Contrasting Muscimol and Benzodiazepines in Anxiety Treatments
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Anxiety Treatment Evolution
The evolution of anxiety treatments has been a journey marked by significant advancements in understanding the neurobiological basis of anxiety disorders and the development of targeted pharmacological interventions. Early treatments primarily relied on non-specific sedatives and barbiturates, which, while effective in reducing anxiety symptoms, often came with severe side effects and high potential for dependence.
The 1960s saw a major breakthrough with the introduction of benzodiazepines, such as diazepam (Valium) and alprazolam (Xanax). These drugs revolutionized anxiety treatment by offering rapid symptom relief with fewer side effects compared to their predecessors. Benzodiazepines work by enhancing the effect of gamma-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the central nervous system, thereby reducing neuronal excitability and anxiety.
As research progressed, the limitations of benzodiazepines became apparent, including the risk of tolerance, dependence, and cognitive impairment with long-term use. This led to the exploration of alternative pharmacological approaches, including the development of selective serotonin reuptake inhibitors (SSRIs) in the 1980s. SSRIs, while primarily used for depression, proved effective in treating various anxiety disorders, offering a different mechanism of action and safety profile compared to benzodiazepines.
In recent years, there has been growing interest in compounds that modulate the GABAergic system more selectively than benzodiazepines. Muscimol, a naturally occurring GABA agonist found in certain mushroom species, has emerged as a potential candidate for anxiety treatment. Unlike benzodiazepines, which act as positive allosteric modulators of GABA-A receptors, muscimol directly activates these receptors, potentially offering a more targeted approach to anxiety reduction.
The contrast between muscimol and benzodiazepines represents a shift towards more precise pharmacological interventions. While benzodiazepines affect multiple GABA-A receptor subtypes, leading to their broad anxiolytic effects and side effect profile, muscimol's more specific action may provide anxiolytic benefits with potentially fewer adverse effects. This evolution reflects the ongoing pursuit of treatments that balance efficacy with improved safety and tolerability.
Current research is focused on understanding the unique properties of muscimol and its potential advantages over benzodiazepines in anxiety treatment. This includes investigating its effects on different anxiety subtypes, its potential for reduced tolerance and dependence, and its impact on cognitive function. The exploration of muscimol and similar compounds represents a continuation of the trend towards more targeted, neuroscience-based approaches to anxiety treatment, aiming to address the limitations of current therapies while maintaining or improving therapeutic efficacy.
The 1960s saw a major breakthrough with the introduction of benzodiazepines, such as diazepam (Valium) and alprazolam (Xanax). These drugs revolutionized anxiety treatment by offering rapid symptom relief with fewer side effects compared to their predecessors. Benzodiazepines work by enhancing the effect of gamma-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the central nervous system, thereby reducing neuronal excitability and anxiety.
As research progressed, the limitations of benzodiazepines became apparent, including the risk of tolerance, dependence, and cognitive impairment with long-term use. This led to the exploration of alternative pharmacological approaches, including the development of selective serotonin reuptake inhibitors (SSRIs) in the 1980s. SSRIs, while primarily used for depression, proved effective in treating various anxiety disorders, offering a different mechanism of action and safety profile compared to benzodiazepines.
In recent years, there has been growing interest in compounds that modulate the GABAergic system more selectively than benzodiazepines. Muscimol, a naturally occurring GABA agonist found in certain mushroom species, has emerged as a potential candidate for anxiety treatment. Unlike benzodiazepines, which act as positive allosteric modulators of GABA-A receptors, muscimol directly activates these receptors, potentially offering a more targeted approach to anxiety reduction.
The contrast between muscimol and benzodiazepines represents a shift towards more precise pharmacological interventions. While benzodiazepines affect multiple GABA-A receptor subtypes, leading to their broad anxiolytic effects and side effect profile, muscimol's more specific action may provide anxiolytic benefits with potentially fewer adverse effects. This evolution reflects the ongoing pursuit of treatments that balance efficacy with improved safety and tolerability.
Current research is focused on understanding the unique properties of muscimol and its potential advantages over benzodiazepines in anxiety treatment. This includes investigating its effects on different anxiety subtypes, its potential for reduced tolerance and dependence, and its impact on cognitive function. The exploration of muscimol and similar compounds represents a continuation of the trend towards more targeted, neuroscience-based approaches to anxiety treatment, aiming to address the limitations of current therapies while maintaining or improving therapeutic efficacy.
Market Analysis for Anxiolytics
The global anxiolytics market has shown significant growth in recent years, driven by increasing prevalence of anxiety disorders and rising awareness about mental health. The market is segmented into various drug classes, with benzodiazepines and selective serotonin reuptake inhibitors (SSRIs) being the dominant players. However, there is a growing interest in alternative treatments, including GABAergic compounds like muscimol.
Benzodiazepines have long been a mainstay in anxiety treatment, valued for their rapid onset of action and effectiveness in acute anxiety situations. They hold a substantial market share due to their well-established efficacy profile and widespread familiarity among healthcare providers. However, concerns about dependence and side effects have led to a shift towards newer alternatives.
Muscimol, a naturally occurring GABA agonist, represents an emerging segment in the anxiolytics market. While not yet widely used in clinical practice, it has garnered attention due to its potential for anxiety relief without the risk of dependence associated with benzodiazepines. The market for muscimol-based treatments is still in its infancy but shows promise for growth as research progresses.
The anxiolytics market is characterized by a mix of branded and generic products. Generic versions of benzodiazepines have significantly impacted market dynamics, leading to price competition and market share erosion for branded products. This trend has prompted pharmaceutical companies to invest in the development of novel anxiolytic compounds with improved safety profiles and efficacy.
Regionally, North America dominates the anxiolytics market, followed by Europe. These regions benefit from advanced healthcare infrastructure, higher awareness of mental health issues, and greater access to treatment. However, emerging markets in Asia-Pacific and Latin America are expected to show rapid growth due to improving healthcare access and rising mental health awareness.
The market is also witnessing a shift towards personalized medicine approaches in anxiety treatment. This trend is driving research into biomarkers and genetic factors that can predict treatment response, potentially leading to more targeted therapies and opening new market opportunities for specialized anxiolytic treatments.
In conclusion, the anxiolytics market is dynamic and evolving, with traditional treatments like benzodiazepines facing challenges from newer alternatives and emerging compounds like muscimol. The market's future growth will likely be shaped by the development of safer, more effective treatments and a growing emphasis on personalized approaches to anxiety management.
Benzodiazepines have long been a mainstay in anxiety treatment, valued for their rapid onset of action and effectiveness in acute anxiety situations. They hold a substantial market share due to their well-established efficacy profile and widespread familiarity among healthcare providers. However, concerns about dependence and side effects have led to a shift towards newer alternatives.
Muscimol, a naturally occurring GABA agonist, represents an emerging segment in the anxiolytics market. While not yet widely used in clinical practice, it has garnered attention due to its potential for anxiety relief without the risk of dependence associated with benzodiazepines. The market for muscimol-based treatments is still in its infancy but shows promise for growth as research progresses.
The anxiolytics market is characterized by a mix of branded and generic products. Generic versions of benzodiazepines have significantly impacted market dynamics, leading to price competition and market share erosion for branded products. This trend has prompted pharmaceutical companies to invest in the development of novel anxiolytic compounds with improved safety profiles and efficacy.
Regionally, North America dominates the anxiolytics market, followed by Europe. These regions benefit from advanced healthcare infrastructure, higher awareness of mental health issues, and greater access to treatment. However, emerging markets in Asia-Pacific and Latin America are expected to show rapid growth due to improving healthcare access and rising mental health awareness.
The market is also witnessing a shift towards personalized medicine approaches in anxiety treatment. This trend is driving research into biomarkers and genetic factors that can predict treatment response, potentially leading to more targeted therapies and opening new market opportunities for specialized anxiolytic treatments.
In conclusion, the anxiolytics market is dynamic and evolving, with traditional treatments like benzodiazepines facing challenges from newer alternatives and emerging compounds like muscimol. The market's future growth will likely be shaped by the development of safer, more effective treatments and a growing emphasis on personalized approaches to anxiety management.
Current Challenges in Anxiety Pharmacology
The field of anxiety pharmacology faces several significant challenges that hinder the development of more effective and safer treatments. One of the primary issues is the limited understanding of the precise neurobiological mechanisms underlying anxiety disorders. While the involvement of neurotransmitter systems such as GABA and serotonin is well-established, the complex interplay between these systems and other neurological factors remains unclear.
The current gold standard for anxiety treatment, benzodiazepines, presents its own set of challenges. These drugs, while effective in the short term, are associated with numerous side effects, including sedation, cognitive impairment, and a high risk of dependence and abuse. The development of tolerance to their anxiolytic effects also limits their long-term efficacy. Finding alternatives that maintain the therapeutic benefits while minimizing these drawbacks is a major focus of current research.
Another significant challenge is the heterogeneity of anxiety disorders. Different subtypes of anxiety may respond differently to various pharmacological interventions, making it difficult to develop a one-size-fits-all approach. This variability necessitates more personalized treatment strategies, which are currently limited by our incomplete understanding of individual patient factors that influence treatment response.
The exploration of novel targets, such as the GABAergic system modulated by muscimol, presents both opportunities and challenges. While compounds like muscimol show promise in preclinical studies, translating these findings into safe and effective human treatments remains a significant hurdle. Issues such as blood-brain barrier penetration, off-target effects, and potential long-term consequences of altering GABAergic signaling need to be carefully addressed.
Furthermore, the development of new anxiolytic drugs is hampered by the limitations of animal models in predicting human responses. The complexity of human anxiety and the subjective nature of its symptoms make it challenging to fully replicate these conditions in preclinical studies. This gap between animal research and human clinical outcomes often leads to failures in late-stage drug development, increasing the cost and risk associated with bringing new anxiety treatments to market.
Lastly, there is a growing need for treatments that not only alleviate symptoms but also address the underlying causes of anxiety disorders. Current pharmacological approaches primarily focus on symptom management rather than disease modification. Developing interventions that can potentially alter the course of anxiety disorders or provide long-lasting remission remains a significant challenge in the field.
The current gold standard for anxiety treatment, benzodiazepines, presents its own set of challenges. These drugs, while effective in the short term, are associated with numerous side effects, including sedation, cognitive impairment, and a high risk of dependence and abuse. The development of tolerance to their anxiolytic effects also limits their long-term efficacy. Finding alternatives that maintain the therapeutic benefits while minimizing these drawbacks is a major focus of current research.
Another significant challenge is the heterogeneity of anxiety disorders. Different subtypes of anxiety may respond differently to various pharmacological interventions, making it difficult to develop a one-size-fits-all approach. This variability necessitates more personalized treatment strategies, which are currently limited by our incomplete understanding of individual patient factors that influence treatment response.
The exploration of novel targets, such as the GABAergic system modulated by muscimol, presents both opportunities and challenges. While compounds like muscimol show promise in preclinical studies, translating these findings into safe and effective human treatments remains a significant hurdle. Issues such as blood-brain barrier penetration, off-target effects, and potential long-term consequences of altering GABAergic signaling need to be carefully addressed.
Furthermore, the development of new anxiolytic drugs is hampered by the limitations of animal models in predicting human responses. The complexity of human anxiety and the subjective nature of its symptoms make it challenging to fully replicate these conditions in preclinical studies. This gap between animal research and human clinical outcomes often leads to failures in late-stage drug development, increasing the cost and risk associated with bringing new anxiety treatments to market.
Lastly, there is a growing need for treatments that not only alleviate symptoms but also address the underlying causes of anxiety disorders. Current pharmacological approaches primarily focus on symptom management rather than disease modification. Developing interventions that can potentially alter the course of anxiety disorders or provide long-lasting remission remains a significant challenge in the field.
Mechanism of Action Comparison
01 Comparative effectiveness of muscimol and benzodiazepines
Studies have been conducted to compare the effectiveness of muscimol, a GABA receptor agonist, with benzodiazepines in treating anxiety disorders. Both compounds act on the GABAergic system, but their mechanisms of action and efficacy profiles differ. Research suggests that muscimol may offer potential advantages in certain anxiety conditions, with possibly fewer side effects than traditional benzodiazepines.- Comparative effectiveness of muscimol and benzodiazepines: Studies have been conducted to compare the effectiveness of muscimol, a GABA receptor agonist, with benzodiazepines in treating anxiety disorders. Research suggests that muscimol may offer similar anxiolytic effects to benzodiazepines but with potentially fewer side effects and lower risk of dependence.
- Novel formulations and delivery methods: Researchers have developed new formulations and delivery methods for muscimol and benzodiazepines to enhance their effectiveness in anxiety treatment. These innovations include extended-release formulations, transdermal patches, and intranasal delivery systems, which may improve bioavailability and duration of action.
- Combination therapies for enhanced efficacy: Combination therapies involving muscimol or benzodiazepines with other anxiolytic agents or complementary treatments have been explored to enhance overall efficacy in anxiety management. These combinations aim to address multiple aspects of anxiety disorders and potentially reduce the required dosages of individual components.
- Genetic factors influencing treatment response: Research has identified genetic factors that may influence individual responses to muscimol and benzodiazepines in anxiety treatment. This knowledge could lead to more personalized treatment approaches, allowing for better prediction of treatment outcomes and potential side effects based on a patient's genetic profile.
- Long-term effects and safety profiles: Studies have been conducted to evaluate the long-term effects and safety profiles of muscimol and benzodiazepines in anxiety treatment. These investigations aim to better understand the potential risks and benefits associated with prolonged use, including cognitive effects, tolerance development, and withdrawal symptoms.
02 Novel formulations and delivery methods
Researchers have developed new formulations and delivery methods for both muscimol and benzodiazepines to enhance their effectiveness in anxiety treatment. These innovations include extended-release preparations, transdermal patches, and intranasal delivery systems. Such advancements aim to improve bioavailability, reduce side effects, and provide more targeted anxiety relief.Expand Specific Solutions03 Combination therapies and synergistic effects
Investigations into combination therapies involving muscimol, benzodiazepines, and other anxiolytic compounds have revealed potential synergistic effects. These combinations may offer enhanced anxiety relief with lower doses of individual components, potentially reducing side effects and improving overall treatment outcomes for patients with various anxiety disorders.Expand Specific Solutions04 Genetic factors influencing treatment response
Research has identified genetic factors that may influence individual responses to muscimol and benzodiazepines in anxiety treatment. Genetic variations in GABA receptor subunits and neurotransmitter transporters can affect drug efficacy and side effect profiles. This knowledge is contributing to the development of personalized treatment approaches for anxiety disorders.Expand Specific Solutions05 Long-term effects and addiction potential
Studies have examined the long-term effects and addiction potential of muscimol and benzodiazepines in anxiety treatment. While benzodiazepines are known for their potential for dependence and withdrawal symptoms, research on muscimol suggests it may have a lower risk of addiction. However, further investigation is needed to fully understand the long-term implications of both compounds in anxiety management.Expand Specific Solutions
Key Players in Anxiolytic Research
The competitive landscape for contrasting muscimol and benzodiazepines in anxiety treatments is in a mature stage, with established players like Pfizer, Janssen Pharmaceutica, and Eli Lilly dominating the market. The global anxiety disorders and depression treatment market is substantial, valued at over $15 billion annually. Technologically, benzodiazepines are well-established, while muscimol-based treatments are still emerging. Companies like Vivoryon Therapeutics and Intra-Cellular Therapies are exploring novel approaches, potentially disrupting the market. Major pharmaceutical firms continue to invest in R&D, seeking improved efficacy and reduced side effects in anxiety treatments.
Pfizer Inc.
Technical Solution: Pfizer has developed a novel approach to anxiety treatment by focusing on the GABAergic system, which is the target of both muscimol and benzodiazepines. Their research has led to the development of compounds that selectively modulate GABA-A receptor subtypes, aiming to provide anxiolytic effects with reduced side effects compared to traditional benzodiazepines[1]. Pfizer's compounds demonstrate high affinity for α2/α3 subunit-containing GABA-A receptors, which are believed to mediate anxiolytic effects without the sedation and addiction potential associated with α1 subunit-targeting benzodiazepines[2]. In preclinical studies, these compounds have shown promising results in anxiety models with a better safety profile than classical benzodiazepines[3].
Strengths: Targeted approach may lead to reduced side effects and addiction potential. Potentially faster onset of action compared to SSRIs. Weaknesses: May still carry some risk of tolerance and withdrawal symptoms. Long-term efficacy and safety data in humans are still needed.
Janssen Pharmaceutica NV
Technical Solution: Janssen has been exploring the use of esketamine, a derivative of ketamine, for treatment-resistant depression and anxiety disorders. While not directly related to muscimol or benzodiazepines, this approach represents a novel mechanism targeting the glutamatergic system, which interacts with GABAergic pathways[4]. Their nasal spray formulation of esketamine (Spravato) has shown rapid onset of action in reducing anxiety symptoms associated with major depressive disorder[5]. Janssen is also investigating compounds that modulate both GABA and glutamate systems, aiming to provide a more balanced approach to anxiety treatment with potentially fewer side effects than traditional anxiolytics[6].
Strengths: Rapid onset of action, potentially effective for treatment-resistant cases. Novel mechanism of action may address limitations of current therapies. Weaknesses: Administration requires medical supervision. Long-term safety profile still being established. Potential for abuse and dissociative side effects.
Innovative Approaches in Anxiety Treatment
Antianxiety compound 7-(2-(2-(3-(4-chlorophenyl) acryloyl) phenoxy) ethoxy)-4-methyl-2h-chromen-2-one and synthesis thereof
PatentPendingIN202211044302A
Innovation
- The synthesis and characterization of the novel antianxiety compound 7-(2-(2-(3-(4-chlorophenyl) acryloyl) phenoxy) ethoxy)-4-methyl-2H-chromen-2-one, which is evaluated for its anxiolytic activity using the elevated plus maze method in animal models, demonstrating its effectiveness as a potential therapeutic agent.
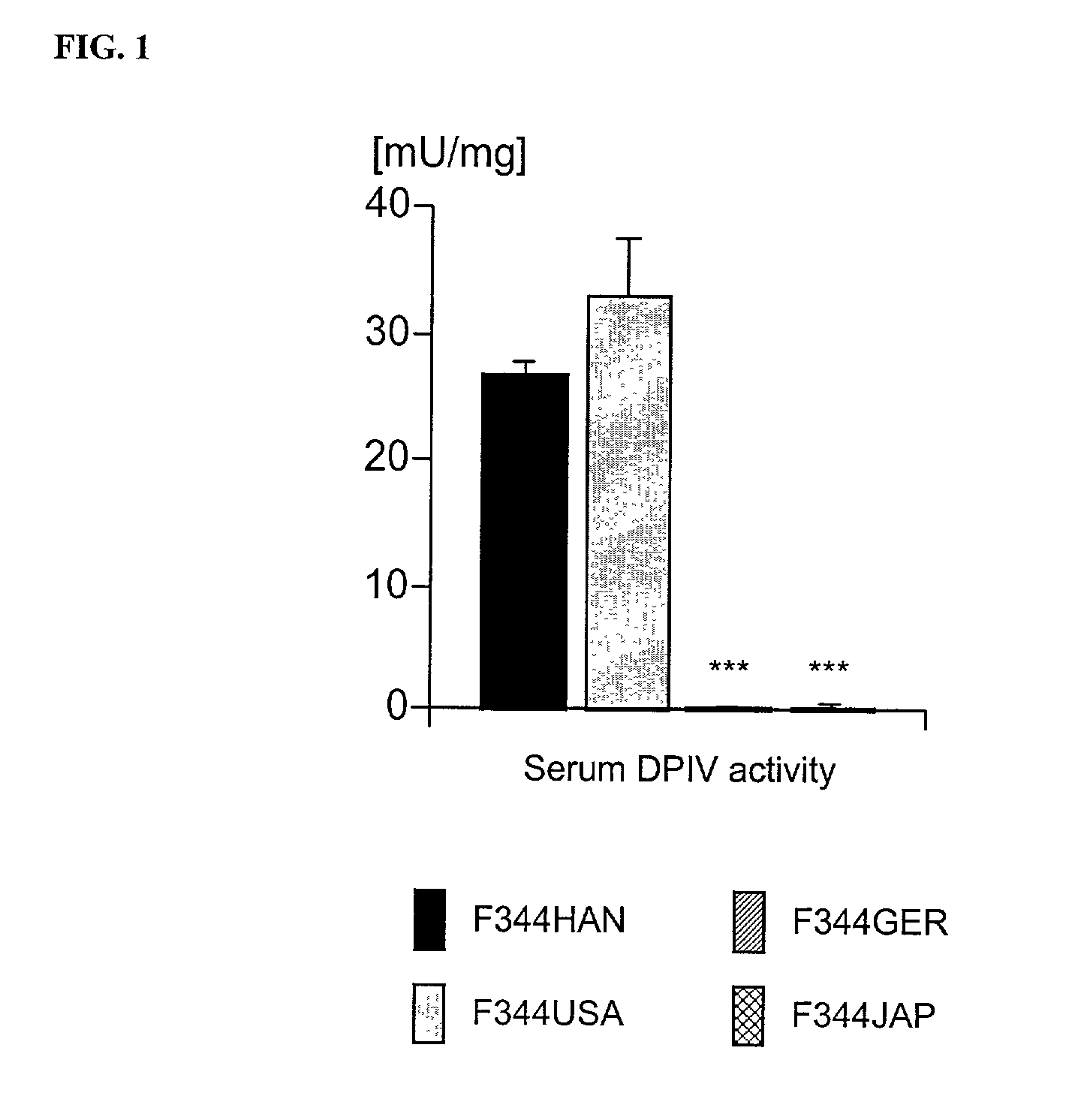
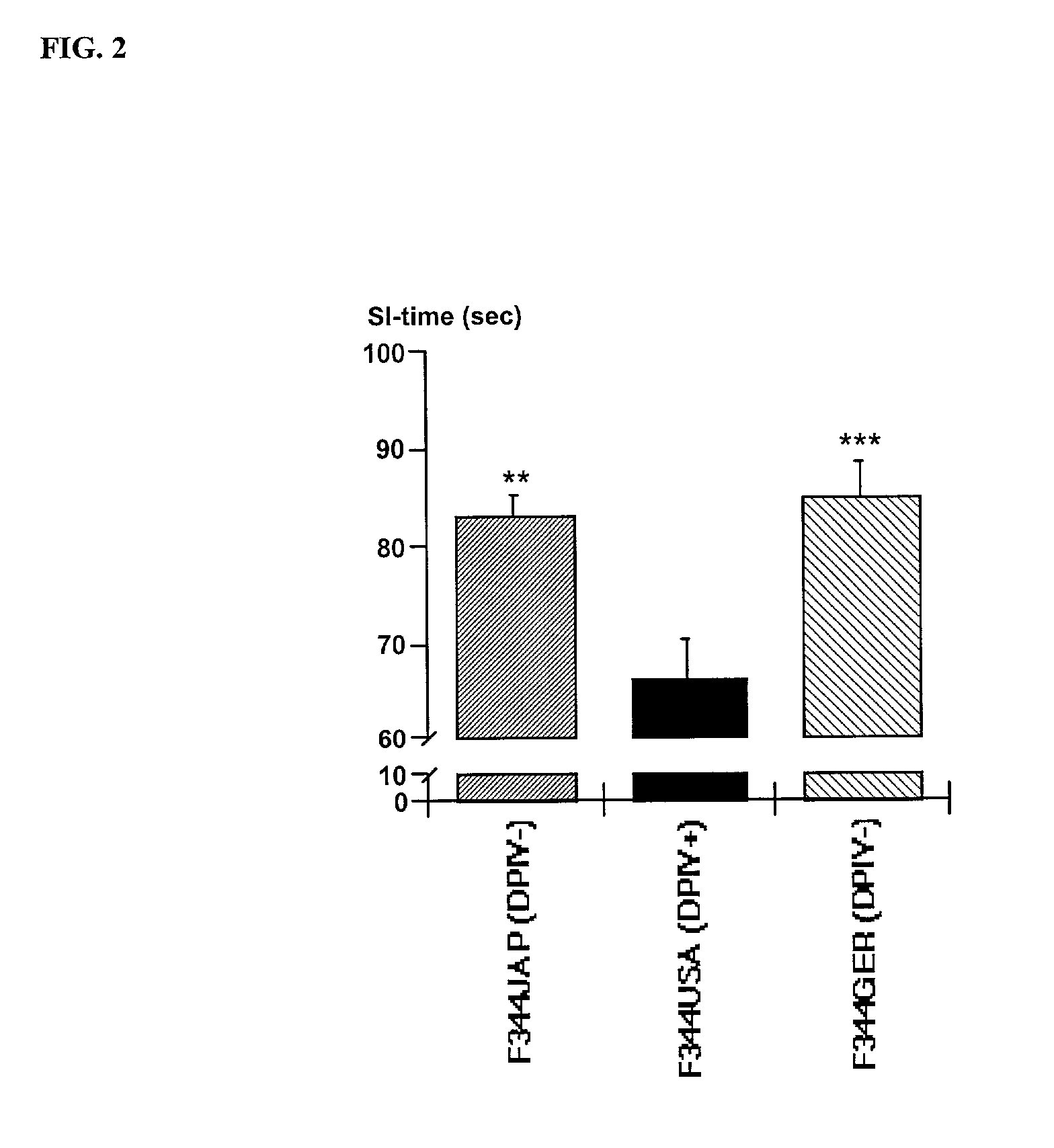
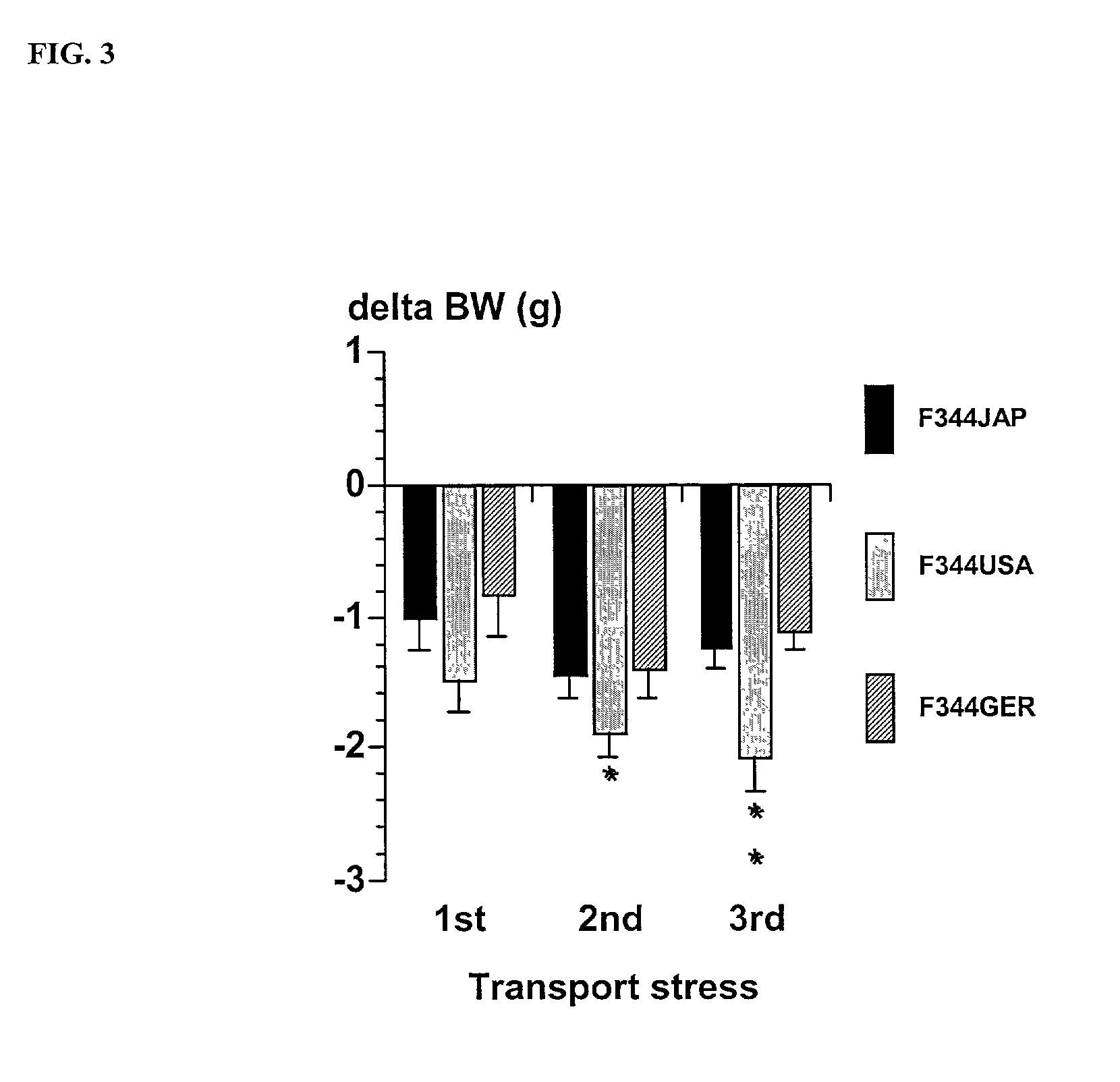
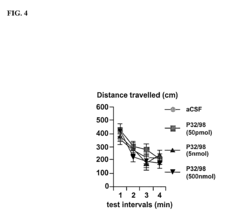
Modulation of central nervous system (CNS) dipeptidyl peptidase IV (DPIV) -like activity for the treatment of neurological and neuropsychological disorders
PatentInactiveUS7132104B1
Innovation
- Inhibiting dipeptidyl peptidase IV (DPIV) activity in the central nervous system (CNS) to delay the degradation of NPY, thereby enhancing NPY Y1 receptor activity and providing anxiolytic, anti-hypertensive, and other therapeutic effects through the use of CNS-targeted DPIV inhibitors.
Regulatory Framework for Anxiolytics
The regulatory framework for anxiolytics plays a crucial role in ensuring the safety, efficacy, and appropriate use of anxiety treatments. In the context of contrasting muscimol and benzodiazepines, it is essential to understand the specific regulations governing these substances and their use in anxiety treatments.
Benzodiazepines, as widely used anxiolytics, are subject to stringent regulatory controls due to their potential for abuse and dependence. In most countries, they are classified as controlled substances, requiring prescription from licensed healthcare providers. The regulatory framework typically includes restrictions on manufacturing, distribution, and prescribing practices. Monitoring systems are often in place to track prescriptions and prevent misuse.
Muscimol, on the other hand, is not as widely regulated as benzodiazepines. As a naturally occurring psychoactive compound found in certain mushroom species, its regulatory status varies across jurisdictions. In some countries, it may be classified as a controlled substance, while in others, it may fall under broader regulations governing natural products or dietary supplements.
The development and approval process for anxiolytic medications involves rigorous clinical trials and safety assessments. Regulatory bodies such as the FDA in the United States or the EMA in Europe require extensive documentation on efficacy, safety profiles, and potential side effects before granting market authorization. This process is well-established for benzodiazepines, but may be less clear for novel compounds like muscimol when considered for anxiety treatment.
Post-market surveillance is another critical aspect of the regulatory framework. Manufacturers are required to report adverse events and conduct ongoing safety monitoring. This is particularly important for benzodiazepines due to their known risks of dependence and withdrawal symptoms. For muscimol-based treatments, if developed, similar post-market surveillance would likely be required to ensure long-term safety and efficacy.
Prescribing guidelines and patient education materials are also part of the regulatory landscape. Healthcare providers must adhere to established protocols for prescribing anxiolytics, including assessing patient suitability, monitoring for side effects, and managing potential risks. These guidelines may need to be adapted or developed specifically for muscimol-based treatments if they become approved for anxiety management.
International harmonization efforts, such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), aim to standardize regulatory requirements across different regions. This is particularly relevant when comparing novel treatments like muscimol-based therapies with established anxiolytics like benzodiazepines, ensuring consistent safety and efficacy standards globally.
Benzodiazepines, as widely used anxiolytics, are subject to stringent regulatory controls due to their potential for abuse and dependence. In most countries, they are classified as controlled substances, requiring prescription from licensed healthcare providers. The regulatory framework typically includes restrictions on manufacturing, distribution, and prescribing practices. Monitoring systems are often in place to track prescriptions and prevent misuse.
Muscimol, on the other hand, is not as widely regulated as benzodiazepines. As a naturally occurring psychoactive compound found in certain mushroom species, its regulatory status varies across jurisdictions. In some countries, it may be classified as a controlled substance, while in others, it may fall under broader regulations governing natural products or dietary supplements.
The development and approval process for anxiolytic medications involves rigorous clinical trials and safety assessments. Regulatory bodies such as the FDA in the United States or the EMA in Europe require extensive documentation on efficacy, safety profiles, and potential side effects before granting market authorization. This process is well-established for benzodiazepines, but may be less clear for novel compounds like muscimol when considered for anxiety treatment.
Post-market surveillance is another critical aspect of the regulatory framework. Manufacturers are required to report adverse events and conduct ongoing safety monitoring. This is particularly important for benzodiazepines due to their known risks of dependence and withdrawal symptoms. For muscimol-based treatments, if developed, similar post-market surveillance would likely be required to ensure long-term safety and efficacy.
Prescribing guidelines and patient education materials are also part of the regulatory landscape. Healthcare providers must adhere to established protocols for prescribing anxiolytics, including assessing patient suitability, monitoring for side effects, and managing potential risks. These guidelines may need to be adapted or developed specifically for muscimol-based treatments if they become approved for anxiety management.
International harmonization efforts, such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), aim to standardize regulatory requirements across different regions. This is particularly relevant when comparing novel treatments like muscimol-based therapies with established anxiolytics like benzodiazepines, ensuring consistent safety and efficacy standards globally.
Safety and Efficacy Profiles
The safety and efficacy profiles of muscimol and benzodiazepines in anxiety treatments present distinct characteristics that warrant careful consideration. Muscimol, a GABA-A receptor agonist derived from the Amanita muscaria mushroom, exhibits a more selective binding profile compared to benzodiazepines. This selectivity potentially results in fewer side effects and a reduced risk of dependence.
Benzodiazepines, while highly effective in managing acute anxiety symptoms, are associated with a range of side effects including sedation, cognitive impairment, and a significant risk of dependence with long-term use. These concerns have led to increased scrutiny and restrictions on their prescription in many countries. However, their rapid onset of action and well-established efficacy in short-term anxiety management continue to make them valuable in certain clinical scenarios.
Muscimol, in contrast, shows promise in potentially offering anxiolytic effects with a lower risk of sedation and cognitive impairment. Preclinical studies have demonstrated its anxiolytic properties in animal models, with some evidence suggesting it may have a more favorable side effect profile than benzodiazepines. However, human clinical data on muscimol's efficacy and safety in anxiety treatment remains limited, necessitating further research.
The efficacy of benzodiazepines in treating anxiety disorders is well-documented through numerous clinical trials and decades of clinical use. They are particularly effective in managing panic disorder, generalized anxiety disorder, and social anxiety disorder. However, their long-term efficacy is questioned due to the development of tolerance and potential for rebound anxiety upon discontinuation.
Muscimol's efficacy profile in anxiety treatment is still being elucidated. While preliminary data suggest potential anxiolytic effects, large-scale clinical trials are needed to establish its efficacy compared to existing treatments. The compound's unique pharmacological profile may offer advantages in certain anxiety subtypes or in patients who do not respond well to conventional treatments.
Safety considerations for muscimol include potential interactions with other GABAergic compounds and the need for careful dosing to avoid excessive GABA-A receptor activation. Long-term safety data is currently lacking, which is a critical area for future research. Benzodiazepines, while having a well-established safety profile, carry risks of respiratory depression, especially when combined with other central nervous system depressants, and can be particularly problematic in elderly patients or those with compromised liver function.
Benzodiazepines, while highly effective in managing acute anxiety symptoms, are associated with a range of side effects including sedation, cognitive impairment, and a significant risk of dependence with long-term use. These concerns have led to increased scrutiny and restrictions on their prescription in many countries. However, their rapid onset of action and well-established efficacy in short-term anxiety management continue to make them valuable in certain clinical scenarios.
Muscimol, in contrast, shows promise in potentially offering anxiolytic effects with a lower risk of sedation and cognitive impairment. Preclinical studies have demonstrated its anxiolytic properties in animal models, with some evidence suggesting it may have a more favorable side effect profile than benzodiazepines. However, human clinical data on muscimol's efficacy and safety in anxiety treatment remains limited, necessitating further research.
The efficacy of benzodiazepines in treating anxiety disorders is well-documented through numerous clinical trials and decades of clinical use. They are particularly effective in managing panic disorder, generalized anxiety disorder, and social anxiety disorder. However, their long-term efficacy is questioned due to the development of tolerance and potential for rebound anxiety upon discontinuation.
Muscimol's efficacy profile in anxiety treatment is still being elucidated. While preliminary data suggest potential anxiolytic effects, large-scale clinical trials are needed to establish its efficacy compared to existing treatments. The compound's unique pharmacological profile may offer advantages in certain anxiety subtypes or in patients who do not respond well to conventional treatments.
Safety considerations for muscimol include potential interactions with other GABAergic compounds and the need for careful dosing to avoid excessive GABA-A receptor activation. Long-term safety data is currently lacking, which is a critical area for future research. Benzodiazepines, while having a well-established safety profile, carry risks of respiratory depression, especially when combined with other central nervous system depressants, and can be particularly problematic in elderly patients or those with compromised liver function.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!