Cycling Stability And Pulverization Control In Hydride Storage Media
AUG 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydride Storage Media Evolution and Research Objectives
Hydride storage media have evolved significantly over the past decades, transitioning from simple metal hydrides to complex multi-component systems engineered at the nanoscale. The journey began with conventional metal hydrides like LaNi5 and TiFe in the 1970s, which demonstrated the fundamental principles of hydrogen storage but suffered from limited capacity and poor cycling performance. The 1990s witnessed the emergence of complex hydrides such as alanates and borohydrides, offering higher theoretical storage capacities but presenting new challenges in terms of kinetics and reversibility.
The early 2000s marked a paradigm shift with the introduction of nanostructured hydrides, where researchers discovered that reducing particle size to nanoscale dimensions could dramatically improve hydrogen sorption kinetics and partially address cycling stability issues. This period also saw the development of catalyst-enhanced systems that lowered activation energies for hydrogen absorption and desorption processes.
Recent advancements have focused on composite systems that combine different hydride families to create synergistic effects. These reactive hydride composites (RHCs) leverage the strengths of individual components while mitigating their weaknesses, particularly regarding thermodynamic properties and cycling degradation. Parallel to material development, innovative microstructural engineering approaches have emerged to control pulverization during hydrogen cycling.
The current research landscape is increasingly oriented toward understanding degradation mechanisms at atomic and molecular levels. Advanced characterization techniques, including in-situ TEM and neutron scattering, have revealed critical insights into how hydride particles evolve during cycling, identifying key failure modes such as phase segregation, grain boundary weakening, and surface passivation.
The primary research objectives in this field now center on achieving practical cycling stability that meets commercial requirements—typically 1,000+ cycles with less than 20% capacity degradation. This involves developing robust strategies to control pulverization through elastic constraint mechanisms, core-shell architectures, and self-healing material designs. Researchers aim to establish fundamental structure-property relationships that connect material composition and microstructure to mechanical resilience during volume changes.
Another critical objective is to balance competing requirements: while nanoscaling improves kinetics and somewhat enhances mechanical stability, it can introduce new challenges related to surface oxidation and agglomeration. The field is therefore pursuing hierarchical material designs that optimize performance across multiple length scales, from atomic to macroscopic.
Looking forward, research goals include developing predictive computational models that can accelerate material discovery and optimization, establishing standardized testing protocols for cycling stability assessment, and creating economically viable manufacturing processes that can translate laboratory successes into commercial hydrogen storage solutions.
The early 2000s marked a paradigm shift with the introduction of nanostructured hydrides, where researchers discovered that reducing particle size to nanoscale dimensions could dramatically improve hydrogen sorption kinetics and partially address cycling stability issues. This period also saw the development of catalyst-enhanced systems that lowered activation energies for hydrogen absorption and desorption processes.
Recent advancements have focused on composite systems that combine different hydride families to create synergistic effects. These reactive hydride composites (RHCs) leverage the strengths of individual components while mitigating their weaknesses, particularly regarding thermodynamic properties and cycling degradation. Parallel to material development, innovative microstructural engineering approaches have emerged to control pulverization during hydrogen cycling.
The current research landscape is increasingly oriented toward understanding degradation mechanisms at atomic and molecular levels. Advanced characterization techniques, including in-situ TEM and neutron scattering, have revealed critical insights into how hydride particles evolve during cycling, identifying key failure modes such as phase segregation, grain boundary weakening, and surface passivation.
The primary research objectives in this field now center on achieving practical cycling stability that meets commercial requirements—typically 1,000+ cycles with less than 20% capacity degradation. This involves developing robust strategies to control pulverization through elastic constraint mechanisms, core-shell architectures, and self-healing material designs. Researchers aim to establish fundamental structure-property relationships that connect material composition and microstructure to mechanical resilience during volume changes.
Another critical objective is to balance competing requirements: while nanoscaling improves kinetics and somewhat enhances mechanical stability, it can introduce new challenges related to surface oxidation and agglomeration. The field is therefore pursuing hierarchical material designs that optimize performance across multiple length scales, from atomic to macroscopic.
Looking forward, research goals include developing predictive computational models that can accelerate material discovery and optimization, establishing standardized testing protocols for cycling stability assessment, and creating economically viable manufacturing processes that can translate laboratory successes into commercial hydrogen storage solutions.
Market Analysis for Hydrogen Storage Technologies
The global hydrogen storage market is experiencing significant growth, projected to reach $25.4 billion by 2027, with a CAGR of 6.5% from 2022. This expansion is primarily driven by increasing adoption of hydrogen as a clean energy carrier across various sectors including transportation, power generation, and industrial applications. The market for hydride-based storage technologies specifically represents approximately 18% of the total hydrogen storage market, with projections indicating growth to 23% by 2030.
Demand for advanced hydrogen storage solutions is particularly strong in regions pursuing aggressive decarbonization strategies, with Europe, Japan, South Korea, and parts of North America leading adoption. The automotive sector remains the largest end-user segment, accounting for approximately 35% of market demand, followed by industrial applications at 28% and power generation at 22%.
Material-based storage technologies, particularly metal hydrides, are gaining traction due to their higher volumetric storage capacity compared to compressed gas solutions. However, market penetration is constrained by cycling stability issues and pulverization challenges that limit commercial viability. Current market solutions typically achieve 500-1000 charge-discharge cycles before significant degradation, falling short of the 1500+ cycles required for commercial transportation applications.
Consumer demand increasingly prioritizes storage solutions offering longer operational lifetimes and reduced maintenance requirements. Market research indicates willingness to pay a 15-20% premium for storage systems demonstrating superior cycling stability, particularly in high-value applications such as fuel cell vehicles and grid-scale energy storage.
The competitive landscape features established industrial gas companies like Air Liquide and Linde, alongside specialized materials technology firms such as McPhy Energy and Hydrogenious Technologies. Recent market entrants include several technology startups focused specifically on addressing cycling stability through novel material compositions and nanostructuring approaches.
Regulatory frameworks are increasingly favorable, with government initiatives worldwide allocating over $70 billion collectively toward hydrogen infrastructure development. The EU Hydrogen Strategy and similar programs in Japan and South Korea specifically highlight the need for improved storage technologies, creating market pull for solutions addressing cycling stability challenges.
Market forecasts suggest that breakthrough innovations in hydride cycling stability could unlock an additional $3.8 billion market opportunity by 2030, particularly in stationary storage applications where longer service life directly translates to improved return on investment. The market segment specifically addressing pulverization control technologies is expected to grow at 9.2% annually, outpacing the broader hydrogen storage market.
Demand for advanced hydrogen storage solutions is particularly strong in regions pursuing aggressive decarbonization strategies, with Europe, Japan, South Korea, and parts of North America leading adoption. The automotive sector remains the largest end-user segment, accounting for approximately 35% of market demand, followed by industrial applications at 28% and power generation at 22%.
Material-based storage technologies, particularly metal hydrides, are gaining traction due to their higher volumetric storage capacity compared to compressed gas solutions. However, market penetration is constrained by cycling stability issues and pulverization challenges that limit commercial viability. Current market solutions typically achieve 500-1000 charge-discharge cycles before significant degradation, falling short of the 1500+ cycles required for commercial transportation applications.
Consumer demand increasingly prioritizes storage solutions offering longer operational lifetimes and reduced maintenance requirements. Market research indicates willingness to pay a 15-20% premium for storage systems demonstrating superior cycling stability, particularly in high-value applications such as fuel cell vehicles and grid-scale energy storage.
The competitive landscape features established industrial gas companies like Air Liquide and Linde, alongside specialized materials technology firms such as McPhy Energy and Hydrogenious Technologies. Recent market entrants include several technology startups focused specifically on addressing cycling stability through novel material compositions and nanostructuring approaches.
Regulatory frameworks are increasingly favorable, with government initiatives worldwide allocating over $70 billion collectively toward hydrogen infrastructure development. The EU Hydrogen Strategy and similar programs in Japan and South Korea specifically highlight the need for improved storage technologies, creating market pull for solutions addressing cycling stability challenges.
Market forecasts suggest that breakthrough innovations in hydride cycling stability could unlock an additional $3.8 billion market opportunity by 2030, particularly in stationary storage applications where longer service life directly translates to improved return on investment. The market segment specifically addressing pulverization control technologies is expected to grow at 9.2% annually, outpacing the broader hydrogen storage market.
Current Challenges in Cycling Stability of Hydride Materials
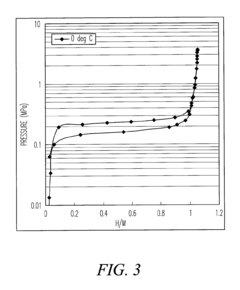
Hydride-based materials have emerged as promising candidates for hydrogen storage applications due to their high volumetric and gravimetric hydrogen densities. However, the widespread adoption of these materials faces significant challenges, particularly in terms of cycling stability. The repeated hydrogen absorption and desorption processes lead to substantial structural changes within the material, resulting in pulverization, capacity degradation, and ultimately, failure of the storage system.
One of the primary challenges is the volume expansion and contraction during hydrogenation and dehydrogenation cycles. Many metal hydrides experience volume changes of up to 30%, creating significant mechanical stress within the material structure. This stress manifests as micro-cracks that propagate with each cycle, eventually leading to particle fragmentation and pulverization. The reduced particle size increases surface area, accelerating oxidation and contamination processes that further degrade performance.
Kinetic limitations represent another critical challenge. As cycling progresses, the reaction kinetics often deteriorate due to surface passivation, agglomeration of catalyst particles, and segregation of alloying elements. These phenomena create diffusion barriers that slow hydrogen transport within the material, extending charging/discharging times and reducing practical usability in real-world applications.
Thermal management during cycling poses additional difficulties. The exothermic nature of hydrogen absorption and endothermic desorption creates temperature fluctuations that can accelerate degradation mechanisms. Inadequate heat dissipation during absorption can lead to localized hotspots, promoting sintering and undesired phase transformations that permanently alter material properties.
Chemical stability issues further complicate cycling performance. Many promising hydride materials are sensitive to common impurities in hydrogen gas streams, particularly oxygen, water vapor, and carbon dioxide. These contaminants react irreversibly with active material surfaces, forming stable oxide or hydroxide layers that block hydrogen diffusion pathways and catalytically active sites.
The trade-off between hydrogen capacity and cycling stability presents a fundamental dilemma. Materials with the highest theoretical hydrogen capacities, such as complex hydrides, often exhibit the poorest cycling behavior due to their complex decomposition pathways and limited reversibility. Conversely, materials with excellent cycling properties typically store less hydrogen, limiting their practical energy density.
Recent research has identified several promising approaches to address these challenges, including nanostructuring, composite formation, catalyst optimization, and surface treatments. However, a comprehensive solution that simultaneously addresses all degradation mechanisms while maintaining high hydrogen capacity remains elusive, highlighting the need for continued fundamental research in this critical area.
One of the primary challenges is the volume expansion and contraction during hydrogenation and dehydrogenation cycles. Many metal hydrides experience volume changes of up to 30%, creating significant mechanical stress within the material structure. This stress manifests as micro-cracks that propagate with each cycle, eventually leading to particle fragmentation and pulverization. The reduced particle size increases surface area, accelerating oxidation and contamination processes that further degrade performance.
Kinetic limitations represent another critical challenge. As cycling progresses, the reaction kinetics often deteriorate due to surface passivation, agglomeration of catalyst particles, and segregation of alloying elements. These phenomena create diffusion barriers that slow hydrogen transport within the material, extending charging/discharging times and reducing practical usability in real-world applications.
Thermal management during cycling poses additional difficulties. The exothermic nature of hydrogen absorption and endothermic desorption creates temperature fluctuations that can accelerate degradation mechanisms. Inadequate heat dissipation during absorption can lead to localized hotspots, promoting sintering and undesired phase transformations that permanently alter material properties.
Chemical stability issues further complicate cycling performance. Many promising hydride materials are sensitive to common impurities in hydrogen gas streams, particularly oxygen, water vapor, and carbon dioxide. These contaminants react irreversibly with active material surfaces, forming stable oxide or hydroxide layers that block hydrogen diffusion pathways and catalytically active sites.
The trade-off between hydrogen capacity and cycling stability presents a fundamental dilemma. Materials with the highest theoretical hydrogen capacities, such as complex hydrides, often exhibit the poorest cycling behavior due to their complex decomposition pathways and limited reversibility. Conversely, materials with excellent cycling properties typically store less hydrogen, limiting their practical energy density.
Recent research has identified several promising approaches to address these challenges, including nanostructuring, composite formation, catalyst optimization, and surface treatments. However, a comprehensive solution that simultaneously addresses all degradation mechanisms while maintaining high hydrogen capacity remains elusive, highlighting the need for continued fundamental research in this critical area.
Current Approaches to Pulverization Control in Hydrides
01 Alloying and composition modifications for cycling stability
Modifying the composition of hydride storage materials through alloying can significantly improve cycling stability and reduce pulverization. By incorporating specific elements or compounds, the lattice expansion during hydrogen absorption/desorption can be controlled, resulting in reduced mechanical stress and improved structural integrity over multiple cycles. These compositional modifications can include rare earth elements, transition metals, or other stabilizing additives that enhance the mechanical properties of the storage media.- Alloying and composition modifications for stability enhancement: Modifying the composition of hydride storage materials through alloying can significantly improve cycling stability and reduce pulverization. By incorporating specific elements or compounds, the crystal structure can be stabilized during hydrogen absorption and desorption cycles. These modifications can create more robust lattice structures that resist fracturing during volume changes, thereby extending the operational lifespan of the storage media.
- Surface coating and encapsulation techniques: Applying protective coatings or encapsulation layers to hydride particles can effectively control pulverization during cycling. These coatings act as flexible barriers that accommodate volume changes while maintaining structural integrity. Various coating materials including polymers, carbon-based materials, and metal oxides can be employed to create core-shell structures that prevent particle fragmentation while maintaining hydrogen diffusion properties.
- Nanostructuring and particle size optimization: Controlling the particle size and implementing nanostructuring approaches can significantly improve cycling stability of hydride storage media. Nanoscale materials offer shorter diffusion paths for hydrogen and can better accommodate strain during hydrogen cycling. The reduced particle size limits crack propagation and decreases the overall stress within the material during volume changes, thereby minimizing pulverization effects and extending cycling life.
- Thermal management systems for cycling stability: Implementing effective thermal management systems can significantly improve the cycling stability of hydride storage media. Controlled heating and cooling during absorption and desorption processes help minimize thermal stress and prevent rapid expansion or contraction that leads to pulverization. These systems can include heat exchangers, thermal buffers, and temperature-controlled reaction chambers that optimize operating conditions and extend material lifespan.
- Composite and matrix-supported hydride structures: Incorporating hydride materials into composite structures or supporting matrices can effectively control pulverization during cycling. These designs distribute stress throughout the structure and provide mechanical support during volume changes. Matrix materials such as porous metals, polymers, or carbon scaffolds can physically constrain the hydride particles while allowing hydrogen diffusion, resulting in improved cycling stability and reduced degradation over multiple absorption-desorption cycles.
02 Surface coatings and treatments to prevent pulverization
Applying protective coatings or surface treatments to hydride storage materials can effectively control pulverization during cycling. These coatings create a barrier that maintains structural integrity while allowing hydrogen diffusion. Surface modification techniques include carbon coating, oxide layer formation, or polymer encapsulation, which can absorb mechanical stress during volume changes. These treatments also help prevent oxidation and contamination of the hydride material, further enhancing cycling stability.Expand Specific Solutions03 Nanostructured hydride materials for improved stability
Developing nanostructured hydride storage materials offers superior cycling stability compared to bulk materials. The reduced particle size shortens hydrogen diffusion paths and provides better accommodation of volume changes during cycling. Nanostructuring approaches include ball milling, chemical synthesis of nanoparticles, or creation of core-shell structures. These materials demonstrate enhanced resistance to pulverization due to their ability to better distribute mechanical stress throughout the structure.Expand Specific Solutions04 Thermal management systems for cycling stability
Implementing effective thermal management systems can significantly improve the cycling stability of hydride storage media. Controlled heating and cooling during absorption and desorption processes help minimize thermal stress that contributes to pulverization. These systems can include heat exchangers, thermal fluids, or phase change materials that maintain optimal temperature conditions. Proper thermal management also enables more uniform hydrogen distribution within the storage material, reducing localized stress points.Expand Specific Solutions05 Composite and matrix-supported hydride structures
Incorporating hydride materials into composite structures or supporting matrices provides mechanical reinforcement that limits pulverization during cycling. These composites typically consist of a hydride material dispersed within a ductile or porous matrix that can accommodate volume changes. Materials used for matrices include polymers, metals, or carbon-based materials. The matrix structure constrains particle growth during cycling and provides pathways for hydrogen diffusion while maintaining structural integrity of the storage media.Expand Specific Solutions
Leading Companies and Research Institutions in Hydride Storage
The cycling stability and pulverization control in hydride storage media market is currently in a growth phase, with increasing focus on sustainable energy storage solutions. The global market is expanding rapidly, driven by clean energy initiatives and hydrogen economy development. Technologically, the field shows moderate maturity with ongoing innovation challenges. Leading companies like Intelligent Energy and Nippon Shokubai are advancing commercial applications, while research institutions such as CNRS and Zhejiang University are developing fundamental solutions. Industrial players including DuPont and Resonac are focusing on material improvements, while specialized entities like Huaneng Clean Energy Research Institute are integrating these technologies into broader energy systems, creating a competitive landscape balanced between established corporations and emerging specialists.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed advanced nanostructured hydride composites to address cycling stability issues in hydrogen storage materials. Their approach focuses on creating core-shell structures where the hydride material is encapsulated within a flexible, conductive matrix that accommodates volume changes during hydrogen absorption/desorption cycles. This matrix typically consists of carbon-based materials or metallic nanoparticles that provide mechanical support while allowing hydrogen diffusion. CNRS researchers have demonstrated that incorporating titanium-based catalysts into magnesium hydride systems can significantly improve cycling performance by facilitating hydrogen dissociation and recombination at lower temperatures. Their proprietary process involves controlled ball milling under specific atmospheric conditions to create optimized particle sizes (typically 50-100 nm) that minimize diffusion distances while maintaining structural integrity. Recent studies have shown their nanocomposites can maintain over 80% of initial hydrogen capacity after 1000 cycles, compared to conventional materials that degrade after 50-100 cycles.
Strengths: Superior cycling stability through engineered nanostructures; reduced activation energy requirements; excellent resistance to pulverization through controlled particle morphology. Weaknesses: Complex synthesis procedures increase production costs; potential for oxidation during manufacturing requires stringent handling protocols; scale-up challenges for industrial applications remain significant.
Zhejiang University
Technical Solution: Zhejiang University has developed an innovative approach to hydride stability through their patented "gradient functionality" design. This technology creates hydride particles with compositionally varied layers that distribute mechanical stress during hydrogen absorption/desorption cycles. Their core-gradient-shell structure features a hydrogen-absorbing core surrounded by progressively more elastic outer layers that accommodate volume changes without fracturing. The university's research team has successfully implemented this design in magnesium-based hydrides by incorporating transition metal dopants (Ni, Ti, Fe) in precisely controlled concentration gradients. Their synthesis method combines solution-based chemistry with controlled thermal processing to achieve nanoscale control over composition. Recent publications demonstrate their materials maintain over 90% capacity retention after 300 cycles at practical operating temperatures (250-300°C), compared to conventional materials that typically lose 50% capacity within 100 cycles. Additionally, their materials show remarkably consistent hydrogen sorption kinetics throughout cycling, indicating minimal surface passivation or structural degradation.
Strengths: Exceptional resistance to pulverization through stress distribution across gradient layers; maintains fast kinetics throughout cycling life; scalable synthesis methods compatible with industrial production. Weaknesses: Requires precise control of multiple processing parameters; higher initial material costs compared to simple hydrides; performance advantages diminish at very high temperatures (>350°C).
Key Patents and Innovations in Hydride Stability Enhancement
Composite hydrogen storage material and methods related thereto
PatentActiveUS7708815B2
Innovation
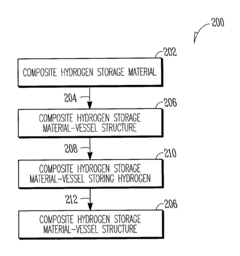
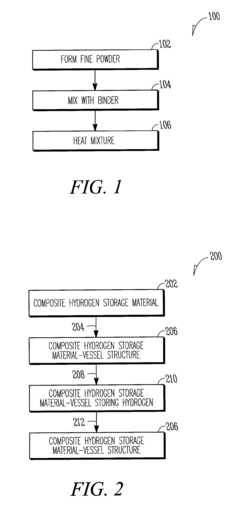
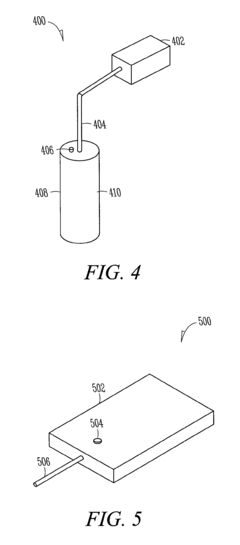
- A composite hydrogen storage material comprising active material particles and a binder that immobilizes the particles to maintain relative spatial relationships, reducing particle bed instability and allowing for safer, more efficient hydrogen occlusion and desorption while acting as a load-bearing member within a storage vessel.
Hydrogen storage tank comprising a textile filter material
PatentWO2018104657A2
Innovation
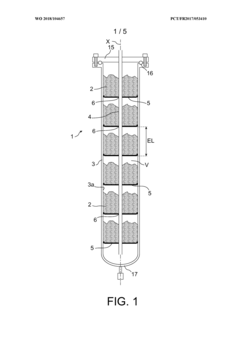
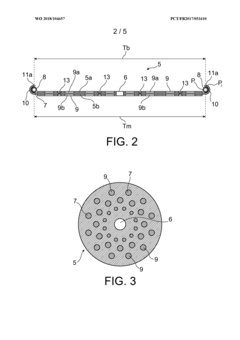
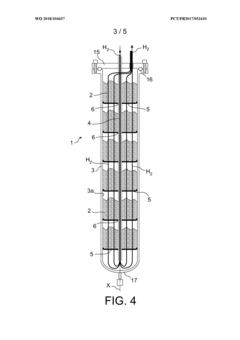
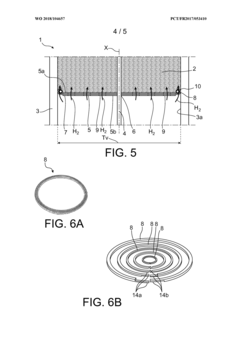
- A hydrogen storage tank design featuring a stack of separation elements with filtering textile material that allows hydrogen and flushing gases to pass while preventing the hydride powder from moving, distributed in multiple shallow beds within a cylindrical envelope, ensuring mechanical stability and efficient gas circulation.
Safety Standards and Testing Protocols for Hydride Storage Systems
The development of comprehensive safety standards and testing protocols for hydride storage systems represents a critical foundation for the widespread adoption of hydrogen storage technologies. Current international standards, such as ISO/TS 19883 and SAE J2579, provide baseline requirements for hydrogen storage vessels but require significant expansion to address the unique characteristics of metal hydride systems, particularly regarding cycling stability and pulverization control.
Safety protocols must specifically address the thermal management challenges inherent in hydride storage media. During hydrogen absorption, these materials generate substantial heat (exothermic reaction), while hydrogen release requires heat input (endothermic process). Testing protocols must verify that thermal management systems can effectively handle these fluctuations without compromising structural integrity or creating unsafe conditions, even after thousands of cycling operations.
Material pulverization presents distinct safety concerns that necessitate specialized testing methodologies. As hydride materials undergo repeated expansion and contraction during hydrogen cycling, the resulting particle size reduction can lead to increased reactivity, potential dust hazards, and compromised system performance. Current standards inadequately address these progressive degradation mechanisms, creating a significant gap in safety assurance.
Accelerated aging tests have emerged as essential components of modern safety protocols. These tests simulate extended cycling under various environmental conditions to predict long-term stability and identify potential failure modes. The correlation between accelerated test results and real-world performance remains an active area of research, with organizations like Sandia National Laboratories and the European Joint Research Centre developing standardized methodologies.
Failure mode analysis specific to hydride systems must be incorporated into safety standards. This includes testing for resistance to mechanical shock, vibration, external fire, and over-pressurization events. The behavior of partially degraded hydride materials during emergency scenarios requires particular attention, as pulverized media may exhibit different reaction kinetics and thermal properties compared to fresh materials.
International harmonization of safety standards remains challenging but essential. Different regulatory frameworks across North America, Europe, and Asia have created inconsistent requirements for hydride storage systems. The International Partnership for Hydrogen and Fuel Cells in the Economy (IPHE) has established working groups focused on developing globally recognized testing protocols that specifically address cycling stability and material degradation in hydride storage systems.
Safety protocols must specifically address the thermal management challenges inherent in hydride storage media. During hydrogen absorption, these materials generate substantial heat (exothermic reaction), while hydrogen release requires heat input (endothermic process). Testing protocols must verify that thermal management systems can effectively handle these fluctuations without compromising structural integrity or creating unsafe conditions, even after thousands of cycling operations.
Material pulverization presents distinct safety concerns that necessitate specialized testing methodologies. As hydride materials undergo repeated expansion and contraction during hydrogen cycling, the resulting particle size reduction can lead to increased reactivity, potential dust hazards, and compromised system performance. Current standards inadequately address these progressive degradation mechanisms, creating a significant gap in safety assurance.
Accelerated aging tests have emerged as essential components of modern safety protocols. These tests simulate extended cycling under various environmental conditions to predict long-term stability and identify potential failure modes. The correlation between accelerated test results and real-world performance remains an active area of research, with organizations like Sandia National Laboratories and the European Joint Research Centre developing standardized methodologies.
Failure mode analysis specific to hydride systems must be incorporated into safety standards. This includes testing for resistance to mechanical shock, vibration, external fire, and over-pressurization events. The behavior of partially degraded hydride materials during emergency scenarios requires particular attention, as pulverized media may exhibit different reaction kinetics and thermal properties compared to fresh materials.
International harmonization of safety standards remains challenging but essential. Different regulatory frameworks across North America, Europe, and Asia have created inconsistent requirements for hydride storage systems. The International Partnership for Hydrogen and Fuel Cells in the Economy (IPHE) has established working groups focused on developing globally recognized testing protocols that specifically address cycling stability and material degradation in hydride storage systems.
Environmental Impact and Sustainability of Hydride Storage Technologies
The environmental implications of hydride storage technologies extend far beyond their technical performance metrics. As these materials become increasingly central to clean energy systems, their full lifecycle environmental impact requires thorough assessment. Hydride-based storage systems offer significant potential for reducing greenhouse gas emissions when integrated into renewable energy infrastructures, potentially displacing carbon-intensive energy storage alternatives.
The manufacturing processes for hydride storage media currently present notable environmental challenges. Production often involves energy-intensive metallurgical processes and chemical treatments that generate substantial carbon footprints. Additionally, some hydride formulations incorporate rare earth elements or strategic metals with environmentally problematic extraction profiles. The environmental burden is particularly pronounced in the initial production phase, though this may be offset by the long-term environmental benefits during operational use.
Water consumption represents another critical environmental consideration. Certain hydride production methods require significant water resources, while the hydrogen generation process itself may involve water electrolysis. In water-stressed regions, this dependency creates additional sustainability concerns that must be factored into deployment strategies.
The cycling stability challenges directly impact the sustainability profile of hydride storage technologies. Materials requiring frequent replacement due to pulverization or degradation increase the lifecycle resource requirements and waste generation. Conversely, advances in cycling stability that extend operational lifetimes significantly improve the overall environmental performance by reducing material throughput and replacement frequency.
End-of-life management presents both challenges and opportunities. While some hydride materials can be effectively recycled, current recycling infrastructure remains underdeveloped. The potential for closed-loop material systems exists but requires further development of specialized recovery processes that can handle the unique properties of degraded hydride materials.
Comparative lifecycle assessments indicate that despite manufacturing impacts, hydride storage technologies generally demonstrate favorable environmental profiles compared to conventional energy storage alternatives when evaluated across their complete operational lifespan. The environmental advantage becomes particularly pronounced in scenarios involving renewable energy integration, where hydrides enable higher penetration of intermittent renewable sources.
Future sustainability improvements will likely emerge from research focused on reducing rare metal content, developing less energy-intensive synthesis methods, and creating more robust recycling pathways. These advancements, coupled with improvements in cycling stability, will be essential for positioning hydride storage as a truly sustainable component of future energy systems.
The manufacturing processes for hydride storage media currently present notable environmental challenges. Production often involves energy-intensive metallurgical processes and chemical treatments that generate substantial carbon footprints. Additionally, some hydride formulations incorporate rare earth elements or strategic metals with environmentally problematic extraction profiles. The environmental burden is particularly pronounced in the initial production phase, though this may be offset by the long-term environmental benefits during operational use.
Water consumption represents another critical environmental consideration. Certain hydride production methods require significant water resources, while the hydrogen generation process itself may involve water electrolysis. In water-stressed regions, this dependency creates additional sustainability concerns that must be factored into deployment strategies.
The cycling stability challenges directly impact the sustainability profile of hydride storage technologies. Materials requiring frequent replacement due to pulverization or degradation increase the lifecycle resource requirements and waste generation. Conversely, advances in cycling stability that extend operational lifetimes significantly improve the overall environmental performance by reducing material throughput and replacement frequency.
End-of-life management presents both challenges and opportunities. While some hydride materials can be effectively recycled, current recycling infrastructure remains underdeveloped. The potential for closed-loop material systems exists but requires further development of specialized recovery processes that can handle the unique properties of degraded hydride materials.
Comparative lifecycle assessments indicate that despite manufacturing impacts, hydride storage technologies generally demonstrate favorable environmental profiles compared to conventional energy storage alternatives when evaluated across their complete operational lifespan. The environmental advantage becomes particularly pronounced in scenarios involving renewable energy integration, where hydrides enable higher penetration of intermittent renewable sources.
Future sustainability improvements will likely emerge from research focused on reducing rare metal content, developing less energy-intensive synthesis methods, and creating more robust recycling pathways. These advancements, coupled with improvements in cycling stability, will be essential for positioning hydride storage as a truly sustainable component of future energy systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!