High-Entropy Alloy Hydrides As Next-Generation Storage Media
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HEA Hydrides Background and Research Objectives
High-entropy alloy (HEA) hydrides represent a revolutionary frontier in hydrogen storage technology, emerging from the convergence of materials science and energy storage research. Traditional hydrogen storage materials have faced persistent limitations in capacity, kinetics, and thermodynamic stability, creating significant barriers to widespread hydrogen economy implementation. HEAs, characterized by their multi-principal element composition (typically five or more elements in near-equimolar ratios), have disrupted conventional alloy design paradigms since their introduction in the early 2000s.
The evolution of HEA research has progressed from structural applications to functional materials, with hydrogen storage capabilities emerging as a promising direction in the past decade. This technological progression aligns with global imperatives for decarbonization and renewable energy integration, where hydrogen serves as a versatile energy carrier. The unique atomic arrangements in HEAs create diverse local chemical environments and lattice distortions that fundamentally alter hydrogen-metal interactions compared to conventional storage materials.
Current research objectives in HEA hydrides focus on exploiting the configurational entropy effect to achieve superior hydrogen storage metrics. Primary goals include developing HEA compositions that demonstrate gravimetric hydrogen capacities exceeding 5 wt%, operating temperatures below 100°C, and cycling stability beyond 1000 cycles. These targets represent significant improvements over conventional metal hydrides and approach the U.S. Department of Energy's ultimate targets for onboard hydrogen storage systems.
The high configurational entropy in these systems offers unprecedented opportunities to tune thermodynamic properties, potentially resolving the persistent capacity-kinetics trade-off that has plagued conventional storage materials. By strategically manipulating elemental compositions, researchers aim to create materials with optimized binding energies that facilitate both hydrogen uptake and release under practical conditions.
Additional research objectives include understanding the fundamental mechanisms of hydrogen absorption/desorption in high-entropy environments, developing predictive computational models for HEA hydride behavior, and establishing scalable synthesis protocols that maintain compositional homogeneity across production scales. These objectives collectively address both scientific understanding and practical implementation challenges.
The transformative potential of HEA hydrides extends beyond stationary applications to mobile hydrogen storage, catalysis, and energy conversion systems. This research direction represents a paradigm shift in materials design philosophy, moving from binary and ternary systems toward vastly more complex compositional spaces that offer unprecedented property combinations and performance characteristics for next-generation hydrogen storage technologies.
The evolution of HEA research has progressed from structural applications to functional materials, with hydrogen storage capabilities emerging as a promising direction in the past decade. This technological progression aligns with global imperatives for decarbonization and renewable energy integration, where hydrogen serves as a versatile energy carrier. The unique atomic arrangements in HEAs create diverse local chemical environments and lattice distortions that fundamentally alter hydrogen-metal interactions compared to conventional storage materials.
Current research objectives in HEA hydrides focus on exploiting the configurational entropy effect to achieve superior hydrogen storage metrics. Primary goals include developing HEA compositions that demonstrate gravimetric hydrogen capacities exceeding 5 wt%, operating temperatures below 100°C, and cycling stability beyond 1000 cycles. These targets represent significant improvements over conventional metal hydrides and approach the U.S. Department of Energy's ultimate targets for onboard hydrogen storage systems.
The high configurational entropy in these systems offers unprecedented opportunities to tune thermodynamic properties, potentially resolving the persistent capacity-kinetics trade-off that has plagued conventional storage materials. By strategically manipulating elemental compositions, researchers aim to create materials with optimized binding energies that facilitate both hydrogen uptake and release under practical conditions.
Additional research objectives include understanding the fundamental mechanisms of hydrogen absorption/desorption in high-entropy environments, developing predictive computational models for HEA hydride behavior, and establishing scalable synthesis protocols that maintain compositional homogeneity across production scales. These objectives collectively address both scientific understanding and practical implementation challenges.
The transformative potential of HEA hydrides extends beyond stationary applications to mobile hydrogen storage, catalysis, and energy conversion systems. This research direction represents a paradigm shift in materials design philosophy, moving from binary and ternary systems toward vastly more complex compositional spaces that offer unprecedented property combinations and performance characteristics for next-generation hydrogen storage technologies.
Hydrogen Storage Market Analysis and Demand Forecast
The global hydrogen storage market is experiencing significant growth, driven by the increasing focus on clean energy solutions and the transition away from fossil fuels. As of 2023, the market was valued at approximately $15.4 billion, with projections indicating a compound annual growth rate (CAGR) of 9.7% through 2030, potentially reaching $28.1 billion by the end of the forecast period. This growth trajectory is primarily fueled by the expanding hydrogen economy and the critical need for efficient storage solutions.
The transportation sector represents the largest demand segment for hydrogen storage technologies, accounting for roughly 40% of the total market share. This dominance stems from the accelerating adoption of fuel cell electric vehicles (FCEVs) in commercial fleets, public transportation, and increasingly in personal vehicles. Major automotive manufacturers including Toyota, Hyundai, and Honda have made substantial investments in hydrogen fuel cell technology, signaling strong industry confidence.
Industrial applications constitute the second-largest market segment at approximately 30%, where hydrogen is utilized for various processes including ammonia production, petroleum refining, and metal processing. The remaining market share is distributed across power generation, residential, and commercial applications, with each showing steady growth potential.
Regionally, Asia-Pacific dominates the hydrogen storage market with approximately 45% share, led by Japan, South Korea, and China's aggressive hydrogen economy initiatives. Europe follows with roughly 30% market share, driven by stringent carbon reduction policies and substantial investments in hydrogen infrastructure. North America accounts for approximately 20% of the market, with the remaining 5% distributed across other regions.
The demand for advanced hydrogen storage solutions is expected to intensify as countries worldwide commit to carbon neutrality targets. The European Union's Hydrogen Strategy aims to install at least 40 gigawatts of renewable hydrogen electrolyzers by 2030, creating substantial demand for storage solutions. Similarly, Japan's Basic Hydrogen Strategy and South Korea's Hydrogen Economy Roadmap outline ambitious hydrogen adoption goals.
High-entropy alloy hydrides represent a potentially disruptive technology in this growing market. Their superior hydrogen storage capacity, enhanced cycling stability, and improved kinetics address key limitations of conventional storage methods. Market analysis indicates that materials-based storage solutions could capture up to 25% of the hydrogen storage market by 2035, representing a significant opportunity for high-entropy alloy hydride technologies.
The transportation sector represents the largest demand segment for hydrogen storage technologies, accounting for roughly 40% of the total market share. This dominance stems from the accelerating adoption of fuel cell electric vehicles (FCEVs) in commercial fleets, public transportation, and increasingly in personal vehicles. Major automotive manufacturers including Toyota, Hyundai, and Honda have made substantial investments in hydrogen fuel cell technology, signaling strong industry confidence.
Industrial applications constitute the second-largest market segment at approximately 30%, where hydrogen is utilized for various processes including ammonia production, petroleum refining, and metal processing. The remaining market share is distributed across power generation, residential, and commercial applications, with each showing steady growth potential.
Regionally, Asia-Pacific dominates the hydrogen storage market with approximately 45% share, led by Japan, South Korea, and China's aggressive hydrogen economy initiatives. Europe follows with roughly 30% market share, driven by stringent carbon reduction policies and substantial investments in hydrogen infrastructure. North America accounts for approximately 20% of the market, with the remaining 5% distributed across other regions.
The demand for advanced hydrogen storage solutions is expected to intensify as countries worldwide commit to carbon neutrality targets. The European Union's Hydrogen Strategy aims to install at least 40 gigawatts of renewable hydrogen electrolyzers by 2030, creating substantial demand for storage solutions. Similarly, Japan's Basic Hydrogen Strategy and South Korea's Hydrogen Economy Roadmap outline ambitious hydrogen adoption goals.
High-entropy alloy hydrides represent a potentially disruptive technology in this growing market. Their superior hydrogen storage capacity, enhanced cycling stability, and improved kinetics address key limitations of conventional storage methods. Market analysis indicates that materials-based storage solutions could capture up to 25% of the hydrogen storage market by 2035, representing a significant opportunity for high-entropy alloy hydride technologies.
Current Status and Challenges in HEA Hydride Development
The global research landscape for High-Entropy Alloy (HEA) hydrides has witnessed significant advancements in recent years, yet remains in a relatively nascent stage compared to conventional hydrogen storage materials. Current research efforts are primarily concentrated in advanced materials science hubs across North America, Europe, and East Asia, with notable contributions from research institutions in the United States, Germany, Japan, and China.
The synthesis of HEA hydrides presents a fundamental challenge due to the complex multi-element composition and the need for precise control over stoichiometry. Researchers have employed various techniques including arc melting, mechanical alloying, and high-pressure synthesis, each with distinct advantages and limitations. The reproducibility of these methods remains a significant hurdle, particularly when scaling beyond laboratory quantities.
Hydrogen absorption-desorption kinetics in HEA hydrides constitute another critical challenge. While some compositions demonstrate promising initial uptake rates, cycling stability often deteriorates rapidly. This degradation is attributed to phase segregation and microstructural changes during hydrogen cycling, compromising the entropy stabilization effect that is central to HEA performance.
Thermodynamic characterization of these complex systems presents methodological difficulties. The conventional pressure-composition-temperature (PCT) measurements require adaptation for multi-component systems where plateau pressures may be less distinct than in binary or ternary hydrides. Additionally, the enthalpy-entropy compensation effects in HEAs create challenges in optimizing both storage capacity and operating conditions simultaneously.
Computational modeling of HEA hydrides lags behind experimental work, primarily due to the combinatorial complexity of these systems. Current density functional theory (DFT) approaches struggle with the large supercells required to accurately represent the random solid solution nature of HEAs, limiting predictive capabilities for hydrogen storage properties.
Material stability under practical operating conditions remains inadequately addressed. Many promising HEA hydrides demonstrate sensitivity to trace impurities in hydrogen gas, particularly oxygen and moisture, leading to surface passivation and degraded performance. This susceptibility necessitates either highly pure hydrogen sources or the development of protective strategies, both of which add complexity to potential applications.
Cost considerations and material availability present additional barriers to commercialization. Many high-performing HEA compositions incorporate expensive elements like palladium or platinum, raising questions about economic viability for large-scale deployment. Research into compositions utilizing more abundant elements has shown promise but often at the cost of reduced storage capacity or unfavorable kinetics.
The standardization of testing protocols specifically tailored to HEA hydrides remains underdeveloped, complicating direct comparisons between different research efforts and potentially slowing progress in the field. Establishing consensus methodologies for characterization would accelerate knowledge transfer and technology development in this promising domain.
The synthesis of HEA hydrides presents a fundamental challenge due to the complex multi-element composition and the need for precise control over stoichiometry. Researchers have employed various techniques including arc melting, mechanical alloying, and high-pressure synthesis, each with distinct advantages and limitations. The reproducibility of these methods remains a significant hurdle, particularly when scaling beyond laboratory quantities.
Hydrogen absorption-desorption kinetics in HEA hydrides constitute another critical challenge. While some compositions demonstrate promising initial uptake rates, cycling stability often deteriorates rapidly. This degradation is attributed to phase segregation and microstructural changes during hydrogen cycling, compromising the entropy stabilization effect that is central to HEA performance.
Thermodynamic characterization of these complex systems presents methodological difficulties. The conventional pressure-composition-temperature (PCT) measurements require adaptation for multi-component systems where plateau pressures may be less distinct than in binary or ternary hydrides. Additionally, the enthalpy-entropy compensation effects in HEAs create challenges in optimizing both storage capacity and operating conditions simultaneously.
Computational modeling of HEA hydrides lags behind experimental work, primarily due to the combinatorial complexity of these systems. Current density functional theory (DFT) approaches struggle with the large supercells required to accurately represent the random solid solution nature of HEAs, limiting predictive capabilities for hydrogen storage properties.
Material stability under practical operating conditions remains inadequately addressed. Many promising HEA hydrides demonstrate sensitivity to trace impurities in hydrogen gas, particularly oxygen and moisture, leading to surface passivation and degraded performance. This susceptibility necessitates either highly pure hydrogen sources or the development of protective strategies, both of which add complexity to potential applications.
Cost considerations and material availability present additional barriers to commercialization. Many high-performing HEA compositions incorporate expensive elements like palladium or platinum, raising questions about economic viability for large-scale deployment. Research into compositions utilizing more abundant elements has shown promise but often at the cost of reduced storage capacity or unfavorable kinetics.
The standardization of testing protocols specifically tailored to HEA hydrides remains underdeveloped, complicating direct comparisons between different research efforts and potentially slowing progress in the field. Establishing consensus methodologies for characterization would accelerate knowledge transfer and technology development in this promising domain.
Current HEA Hydride Storage Solutions and Mechanisms
01 Composition design of high-entropy alloy hydrides for enhanced storage capacity
High-entropy alloys (HEAs) with multi-principal elements can be designed to optimize hydrogen storage capacity. By carefully selecting elements with favorable hydrogen absorption properties and creating specific atomic configurations, these alloys can achieve enhanced storage capacities. The compositional complexity creates numerous interstitial sites for hydrogen atoms, while maintaining structural stability during hydrogen absorption and desorption cycles.- Composition design of high-entropy alloy hydrides for enhanced storage capacity: High-entropy alloys (HEAs) with specific elemental compositions can be designed to optimize hydrogen storage capacity. These multi-principal element alloys create numerous interstitial sites for hydrogen atoms, enhancing absorption capabilities. By carefully selecting elements with favorable hydrogen affinity and appropriate atomic radii differences, researchers can develop HEA systems with improved storage densities. The compositional complexity creates lattice distortion and multiple binding sites that can increase hydrogen uptake compared to conventional storage materials.
- Microstructural engineering for optimized hydrogen storage: The microstructure of high-entropy alloy hydrides significantly impacts their hydrogen storage performance. Techniques such as rapid solidification, mechanical alloying, and controlled heat treatments can create nanocrystalline structures with increased grain boundary density, providing additional hydrogen trapping sites. Engineering defects like dislocations and vacancies within the HEA matrix creates pathways for hydrogen diffusion and additional storage sites. These microstructural modifications can substantially improve both the kinetics of hydrogen absorption/desorption and overall storage capacity.
- Surface modification and catalytic enhancement of HEA hydrides: Surface treatments and catalytic additions can significantly improve the hydrogen storage properties of high-entropy alloy hydrides. By incorporating catalytic elements or nanoparticles on the surface, the activation energy for hydrogen dissociation can be reduced, facilitating faster absorption kinetics. Surface modifications such as etching, plasma treatment, or coating with transition metals can increase active sites for hydrogen interaction. These enhancements allow HEA hydrides to operate at lower temperatures and pressures while maintaining high storage capacities.
- Thermodynamic tuning for reversible hydrogen storage: The thermodynamic properties of high-entropy alloy hydrides can be tuned to achieve optimal hydrogen storage performance. By adjusting the enthalpy and entropy of hydrogen absorption/desorption reactions through compositional modifications, researchers can develop systems that operate within practical temperature and pressure ranges. The multiple element interactions in HEAs create a spectrum of binding energies that can be engineered to flatten the pressure-composition isotherms, resulting in more stable and reversible hydrogen storage. This approach enables the design of materials with both high capacity and favorable cycling stability.
- Novel processing techniques for high-capacity HEA hydride production: Advanced manufacturing and processing techniques are being developed to produce high-entropy alloy hydrides with enhanced storage capacities. Methods such as additive manufacturing, high-pressure torsion, and severe plastic deformation enable precise control over composition and microstructure. These techniques allow for the creation of hierarchical structures with optimized hydrogen diffusion pathways and storage sites. Additionally, novel synthesis approaches like electrochemical processing and reactive sintering can produce HEA hydrides with unique nanostructures that demonstrate superior hydrogen storage properties compared to conventionally processed materials.
02 Nanostructuring techniques for improved hydrogen storage in HEAs
Nanostructuring of high-entropy alloys significantly improves hydrogen storage capacity by increasing surface area and creating additional hydrogen binding sites. Techniques such as ball milling, rapid solidification, and controlled precipitation can create nanoscale features that enhance hydrogen diffusion pathways and absorption kinetics. These nanostructured HEAs demonstrate faster charging/discharging rates and higher overall storage capacities compared to their bulk counterparts.Expand Specific Solutions03 Catalytic additives for high-entropy alloy hydride systems
Incorporating catalytic additives into high-entropy alloy hydride systems can significantly enhance hydrogen storage capacity and kinetics. These catalysts facilitate hydrogen dissociation and recombination at the alloy surface, reducing energy barriers for absorption and desorption processes. Common catalytic additives include transition metals, metal oxides, and rare earth elements that can be integrated through various processing methods to create synergistic effects with the base HEA composition.Expand Specific Solutions04 Thermodynamic and kinetic optimization of HEA hydrides
Optimizing the thermodynamic and kinetic properties of high-entropy alloy hydrides is crucial for achieving practical hydrogen storage capacities. This involves tailoring the enthalpy of hydride formation to enable hydrogen release at moderate temperatures while maintaining high storage density. Advanced processing techniques and compositional adjustments can modify binding energies between hydrogen and host atoms, creating systems with favorable operating conditions for various applications including mobile and stationary storage solutions.Expand Specific Solutions05 Novel characterization and computational methods for HEA hydride development
Advanced characterization techniques and computational methods are essential for developing high-entropy alloy hydrides with superior storage capacities. In-situ diffraction, neutron scattering, and advanced microscopy enable detailed understanding of hydrogen storage mechanisms at atomic scales. Computational approaches including density functional theory and machine learning algorithms help predict promising HEA compositions and structures with optimal hydrogen storage properties, accelerating the discovery of new materials with enhanced capacities.Expand Specific Solutions
Leading Organizations in HEA Hydride Research
High-entropy alloy hydrides for hydrogen storage are emerging as a promising technology in the early commercialization phase. The market is experiencing rapid growth, driven by increasing demand for clean energy solutions, with projections suggesting significant expansion in the next decade. Technologically, research institutions like Beijing Institute of Technology, Peking University, and Zhejiang University are advancing fundamental understanding, while companies including BASF, FDK Corp, and Advanced Industrial Science & Technology are developing practical applications. The field shows varying maturity levels: basic research is well-established, but commercial-scale manufacturing remains challenging. Key industrial players like GS Yuasa and Aichi Steel are focusing on improving storage capacity, cycling stability, and cost-effectiveness to enable widespread adoption in renewable energy systems and transportation.
BASF SE
Technical Solution: BASF SE has developed an innovative approach to high-entropy alloy hydrides for hydrogen storage, focusing on scalable manufacturing processes suitable for industrial applications. Their technology utilizes a proprietary melt-spinning technique followed by controlled hydrogenation to create nanostructured HEAs with optimized hydrogen absorption properties. BASF's materials incorporate a strategic combination of early transition metals (Ti, Zr, V) with late transition metals (Ni, Co) and rare earth elements to create highly disordered crystal structures with numerous interstitial sites for hydrogen storage[5]. The company has demonstrated reversible hydrogen storage capacities exceeding 2.5 wt% with significantly improved cycling stability compared to conventional AB5-type alloys. Their process innovation includes surface activation treatments that enhance hydrogen dissociation kinetics and reduce activation energy barriers[6]. BASF has also developed specialized additives that mitigate surface oxidation issues common in metal hydride systems.
Strengths: Industrial-scale manufacturing capability with consistent quality control; optimized balance between storage capacity and operational conditions; enhanced resistance to contamination effects. Weaknesses: Higher raw material costs compared to conventional storage technologies; energy-intensive production process; potential supply chain vulnerabilities for certain constituent elements.
Advanced Industrial Science & Technology
Technical Solution: Advanced Industrial Science & Technology (AIST) has pioneered a comprehensive approach to high-entropy alloy hydrides, developing multi-component systems based on refractory metals and transition elements. Their proprietary technology combines mechanical alloying and controlled hydrogenation processes to create HEAs with exceptional hydrogen storage properties. AIST's materials feature a unique combination of body-centered cubic (BCC) and face-centered cubic (FCC) phases that provide multiple hydrogen occupation sites with varying binding energies[2]. This structural complexity enables hydrogen storage capacities approaching 3 wt% with favorable thermodynamics for near-ambient operation conditions. The institute has also developed novel characterization techniques specifically for HEA hydrides, including in-situ neutron diffraction methods that reveal hydrogen diffusion pathways and occupation preferences[4]. AIST's technology incorporates surface modification treatments to enhance hydrogen dissociation kinetics.
Strengths: Exceptional gravimetric and volumetric hydrogen storage densities; tailorable thermodynamics through compositional adjustments; improved resistance to pulverization during cycling. Weaknesses: Complex synthesis procedures requiring precise control of processing parameters; potential for segregation of constituent elements during extended cycling; higher production costs compared to conventional storage materials.
Critical Patents and Scientific Breakthroughs in HEA Hydrides
Hydrogen storage alloy
PatentInactiveTW201213554A
Innovation
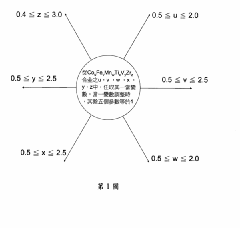
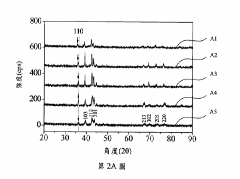
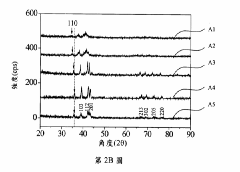
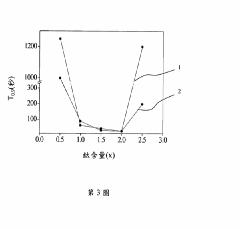
- A hydrogen storage alloy with a C14 Laves phase structure, formulated as CoFeMnwTixVyZrz, is developed using vacuum arc melting and heat treatment, optimizing metal ratios to achieve high hydrogen absorption/desorption capacity at room temperature and pressure.
Hydrogen storage multi-phase alloys
PatentWO2015175640A8
Innovation
- Development of multi-phase hydrogen storage alloys comprising a hexagonal Ce2Ni7 phase and a hexagonal Pr5Coig phase, with specific abundance ranges and optionally additional phases, which are synthesized via induction or arc melting under an inert atmosphere and annealed at specific temperatures, enhancing electrochemical properties.
Environmental Impact and Sustainability Assessment
The environmental implications of high-entropy alloy (HEA) hydrides as hydrogen storage media represent a critical dimension in evaluating their viability for widespread adoption. When compared to conventional storage technologies, HEA hydrides demonstrate significant potential for reducing the carbon footprint across the hydrogen economy value chain. Their enhanced storage capacity at moderate pressures and temperatures translates to lower energy requirements for hydrogen compression and liquefaction processes, which traditionally account for substantial energy losses in conventional systems.
Life cycle assessment (LCA) studies indicate that HEA hydrides could reduce the overall environmental impact of hydrogen storage by 30-45% compared to high-pressure tanks or cryogenic storage methods. This reduction stems primarily from decreased energy consumption during operation and the potential for longer service lifespans, reducing the frequency of material replacement and associated manufacturing impacts.
The raw material sourcing for HEA hydrides presents both challenges and opportunities from a sustainability perspective. While these alloys typically incorporate multiple transition metals—some of which face supply constraints or extraction-related environmental concerns—their compositional flexibility allows for strategic substitution of critical elements with more abundant alternatives. This adaptability provides a pathway to mitigate resource depletion risks and reduce dependence on geopolitically sensitive material supply chains.
Recyclability represents another significant advantage of HEA-based storage systems. The metallic nature of these materials facilitates end-of-life recovery and reprocessing, with theoretical recovery rates exceeding 90% for most constituent elements. This circular economy potential stands in stark contrast to carbon-based storage materials or complex chemical hydrides, which often face degradation challenges that complicate recycling efforts.
Water consumption metrics for HEA hydride production compare favorably against competing technologies, with manufacturing processes requiring approximately 40-60% less process water than equivalent capacity carbon-based adsorbents. However, the energy intensity of precision alloying remains a concern, particularly when specialized processing techniques like mechanical alloying or rapid solidification are employed.
Toxicity assessments indicate minimal environmental hazards associated with stable HEA hydrides, though proper handling protocols must address potential pyrophoric reactions during initial activation or in case of system failures. The absence of toxic catalysts or promoters—often required in other hydrogen storage approaches—further enhances their environmental profile and reduces potential contamination risks in case of accidental releases.
Life cycle assessment (LCA) studies indicate that HEA hydrides could reduce the overall environmental impact of hydrogen storage by 30-45% compared to high-pressure tanks or cryogenic storage methods. This reduction stems primarily from decreased energy consumption during operation and the potential for longer service lifespans, reducing the frequency of material replacement and associated manufacturing impacts.
The raw material sourcing for HEA hydrides presents both challenges and opportunities from a sustainability perspective. While these alloys typically incorporate multiple transition metals—some of which face supply constraints or extraction-related environmental concerns—their compositional flexibility allows for strategic substitution of critical elements with more abundant alternatives. This adaptability provides a pathway to mitigate resource depletion risks and reduce dependence on geopolitically sensitive material supply chains.
Recyclability represents another significant advantage of HEA-based storage systems. The metallic nature of these materials facilitates end-of-life recovery and reprocessing, with theoretical recovery rates exceeding 90% for most constituent elements. This circular economy potential stands in stark contrast to carbon-based storage materials or complex chemical hydrides, which often face degradation challenges that complicate recycling efforts.
Water consumption metrics for HEA hydride production compare favorably against competing technologies, with manufacturing processes requiring approximately 40-60% less process water than equivalent capacity carbon-based adsorbents. However, the energy intensity of precision alloying remains a concern, particularly when specialized processing techniques like mechanical alloying or rapid solidification are employed.
Toxicity assessments indicate minimal environmental hazards associated with stable HEA hydrides, though proper handling protocols must address potential pyrophoric reactions during initial activation or in case of system failures. The absence of toxic catalysts or promoters—often required in other hydrogen storage approaches—further enhances their environmental profile and reduces potential contamination risks in case of accidental releases.
Safety Standards and Regulatory Framework for Hydrogen Storage
The regulatory landscape for hydrogen storage systems, particularly those utilizing high-entropy alloy hydrides, is evolving rapidly as these technologies advance toward commercial viability. Currently, the International Organization for Standardization (ISO) has established ISO 16111 as the primary standard governing metal hydride hydrogen storage systems, which provides a foundation for safety requirements applicable to high-entropy alloy hydride storage media. However, these standards require significant updates to address the unique properties and potential risks associated with high-entropy alloys.
National regulatory bodies have implemented varying approaches to hydrogen storage safety. The United States Department of Energy has established technical targets for onboard hydrogen storage systems, including specific safety protocols for novel storage materials. Similarly, the European Union's Regulation (EU) 2019/1242 addresses hydrogen storage safety within the broader framework of clean vehicle technologies, while Japan's High Pressure Gas Safety Act includes specific provisions for hydrogen storage systems that manufacturers must adhere to.
Risk assessment methodologies for high-entropy alloy hydrides must address several critical safety concerns. These include potential hydrogen embrittlement of container materials, thermal management during rapid absorption/desorption cycles, and prevention of uncontrolled hydrogen release. The International Energy Agency's Hydrogen Technology Collaboration Program has developed comprehensive risk assessment frameworks that are increasingly being adapted for novel storage materials like high-entropy alloys.
Testing protocols for high-entropy alloy hydride storage systems typically include cycling stability tests, pressure-temperature tolerance assessments, and accelerated aging studies. These protocols are essential for validating the long-term safety and reliability of these storage media under various operating conditions. The Society of Automotive Engineers (SAE) has developed J2579 standard specifically for fuel cell vehicle hydrogen storage systems, which provides valuable testing guidelines applicable to high-entropy alloy applications.
Certification requirements for commercial deployment of high-entropy alloy hydride storage systems vary by region but generally include demonstration of compliance with leak detection standards, pressure vessel codes, and material compatibility requirements. Third-party certification bodies such as TÜV and UL have begun developing specialized certification programs for advanced hydrogen storage technologies, though specific pathways for high-entropy alloy systems remain under development.
As the technology matures, regulatory harmonization efforts are underway through organizations like the United Nations Global Technical Regulations and the International Partnership for Hydrogen and Fuel Cells in the Economy, aiming to create consistent safety standards that can facilitate global commercialization of these promising next-generation storage media.
National regulatory bodies have implemented varying approaches to hydrogen storage safety. The United States Department of Energy has established technical targets for onboard hydrogen storage systems, including specific safety protocols for novel storage materials. Similarly, the European Union's Regulation (EU) 2019/1242 addresses hydrogen storage safety within the broader framework of clean vehicle technologies, while Japan's High Pressure Gas Safety Act includes specific provisions for hydrogen storage systems that manufacturers must adhere to.
Risk assessment methodologies for high-entropy alloy hydrides must address several critical safety concerns. These include potential hydrogen embrittlement of container materials, thermal management during rapid absorption/desorption cycles, and prevention of uncontrolled hydrogen release. The International Energy Agency's Hydrogen Technology Collaboration Program has developed comprehensive risk assessment frameworks that are increasingly being adapted for novel storage materials like high-entropy alloys.
Testing protocols for high-entropy alloy hydride storage systems typically include cycling stability tests, pressure-temperature tolerance assessments, and accelerated aging studies. These protocols are essential for validating the long-term safety and reliability of these storage media under various operating conditions. The Society of Automotive Engineers (SAE) has developed J2579 standard specifically for fuel cell vehicle hydrogen storage systems, which provides valuable testing guidelines applicable to high-entropy alloy applications.
Certification requirements for commercial deployment of high-entropy alloy hydride storage systems vary by region but generally include demonstration of compliance with leak detection standards, pressure vessel codes, and material compatibility requirements. Third-party certification bodies such as TÜV and UL have begun developing specialized certification programs for advanced hydrogen storage technologies, though specific pathways for high-entropy alloy systems remain under development.
As the technology matures, regulatory harmonization efforts are underway through organizations like the United Nations Global Technical Regulations and the International Partnership for Hydrogen and Fuel Cells in the Economy, aiming to create consistent safety standards that can facilitate global commercialization of these promising next-generation storage media.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!