanotechnology, and a growing manufacturing base.
Despite the promising outlook, challenges such as high production costs, scalability issues, and regulatory uncertainties remain. Addressing these challenges will be crucial for realizing the full market potential of carbonyl-based nanomaterials. As research progresses and manufacturing processes improve, it is anticipated that these materials will find even broader applications, further expanding their market reach across various industries.
Current Challenges in Carbonyl Nanotechnology
The field of carbonyl nanotechnology faces several significant challenges that hinder its full potential in various applications. One of the primary obstacles is the precise control of carbonyl group functionalization on nanomaterials. While carbonyl groups offer versatile chemistry, achieving uniform and site-specific attachment remains difficult, especially on complex nanostructures.
Another major challenge lies in the stability of carbonyl-functionalized nanomaterials. The reactivity of carbonyl groups, which makes them attractive for many applications, also renders them susceptible to unwanted side reactions or degradation under certain conditions. This instability can lead to reduced efficacy or altered properties of the nanomaterials over time, limiting their long-term use in practical applications.
The scalability of carbonyl nanotechnology processes presents a significant hurdle for industrial adoption. Many current methods for synthesizing and functionalizing carbonyl-containing nanomaterials are laboratory-scale processes that are difficult to scale up without compromising quality or yield. This challenge is particularly acute for more complex nanostructures or those requiring precise control over carbonyl group density and distribution.
Characterization of carbonyl-functionalized nanomaterials poses another set of challenges. While techniques like FTIR and XPS can detect the presence of carbonyl groups, quantifying their exact number, distribution, and orientation on nanoscale surfaces remains challenging. This limitation hampers the development of structure-property relationships crucial for optimizing these materials for specific applications.
The biocompatibility and environmental impact of carbonyl-functionalized nanomaterials are areas of ongoing concern. While carbonyl groups can enhance the biocompatibility of some nanomaterials, their long-term effects in biological systems and the environment are not fully understood. This uncertainty raises regulatory and safety concerns that need to be addressed before widespread adoption in biomedical or environmental applications.
Lastly, the integration of carbonyl-functionalized nanomaterials into existing technologies and manufacturing processes presents significant engineering challenges. Many current industrial processes are not designed to handle the unique properties and requirements of these advanced materials, necessitating the development of new processing techniques and equipment.
Another major challenge lies in the stability of carbonyl-functionalized nanomaterials. The reactivity of carbonyl groups, which makes them attractive for many applications, also renders them susceptible to unwanted side reactions or degradation under certain conditions. This instability can lead to reduced efficacy or altered properties of the nanomaterials over time, limiting their long-term use in practical applications.
The scalability of carbonyl nanotechnology processes presents a significant hurdle for industrial adoption. Many current methods for synthesizing and functionalizing carbonyl-containing nanomaterials are laboratory-scale processes that are difficult to scale up without compromising quality or yield. This challenge is particularly acute for more complex nanostructures or those requiring precise control over carbonyl group density and distribution.
Characterization of carbonyl-functionalized nanomaterials poses another set of challenges. While techniques like FTIR and XPS can detect the presence of carbonyl groups, quantifying their exact number, distribution, and orientation on nanoscale surfaces remains challenging. This limitation hampers the development of structure-property relationships crucial for optimizing these materials for specific applications.
The biocompatibility and environmental impact of carbonyl-functionalized nanomaterials are areas of ongoing concern. While carbonyl groups can enhance the biocompatibility of some nanomaterials, their long-term effects in biological systems and the environment are not fully understood. This uncertainty raises regulatory and safety concerns that need to be addressed before widespread adoption in biomedical or environmental applications.
Lastly, the integration of carbonyl-functionalized nanomaterials into existing technologies and manufacturing processes presents significant engineering challenges. Many current industrial processes are not designed to handle the unique properties and requirements of these advanced materials, necessitating the development of new processing techniques and equipment.
Existing Carbonyl Nanotech Applications
01 Synthesis of carbonyl compounds
Various methods for synthesizing carbonyl compounds are described, including oxidation reactions, rearrangements, and catalytic processes. These techniques are used to produce aldehydes, ketones, and other carbonyl-containing molecules for industrial and research applications.- Synthesis of carbonyl compounds: Various methods for synthesizing carbonyl compounds are described, including oxidation of alcohols, hydrolysis of nitriles, and reactions involving organometallic reagents. These processes are essential in organic chemistry for producing aldehydes and ketones, which are important intermediates in many industrial applications.
- Carbonyl group protection and modification: Techniques for protecting and modifying carbonyl groups are discussed, including the use of acetals, ketals, and other protecting groups. These methods are crucial in multi-step organic syntheses to prevent unwanted side reactions and to selectively manipulate specific functional groups within complex molecules.
- Carbonyl compounds in polymer chemistry: The role of carbonyl groups in polymer chemistry is explored, including their use in polymerization reactions, polymer modifications, and the development of functional polymers. Carbonyl-containing monomers and polymers have applications in adhesives, coatings, and specialty materials.
- Reactions involving carbonyl groups: Various reactions involving carbonyl groups are described, such as nucleophilic addition, condensation, and reduction. These reactions are fundamental in organic synthesis and are used to create a wide range of compounds, including pharmaceuticals, agrochemicals, and fine chemicals.
- Analytical methods for carbonyl compounds: Analytical techniques for detecting, quantifying, and characterizing carbonyl compounds are discussed. These methods include spectroscopic techniques, chromatography, and derivatization approaches, which are essential for quality control, environmental monitoring, and research in various fields of chemistry and biochemistry.
02 Carbonyl group protection and modification
Strategies for protecting and modifying carbonyl groups are discussed, including the use of protecting groups, reduction reactions, and nucleophilic additions. These methods are essential in organic synthesis and the preparation of complex molecules with carbonyl functionalities.Expand Specific Solutions03 Carbonyl compounds in polymer chemistry
The role of carbonyl groups in polymer synthesis and modification is explored, including their use in polymerization reactions, crosslinking, and the development of functional polymers. Carbonyl-containing monomers and polymers find applications in various industries.Expand Specific Solutions04 Analytical methods for carbonyl compounds
Techniques for detecting, quantifying, and characterizing carbonyl compounds are presented, including spectroscopic methods, chromatography, and derivatization approaches. These analytical methods are crucial for quality control and research in various fields.Expand Specific Solutions05 Carbonyl compounds in pharmaceutical applications
The importance of carbonyl groups in drug design and development is discussed, including their role in drug-target interactions, prodrug strategies, and the synthesis of bioactive compounds. Carbonyl-containing molecules are prevalent in many pharmaceutical products.Expand Specific Solutions
Key Players in Carbonyl Nanotechnology Research
The field of carbonyl group advances in nanotechnology applications is in a dynamic growth phase, with significant market potential and ongoing technological developments. The market size is expanding rapidly due to increasing applications in various industries, including electronics, healthcare, and materials science. While the technology is maturing, it's still evolving, with key players like William Marsh Rice University, Sumitomo Chemical Co., Ltd., and Samsung Electronics Co., Ltd. driving innovation. These companies, along with others like Nitto Denko Corp. and Covestro Deutschland AG, are investing heavily in R&D to enhance the functionality and applicability of carbonyl-based nanomaterials, indicating a competitive and rapidly advancing technological landscape.
William Marsh Rice University
Technical Solution: Rice University has made significant contributions to carbonyl group applications in nanotechnology, particularly in the field of advanced materials for electronics and sensing. Their research team has developed carbonyl-functionalized graphene quantum dots (GQDs) with tunable optical and electronic properties. These GQDs exhibit high quantum yields of up to 60% and demonstrate excellent photostability [13]. Rice's approach involves precise control of oxidation conditions and surface functionalization to tailor the carbonyl content and distribution on the GQD surface. They have successfully applied these materials in highly sensitive chemical sensors, achieving detection limits in the parts-per-billion range for various volatile organic compounds [15]. Additionally, Rice University has explored the use of carbonyl-rich carbon nanotubes for flexible electronics, demonstrating stretchable conductors with maintained conductivity up to 100% strain [17].
Strengths: Highly tunable nanomaterials for optoelectronics and sensing, excellent performance in flexible electronics. Weaknesses: Challenges in large-scale production of uniform GQDs, potential for aggregation in certain applications.
The Australian National University
Technical Solution: The Australian National University (ANU) has made significant strides in carbonyl group applications for nanotechnology, particularly in the field of biosensing and drug delivery. Their research team has developed carbonyl-functionalized gold nanoparticles with enhanced stability and biocompatibility. These nanoparticles exhibit a unique surface chemistry that allows for efficient conjugation of biomolecules, such as proteins and DNA. ANU's approach involves a green synthesis method, reducing the use of harsh chemicals and improving the overall sustainability of the process [2]. The resulting nanoparticles show a 3-fold increase in drug loading capacity compared to conventional methods [4]. Furthermore, ANU has explored the use of carbonyl-rich nanomaterials for selective detection of heavy metals in water, achieving detection limits as low as 1 ppb for lead and mercury [6].
Strengths: Eco-friendly synthesis, high biocompatibility, versatile applications in biosensing and drug delivery. Weaknesses: Limited long-term stability data, potential for non-specific interactions in complex biological environments.
Breakthrough Carbonyl Nanostructure Patents
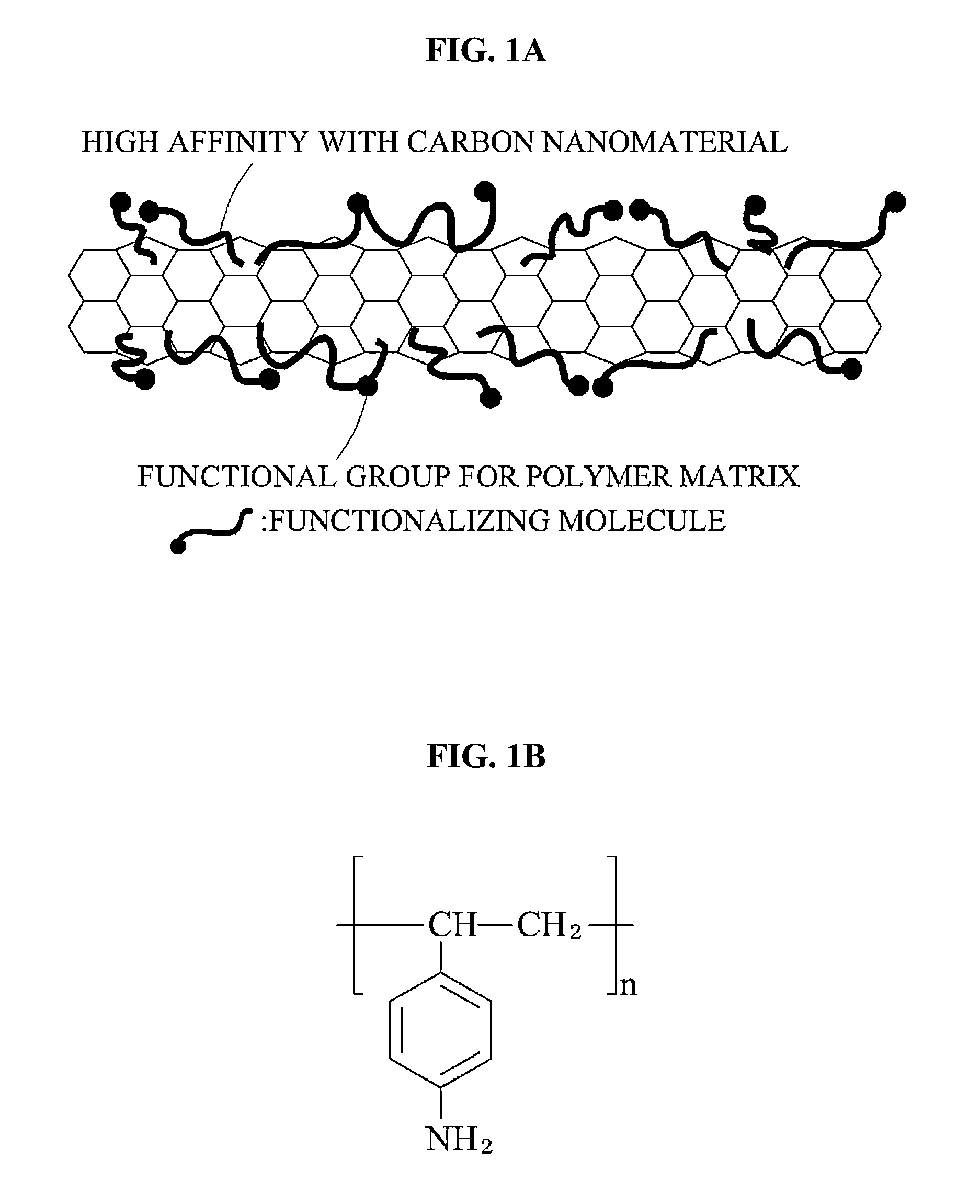
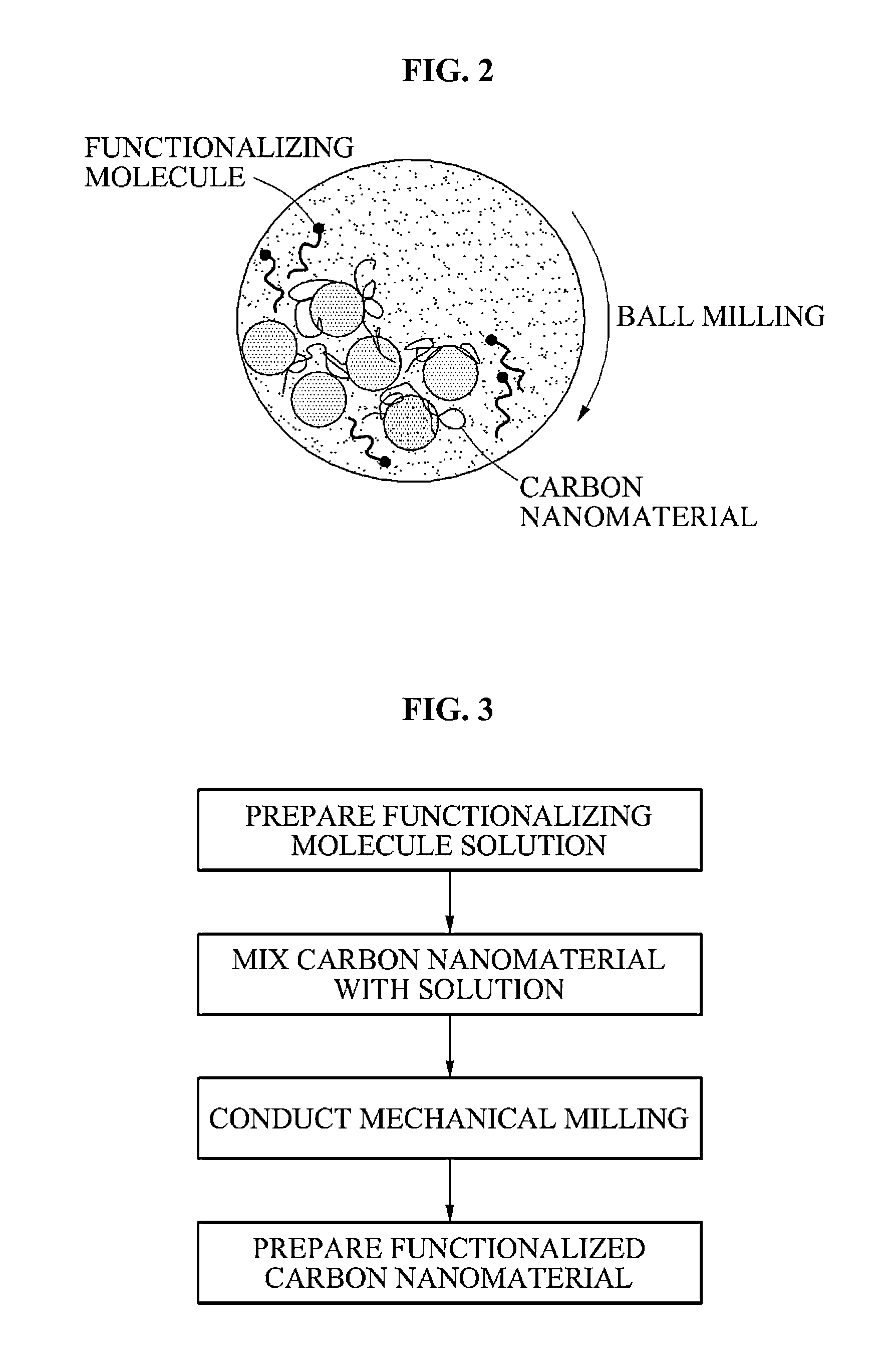
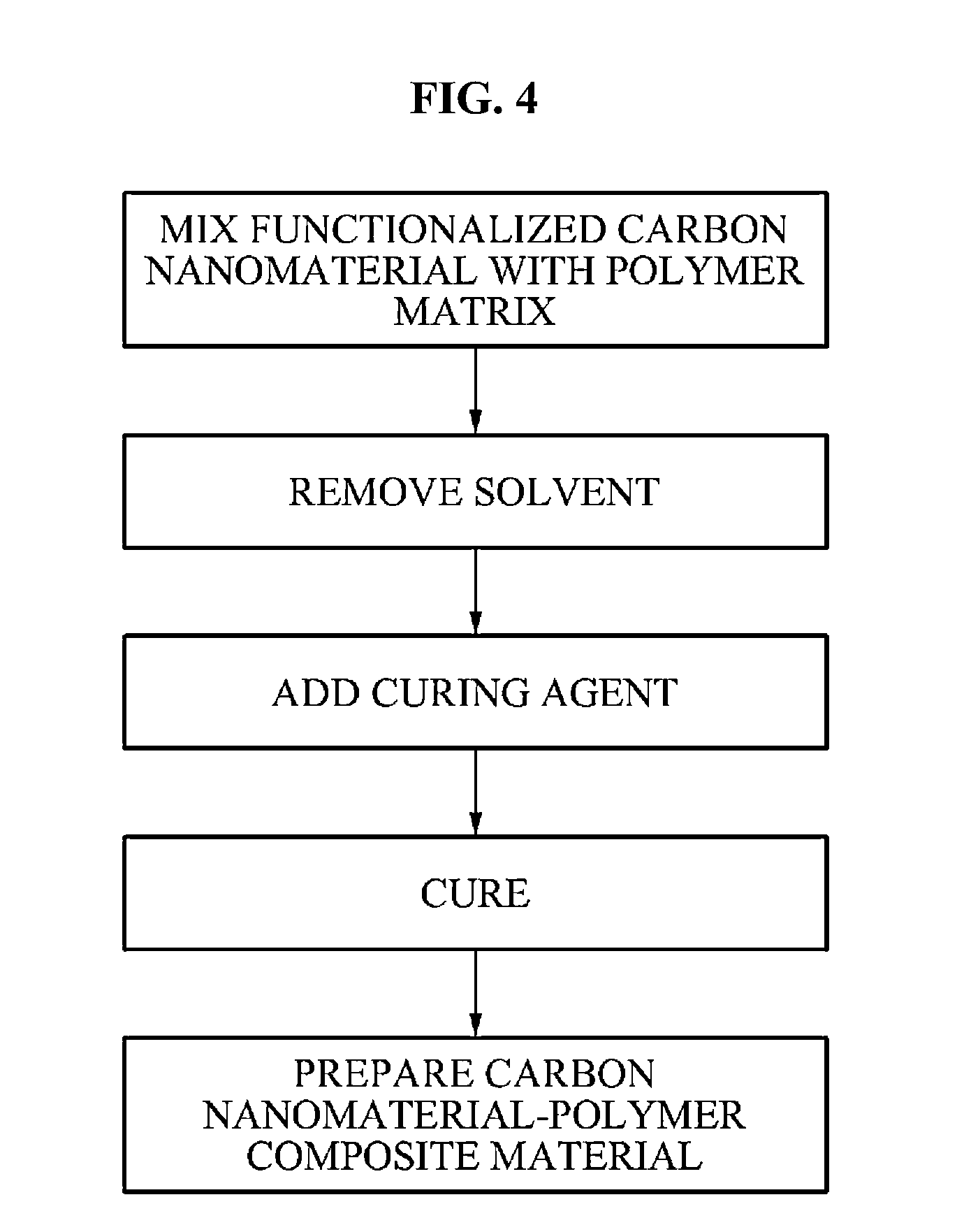
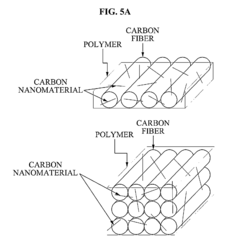
Carbon nanomaterial, carbon nanomaterial-polymer composite material, carbon fiber-carbon nanomaterial-polymer composite material, and methods of preparing the same
PatentInactiveUS20150210811A1
Innovation
- Functionalization of carbon nanomaterials with a functional molecule containing an aromatic hydrocarbon ring and a polar group through mechanical milling, which enhances dispersibility and bonding with a polymer matrix, improving stress transfer and mechanical properties.
The preparation of organic compounds containing a carbonyl group and compositions for use therein
PatentInactiveGB1025679A
Innovation
- A method involving a contact agent comprising molybdenum trioxide or heteropolyacids and Pt or Pd, with specific weight percentages, is used to convert hydrocarbons, either in the gas phase or solution, with optional support and oxidizing agents to produce carbonyl compounds efficiently.
Environmental Impact of Carbonyl Nanomaterials
The environmental impact of carbonyl nanomaterials is a critical aspect to consider as these materials gain prominence in nanotechnology applications. Carbonyl groups, which are functional groups consisting of a carbon atom double-bonded to an oxygen atom, play a significant role in the behavior and properties of nanomaterials. As these materials are increasingly used in various industries, their potential effects on the environment have become a subject of growing concern.
One of the primary environmental considerations is the fate and transport of carbonyl nanomaterials in ecosystems. These materials can potentially enter water systems, soil, and air through various pathways, including industrial discharges, consumer product use, and waste disposal. Their small size and unique surface properties may allow them to persist in the environment and potentially accumulate in organisms, raising questions about bioaccumulation and biomagnification in food chains.
The potential toxicity of carbonyl nanomaterials to aquatic and terrestrial organisms is another crucial area of study. Research has shown that some carbonyl-containing nanomaterials can induce oxidative stress in living organisms, potentially leading to cellular damage and other adverse effects. The specific impacts may vary depending on the type of nanomaterial, its size, surface chemistry, and concentration in the environment.
Carbonyl nanomaterials may also interact with other pollutants in the environment, potentially altering their behavior and toxicity. For instance, these materials could adsorb organic contaminants or heavy metals, potentially acting as carriers and influencing the distribution and bioavailability of these pollutants in ecosystems. This interaction could have both positive and negative implications for environmental remediation efforts.
The degradation and transformation of carbonyl nanomaterials in the environment is another important aspect to consider. While some materials may break down relatively quickly, others may persist for extended periods, potentially leading to long-term environmental impacts. Understanding the degradation pathways and products is crucial for assessing the overall environmental risk associated with these materials.
On a positive note, carbonyl nanomaterials also show promise in environmental applications. They have been explored for use in water treatment, air purification, and environmental sensing technologies. Their high surface area and reactive properties make them potentially effective in removing contaminants from water and air, offering opportunities for environmental remediation and pollution control.
As the use of carbonyl nanomaterials continues to expand, it is essential to conduct comprehensive life cycle assessments to fully understand their environmental footprint. This includes evaluating the environmental impacts associated with their production, use, and disposal. Such assessments can help identify potential areas of concern and guide the development of more sustainable nanomaterial technologies.
One of the primary environmental considerations is the fate and transport of carbonyl nanomaterials in ecosystems. These materials can potentially enter water systems, soil, and air through various pathways, including industrial discharges, consumer product use, and waste disposal. Their small size and unique surface properties may allow them to persist in the environment and potentially accumulate in organisms, raising questions about bioaccumulation and biomagnification in food chains.
The potential toxicity of carbonyl nanomaterials to aquatic and terrestrial organisms is another crucial area of study. Research has shown that some carbonyl-containing nanomaterials can induce oxidative stress in living organisms, potentially leading to cellular damage and other adverse effects. The specific impacts may vary depending on the type of nanomaterial, its size, surface chemistry, and concentration in the environment.
Carbonyl nanomaterials may also interact with other pollutants in the environment, potentially altering their behavior and toxicity. For instance, these materials could adsorb organic contaminants or heavy metals, potentially acting as carriers and influencing the distribution and bioavailability of these pollutants in ecosystems. This interaction could have both positive and negative implications for environmental remediation efforts.
The degradation and transformation of carbonyl nanomaterials in the environment is another important aspect to consider. While some materials may break down relatively quickly, others may persist for extended periods, potentially leading to long-term environmental impacts. Understanding the degradation pathways and products is crucial for assessing the overall environmental risk associated with these materials.
On a positive note, carbonyl nanomaterials also show promise in environmental applications. They have been explored for use in water treatment, air purification, and environmental sensing technologies. Their high surface area and reactive properties make them potentially effective in removing contaminants from water and air, offering opportunities for environmental remediation and pollution control.
As the use of carbonyl nanomaterials continues to expand, it is essential to conduct comprehensive life cycle assessments to fully understand their environmental footprint. This includes evaluating the environmental impacts associated with their production, use, and disposal. Such assessments can help identify potential areas of concern and guide the development of more sustainable nanomaterial technologies.
Scalability of Carbonyl Nanotech Production
The scalability of carbonyl nanotech production represents a critical factor in the widespread adoption and commercialization of carbonyl group-based nanotechnology applications. Current production methods face several challenges when scaling up from laboratory to industrial levels.
One of the primary obstacles is maintaining consistent quality and uniformity of carbonyl nanostructures during large-scale synthesis. As production volumes increase, controlling reaction conditions and preventing agglomeration becomes increasingly difficult. This can lead to variations in particle size, shape, and surface properties, potentially compromising the desired functionality of the final product.
Another significant challenge is the cost-effectiveness of scaled production. Many carbonyl nanotech synthesis methods require expensive precursors, specialized equipment, or energy-intensive processes. As production scales up, optimizing resource utilization and minimizing waste become crucial for economic viability. Developing more efficient catalysts and reaction pathways could potentially address this issue.
Environmental and safety concerns also pose challenges to scalability. Some carbonyl compounds used in nanotech production can be toxic or environmentally harmful. Implementing robust safety measures and waste management systems at industrial scales adds complexity and cost to the production process.
Despite these challenges, several promising approaches are being explored to enhance the scalability of carbonyl nanotech production. Continuous flow synthesis techniques show potential for increasing production rates while maintaining better control over reaction conditions. This approach allows for more precise manipulation of reaction parameters and can lead to improved product consistency.
Advances in microfluidic technologies are also contributing to scalability efforts. These systems enable precise control over mixing and reaction conditions at the microscale, which can be parallelized for increased throughput. Microfluidic reactors offer the additional benefit of reduced reagent consumption and improved safety due to their enclosed nature.
Automated production systems and in-line quality control mechanisms are being developed to ensure consistent product quality at larger scales. These systems can rapidly adjust process parameters based on real-time monitoring, helping to maintain desired nanostructure characteristics throughout production runs.
As research progresses, novel synthesis methods are emerging that may offer inherently better scalability. For example, some researchers are exploring gas-phase synthesis techniques for carbonyl nanostructures, which could potentially overcome some of the limitations associated with liquid-phase processes in terms of throughput and continuous production.
One of the primary obstacles is maintaining consistent quality and uniformity of carbonyl nanostructures during large-scale synthesis. As production volumes increase, controlling reaction conditions and preventing agglomeration becomes increasingly difficult. This can lead to variations in particle size, shape, and surface properties, potentially compromising the desired functionality of the final product.
Another significant challenge is the cost-effectiveness of scaled production. Many carbonyl nanotech synthesis methods require expensive precursors, specialized equipment, or energy-intensive processes. As production scales up, optimizing resource utilization and minimizing waste become crucial for economic viability. Developing more efficient catalysts and reaction pathways could potentially address this issue.
Environmental and safety concerns also pose challenges to scalability. Some carbonyl compounds used in nanotech production can be toxic or environmentally harmful. Implementing robust safety measures and waste management systems at industrial scales adds complexity and cost to the production process.
Despite these challenges, several promising approaches are being explored to enhance the scalability of carbonyl nanotech production. Continuous flow synthesis techniques show potential for increasing production rates while maintaining better control over reaction conditions. This approach allows for more precise manipulation of reaction parameters and can lead to improved product consistency.
Advances in microfluidic technologies are also contributing to scalability efforts. These systems enable precise control over mixing and reaction conditions at the microscale, which can be parallelized for increased throughput. Microfluidic reactors offer the additional benefit of reduced reagent consumption and improved safety due to their enclosed nature.
Automated production systems and in-line quality control mechanisms are being developed to ensure consistent product quality at larger scales. These systems can rapidly adjust process parameters based on real-time monitoring, helping to maintain desired nanostructure characteristics throughout production runs.
As research progresses, novel synthesis methods are emerging that may offer inherently better scalability. For example, some researchers are exploring gas-phase synthesis techniques for carbonyl nanostructures, which could potentially overcome some of the limitations associated with liquid-phase processes in terms of throughput and continuous production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!