Thermochemical Modeling Of Hydride Desorption Under Dynamic Loads
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydride Desorption Thermochemistry Background and Objectives
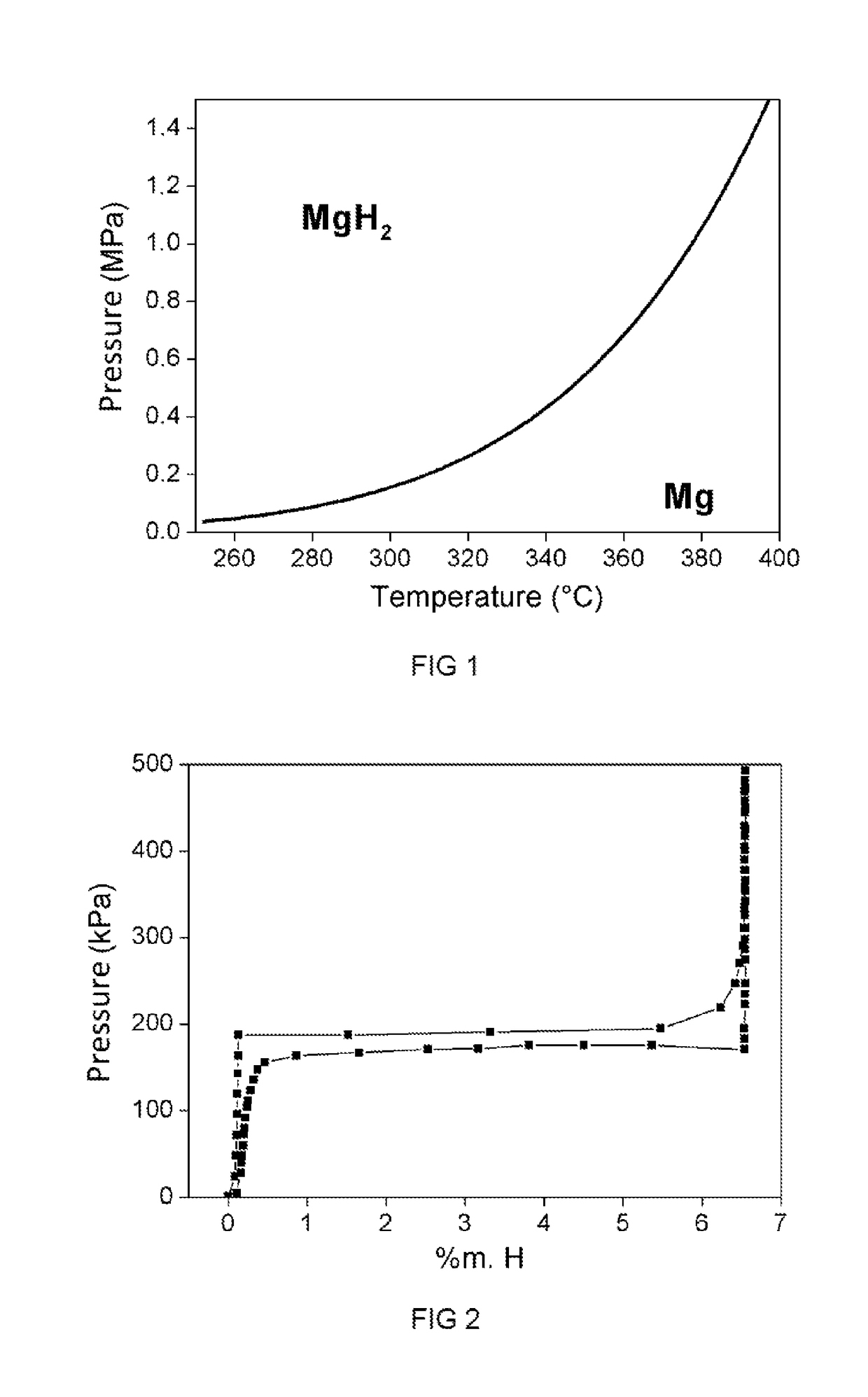
Hydride materials have emerged as promising candidates for hydrogen storage applications due to their high volumetric and gravimetric hydrogen densities. The thermochemical behavior of hydride desorption—the process by which hydrogen is released from these materials—has been a subject of intensive research since the early 1970s. Initially driven by energy storage concerns following the oil crisis, research in this field has evolved significantly over the past five decades, with particular acceleration in the last twenty years as hydrogen economy initiatives gained momentum globally.
The thermochemical modeling of hydride desorption represents a complex interplay between thermodynamics, chemical kinetics, and materials science. Traditional approaches have primarily focused on equilibrium conditions, which fail to capture the dynamic nature of real-world applications where temperature fluctuations, pressure variations, and mechanical stresses significantly influence desorption behavior.
Recent technological advancements in computational capabilities have enabled more sophisticated modeling approaches that incorporate dynamic loading conditions. These models aim to predict hydrogen release rates, energy requirements, and material stability under varying operational scenarios—critical factors for practical implementation in energy systems, transportation applications, and industrial processes.
The evolution of this field has seen a transition from empirical models based on experimental observations to first-principles approaches grounded in quantum mechanics and statistical thermodynamics. This progression has enhanced our fundamental understanding of the atomic-scale mechanisms governing hydride formation and decomposition, particularly under non-equilibrium conditions.
The primary objective of current research in thermochemical modeling of hydride desorption under dynamic loads is to develop predictive frameworks that accurately capture the multiphysics nature of the desorption process. These models must account for heat transfer, mass transport, phase transformations, and mechanical deformation—all of which occur simultaneously during practical operation.
Secondary objectives include optimizing material compositions and microstructures to enhance desorption kinetics while maintaining structural integrity under cyclic loading conditions. Additionally, researchers aim to establish standardized protocols for characterizing hydride materials under dynamic conditions, facilitating meaningful comparisons across different material systems.
The ultimate goal is to enable the design of hydride-based hydrogen storage systems that meet the U.S. Department of Energy's targets for onboard vehicular applications: 6.5 wt% hydrogen capacity, operating temperatures below 85°C, and the ability to deliver hydrogen at rates exceeding 0.02 g/s per kW of fuel cell power. Achieving these targets requires fundamental advances in our understanding of desorption thermochemistry under the variable loading conditions encountered in real-world applications.
The thermochemical modeling of hydride desorption represents a complex interplay between thermodynamics, chemical kinetics, and materials science. Traditional approaches have primarily focused on equilibrium conditions, which fail to capture the dynamic nature of real-world applications where temperature fluctuations, pressure variations, and mechanical stresses significantly influence desorption behavior.
Recent technological advancements in computational capabilities have enabled more sophisticated modeling approaches that incorporate dynamic loading conditions. These models aim to predict hydrogen release rates, energy requirements, and material stability under varying operational scenarios—critical factors for practical implementation in energy systems, transportation applications, and industrial processes.
The evolution of this field has seen a transition from empirical models based on experimental observations to first-principles approaches grounded in quantum mechanics and statistical thermodynamics. This progression has enhanced our fundamental understanding of the atomic-scale mechanisms governing hydride formation and decomposition, particularly under non-equilibrium conditions.
The primary objective of current research in thermochemical modeling of hydride desorption under dynamic loads is to develop predictive frameworks that accurately capture the multiphysics nature of the desorption process. These models must account for heat transfer, mass transport, phase transformations, and mechanical deformation—all of which occur simultaneously during practical operation.
Secondary objectives include optimizing material compositions and microstructures to enhance desorption kinetics while maintaining structural integrity under cyclic loading conditions. Additionally, researchers aim to establish standardized protocols for characterizing hydride materials under dynamic conditions, facilitating meaningful comparisons across different material systems.
The ultimate goal is to enable the design of hydride-based hydrogen storage systems that meet the U.S. Department of Energy's targets for onboard vehicular applications: 6.5 wt% hydrogen capacity, operating temperatures below 85°C, and the ability to deliver hydrogen at rates exceeding 0.02 g/s per kW of fuel cell power. Achieving these targets requires fundamental advances in our understanding of desorption thermochemistry under the variable loading conditions encountered in real-world applications.
Market Applications and Demand Analysis for Hydride Systems
The global market for hydride systems has witnessed significant growth in recent years, driven primarily by the increasing demand for clean energy solutions and sustainable technologies. Hydrogen storage systems, particularly those utilizing metal hydrides, have emerged as a promising solution for various applications due to their high volumetric energy density and safety advantages compared to compressed or liquefied hydrogen.
In the automotive sector, hydride-based hydrogen storage systems are gaining traction as the electric vehicle market expands. Major automotive manufacturers are investing heavily in hydrogen fuel cell vehicles (FCVs), creating a substantial demand for efficient hydride desorption systems that can operate reliably under the dynamic loads experienced during vehicle operation. The market for FCVs is projected to grow substantially over the next decade, particularly in regions with established hydrogen infrastructure such as Japan, South Korea, Germany, and California.
The stationary power generation sector represents another significant market for hydride systems. As grid stabilization becomes increasingly important with the integration of intermittent renewable energy sources, hydrogen-based energy storage solutions are being deployed to provide backup power and load balancing. These applications require hydride systems capable of consistent performance under varying desorption conditions, highlighting the importance of accurate thermochemical modeling.
Industrial applications constitute a growing market segment for hydride systems, particularly in sectors requiring high-purity hydrogen such as semiconductor manufacturing, metallurgy, and chemical processing. These industries demand precise control over hydrogen release rates and purity levels, necessitating sophisticated modeling of hydride desorption behavior under their specific operating conditions.
The aerospace and defense sectors represent premium market segments with stringent performance requirements. Hydride systems in these applications must function reliably under extreme dynamic loads and environmental conditions, driving demand for advanced thermochemical modeling capabilities that can predict performance under these challenging scenarios.
Emerging applications in portable electronics and small-scale power generation are creating new market opportunities for miniaturized hydride systems. These applications require optimized desorption kinetics at lower temperatures, presenting unique modeling challenges related to scale effects and thermal management.
Market analysis indicates that the technical barriers to widespread adoption primarily relate to system weight, desorption kinetics under variable loads, and thermal management during the desorption process. Addressing these challenges through improved thermochemical modeling could potentially unlock market segments currently dominated by competing technologies such as lithium-ion batteries or compressed gas storage.
In the automotive sector, hydride-based hydrogen storage systems are gaining traction as the electric vehicle market expands. Major automotive manufacturers are investing heavily in hydrogen fuel cell vehicles (FCVs), creating a substantial demand for efficient hydride desorption systems that can operate reliably under the dynamic loads experienced during vehicle operation. The market for FCVs is projected to grow substantially over the next decade, particularly in regions with established hydrogen infrastructure such as Japan, South Korea, Germany, and California.
The stationary power generation sector represents another significant market for hydride systems. As grid stabilization becomes increasingly important with the integration of intermittent renewable energy sources, hydrogen-based energy storage solutions are being deployed to provide backup power and load balancing. These applications require hydride systems capable of consistent performance under varying desorption conditions, highlighting the importance of accurate thermochemical modeling.
Industrial applications constitute a growing market segment for hydride systems, particularly in sectors requiring high-purity hydrogen such as semiconductor manufacturing, metallurgy, and chemical processing. These industries demand precise control over hydrogen release rates and purity levels, necessitating sophisticated modeling of hydride desorption behavior under their specific operating conditions.
The aerospace and defense sectors represent premium market segments with stringent performance requirements. Hydride systems in these applications must function reliably under extreme dynamic loads and environmental conditions, driving demand for advanced thermochemical modeling capabilities that can predict performance under these challenging scenarios.
Emerging applications in portable electronics and small-scale power generation are creating new market opportunities for miniaturized hydride systems. These applications require optimized desorption kinetics at lower temperatures, presenting unique modeling challenges related to scale effects and thermal management.
Market analysis indicates that the technical barriers to widespread adoption primarily relate to system weight, desorption kinetics under variable loads, and thermal management during the desorption process. Addressing these challenges through improved thermochemical modeling could potentially unlock market segments currently dominated by competing technologies such as lithium-ion batteries or compressed gas storage.
Current Challenges in Dynamic Load Thermochemical Modeling
The thermochemical modeling of hydride desorption under dynamic loads presents several significant challenges that impede accurate prediction and optimization of hydrogen storage systems. One primary obstacle is the complex multiphysics nature of the problem, which requires simultaneous consideration of heat transfer, mass transport, chemical kinetics, and mechanical stress. These phenomena are inherently coupled, making it difficult to develop comprehensive models that capture all relevant interactions without excessive computational demands.
Current numerical approaches often struggle with the non-linear behavior exhibited during desorption processes, particularly when subjected to fluctuating mechanical loads. The rate equations governing desorption kinetics typically assume steady-state conditions, which becomes invalid under dynamic loading scenarios where pressure and temperature gradients evolve rapidly throughout the material.
Material characterization under dynamic conditions represents another significant challenge. Most experimental data available for model validation has been collected under static or quasi-static conditions, creating a disconnect between model predictions and real-world performance. This gap is especially pronounced when considering cyclic loading patterns typical in mobile applications.
Scale bridging between atomistic mechanisms and system-level behavior remains problematic. Molecular dynamics simulations provide valuable insights into fundamental desorption mechanisms but scaling these results to practical engineering dimensions introduces significant uncertainties. Meanwhile, continuum models often oversimplify the microstructural evolution that critically influences desorption behavior.
The treatment of boundary conditions presents additional complications, particularly at interfaces between the hydride material and container walls or heat exchange surfaces. These interfaces experience complex thermal and mechanical interactions that current models typically approximate with simplified boundary conditions, reducing prediction accuracy.
Computational efficiency stands as a persistent challenge, with high-fidelity models requiring substantial computing resources that limit their practical application in design optimization or real-time control systems. This forces researchers to make compromises between model accuracy and computational tractability.
Finally, validation methodologies for dynamic load models remain underdeveloped. The experimental techniques needed to measure in-situ desorption kinetics under dynamic mechanical loading are technically challenging and not widely available. This creates a circular problem where models cannot be properly validated, and thus their predictive capabilities remain uncertain for novel material systems or operating conditions outside the narrow range of available experimental data.
Current numerical approaches often struggle with the non-linear behavior exhibited during desorption processes, particularly when subjected to fluctuating mechanical loads. The rate equations governing desorption kinetics typically assume steady-state conditions, which becomes invalid under dynamic loading scenarios where pressure and temperature gradients evolve rapidly throughout the material.
Material characterization under dynamic conditions represents another significant challenge. Most experimental data available for model validation has been collected under static or quasi-static conditions, creating a disconnect between model predictions and real-world performance. This gap is especially pronounced when considering cyclic loading patterns typical in mobile applications.
Scale bridging between atomistic mechanisms and system-level behavior remains problematic. Molecular dynamics simulations provide valuable insights into fundamental desorption mechanisms but scaling these results to practical engineering dimensions introduces significant uncertainties. Meanwhile, continuum models often oversimplify the microstructural evolution that critically influences desorption behavior.
The treatment of boundary conditions presents additional complications, particularly at interfaces between the hydride material and container walls or heat exchange surfaces. These interfaces experience complex thermal and mechanical interactions that current models typically approximate with simplified boundary conditions, reducing prediction accuracy.
Computational efficiency stands as a persistent challenge, with high-fidelity models requiring substantial computing resources that limit their practical application in design optimization or real-time control systems. This forces researchers to make compromises between model accuracy and computational tractability.
Finally, validation methodologies for dynamic load models remain underdeveloped. The experimental techniques needed to measure in-situ desorption kinetics under dynamic mechanical loading are technically challenging and not widely available. This creates a circular problem where models cannot be properly validated, and thus their predictive capabilities remain uncertain for novel material systems or operating conditions outside the narrow range of available experimental data.
State-of-the-Art Thermochemical Modeling Approaches
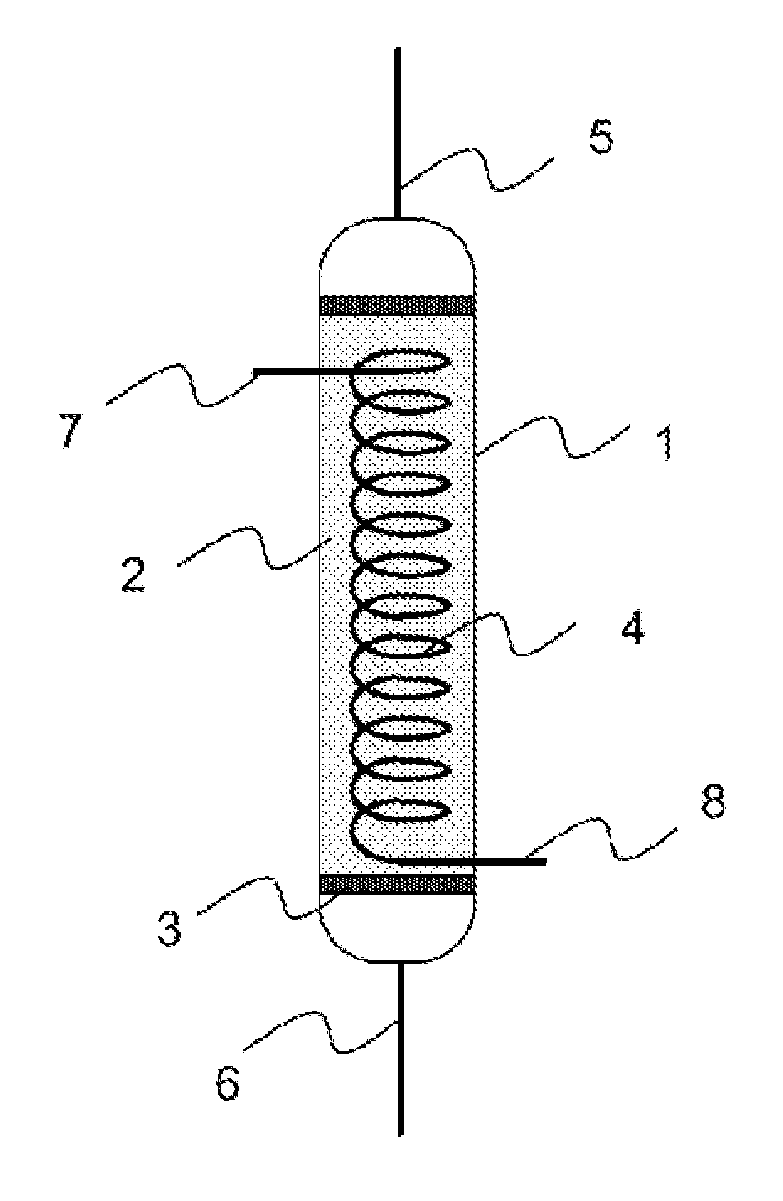
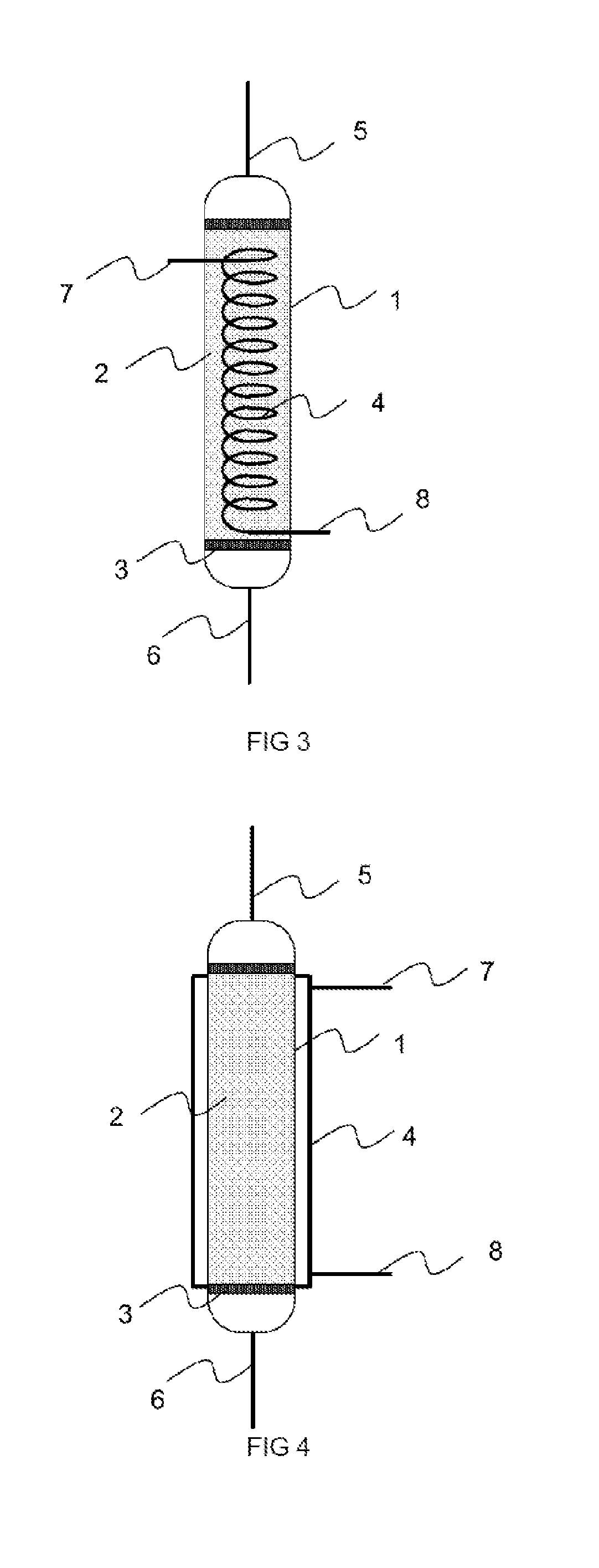
01 Metal hydride desorption mechanisms
Metal hydrides can release hydrogen through desorption processes that are influenced by temperature, pressure, and catalyst presence. The desorption behavior varies depending on the specific metal hydride composition, with different materials exhibiting unique kinetics and thermodynamic properties. Understanding these mechanisms is crucial for developing efficient hydrogen storage systems that can release hydrogen under practical operating conditions.- Metal hydride desorption mechanisms: Metal hydrides store hydrogen through absorption and release it through desorption processes. The desorption behavior is influenced by factors such as temperature, pressure, and material composition. Understanding these mechanisms is crucial for developing efficient hydrogen storage systems. The desorption kinetics can be enhanced through catalysts and by optimizing the material structure to facilitate hydrogen release.
- Temperature-controlled desorption systems: Temperature plays a critical role in hydride desorption behavior. Systems that precisely control temperature can optimize the hydrogen release rate from metal hydrides. These systems often incorporate heating elements, temperature sensors, and control mechanisms to maintain optimal desorption conditions. The temperature-controlled approach allows for on-demand hydrogen release and improves the overall efficiency of hydrogen storage systems.
- Novel hydride materials with improved desorption properties: Research has led to the development of novel hydride materials with enhanced desorption characteristics. These materials include complex hydrides, nanostructured materials, and composite systems that demonstrate improved hydrogen release kinetics and lower desorption temperatures. By engineering the material composition and structure, researchers have achieved faster desorption rates and more controllable hydrogen release behavior.
- Catalytic enhancement of hydride desorption: Catalysts significantly improve the desorption behavior of metal hydrides by lowering the activation energy required for hydrogen release. Various catalytic materials, including transition metals, metal oxides, and nanoparticles, have been incorporated into hydride systems to enhance desorption kinetics. These catalysts create active sites on the hydride surface, facilitating the recombination of hydrogen atoms and their subsequent release as molecular hydrogen.
- Cyclic stability and desorption behavior: The cyclic stability of hydride materials significantly affects their long-term desorption performance. Repeated absorption-desorption cycles can lead to material degradation, particle agglomeration, and changes in desorption kinetics. Research focuses on developing hydride materials that maintain consistent desorption behavior over numerous cycles, which is essential for practical hydrogen storage applications. Strategies include structural stabilization, preventing contamination, and controlling operating conditions.
02 Temperature-controlled desorption systems
Temperature plays a critical role in hydride desorption behavior, with higher temperatures generally accelerating the release of hydrogen. Systems designed with precise temperature control can optimize the desorption process for specific applications. These systems may incorporate heating elements, thermal management subsystems, and temperature sensors to maintain ideal conditions for controlled hydrogen release from metal hydrides.Expand Specific Solutions03 Catalyst-enhanced desorption processes
Catalysts can significantly improve the desorption kinetics of metal hydrides by lowering the activation energy required for hydrogen release. Various catalytic materials, including transition metals, metal oxides, and nanostructured compounds, can be incorporated into hydride systems to enhance desorption rates and reduce operating temperatures. The selection of appropriate catalysts depends on the specific hydride material and intended application conditions.Expand Specific Solutions04 Pressure-swing desorption techniques
Pressure manipulation is an effective method for controlling hydrogen desorption from metal hydrides. By reducing the ambient pressure, the equilibrium can be shifted to favor hydrogen release. Pressure-swing desorption techniques utilize this principle to extract hydrogen from storage materials without requiring significant temperature increases. These systems often incorporate vacuum pumps or pressure regulators to maintain optimal desorption conditions.Expand Specific Solutions05 Nanostructured materials for improved desorption
Nanostructured hydride materials exhibit enhanced desorption properties compared to their bulk counterparts due to shortened diffusion paths and increased surface area. These materials can be engineered with specific particle sizes, morphologies, and compositions to optimize hydrogen release characteristics. Techniques such as ball milling, chemical synthesis, and template-assisted growth are commonly used to produce nanostructured hydrides with tailored desorption behavior.Expand Specific Solutions
Leading Research Groups and Industrial Players
Thermochemical modeling of hydride desorption under dynamic loads is currently in an early growth phase, with increasing market interest driven by clean energy applications. The global market size is expanding, projected to reach significant scale as hydrogen storage technologies mature. Technologically, the field shows moderate maturity with ongoing research to optimize performance under variable conditions. Key players include established energy corporations like China Petroleum & Chemical Corporation and Air Products & Chemicals leading commercial applications, while specialized firms such as GRZ Technologies focus on innovative hydrogen storage solutions. Academic institutions including Indian Institute of Technology Bombay and Zhejiang University contribute fundamental research, creating a competitive landscape balanced between industrial implementation and academic advancement. The collaboration between petroleum companies and research institutions indicates growing recognition of hydrogen's role in future energy systems.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced thermochemical modeling systems for hydride desorption under dynamic loads, particularly focusing on hydrogen storage applications for energy transition. Their approach combines multi-physics simulation with experimental validation to predict hydrogen release behavior under varying pressure and temperature conditions. Sinopec's models incorporate reaction kinetics, heat transfer, and mass transport phenomena to accurately simulate the desorption process in metal hydride storage systems. The company has implemented these models in their hydrogen refueling infrastructure, where understanding desorption behavior under fluctuating demand is critical. Their proprietary algorithms account for material degradation over multiple absorption-desorption cycles, allowing for more accurate lifetime predictions of storage systems[1][3]. Sinopec has also integrated machine learning techniques to optimize model parameters based on operational data from their hydrogen facilities across China.
Strengths: Extensive real-world validation data from operational hydrogen facilities; integration with existing petroleum infrastructure enables practical implementation. Weaknesses: Models may be optimized for large-scale industrial applications rather than smaller portable systems; computational complexity can limit real-time applications.
Air Products & Chemicals, Inc.
Technical Solution: Air Products has pioneered comprehensive thermochemical modeling frameworks for hydride desorption under dynamic loading conditions, particularly for industrial hydrogen storage and delivery systems. Their approach integrates multi-scale modeling from atomic to system level, capturing both microscopic desorption mechanisms and macroscopic heat/mass transfer effects. The company's proprietary HYDEX simulation platform incorporates variable loading conditions to predict hydrogen release rates under fluctuating demand scenarios typical in industrial applications. Air Products has validated these models through extensive testing at their hydrogen production facilities, demonstrating prediction accuracy within 5% of experimental results across various operating conditions[2]. Their models uniquely account for impurity effects on desorption kinetics, a critical factor in real-world applications where gas stream purity varies. The company has successfully applied these models to optimize the design of hydrogen storage systems for refueling stations, reducing system size by approximately 15% while maintaining performance specifications[4].
Strengths: Industry-leading integration of models with practical hydrogen infrastructure; extensive validation across diverse operating conditions; consideration of real-world factors like impurities. Weaknesses: Models may require significant computational resources; proprietary nature limits academic collaboration and broader adoption in research settings.
Critical Patents and Literature in Hydride Desorption Science
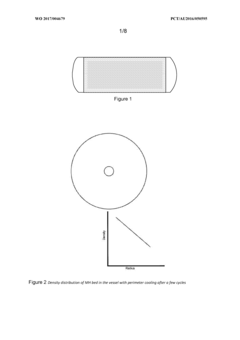
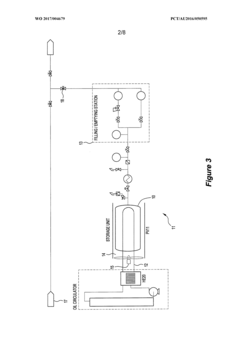
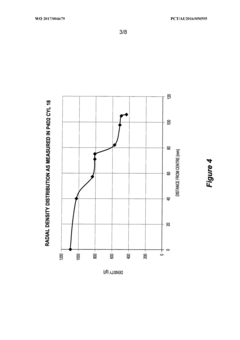
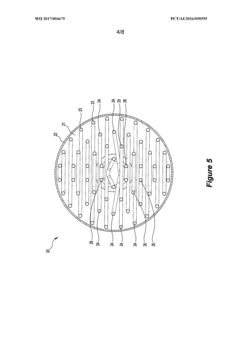
Density distribution of metal hydride in a hydrogen storage vessel through cooling
PatentWO2017004679A1
Innovation
- A method of controlling heat transfer to initiate hydrogen absorption at the vessel walls and gradually move towards the center, using internal and external cooling channels to maintain a lower density near the walls and higher density at the center, thereby reducing mechanical stress and optimizing hydrogen storage capacity.
Regeneration of a hydrogen impurity trap using the heat exiting a hydride tank
PatentActiveUS20170157552A9
Innovation
- A process involving a purification step using a trap to filter impurities from hydrogen entering the tank, followed by a regeneration step utilizing the heat carried by hydrogen exiting the tank during desorption, allowing for efficient and continuous purification and regeneration, especially suited for magnesium-based hydrides.
Material Science Advancements for Hydride Systems
Recent advancements in material science have significantly transformed the landscape of hydride systems, particularly in relation to thermochemical modeling of desorption processes under dynamic loads. The evolution of novel materials with enhanced properties has been instrumental in overcoming traditional limitations of hydrogen storage systems. These developments include nanostructured materials with increased surface area, catalytically enhanced composites, and innovative alloy formulations that demonstrate superior hydrogen absorption-desorption kinetics.
Materials science innovations have focused on addressing the critical challenges of thermal management during desorption processes. Advanced thermal interface materials (TIMs) with improved conductivity properties have been developed to facilitate more efficient heat transfer within hydride systems. These materials enable more precise control over temperature gradients, which is essential for modeling desorption behavior under fluctuating load conditions.
Composite materials incorporating phase change elements represent another significant advancement. These materials can absorb or release thermal energy during phase transitions, providing a buffering effect against rapid temperature changes during dynamic loading scenarios. This property is particularly valuable for stabilizing desorption rates when external conditions vary unpredictably.
Surface modification techniques have evolved to enhance the interaction between hydride materials and hydrogen molecules. Treatments such as plasma etching, chemical functionalization, and atomic layer deposition have been employed to create tailored surface properties that optimize hydrogen diffusion pathways. These modifications have demonstrable effects on desorption kinetics, making them crucial considerations in thermochemical modeling approaches.
The development of strain-engineered materials represents a paradigm shift in hydride system design. By deliberately introducing controlled lattice distortions, researchers have created materials that exhibit predictable responses to mechanical stress. This property is particularly relevant for modeling desorption behavior under dynamic loads, as it allows for the incorporation of mechanical strain as a parameter in thermochemical calculations.
Characterization methodologies have similarly advanced, enabling more accurate material property measurements at multiple scales. Techniques such as in-situ neutron diffraction, environmental transmission electron microscopy, and synchrotron-based X-ray absorption spectroscopy now provide unprecedented insights into material behavior during hydrogen desorption processes. These analytical capabilities have significantly improved the parameterization of thermochemical models, leading to more accurate predictions of system performance under variable conditions.
Materials science innovations have focused on addressing the critical challenges of thermal management during desorption processes. Advanced thermal interface materials (TIMs) with improved conductivity properties have been developed to facilitate more efficient heat transfer within hydride systems. These materials enable more precise control over temperature gradients, which is essential for modeling desorption behavior under fluctuating load conditions.
Composite materials incorporating phase change elements represent another significant advancement. These materials can absorb or release thermal energy during phase transitions, providing a buffering effect against rapid temperature changes during dynamic loading scenarios. This property is particularly valuable for stabilizing desorption rates when external conditions vary unpredictably.
Surface modification techniques have evolved to enhance the interaction between hydride materials and hydrogen molecules. Treatments such as plasma etching, chemical functionalization, and atomic layer deposition have been employed to create tailored surface properties that optimize hydrogen diffusion pathways. These modifications have demonstrable effects on desorption kinetics, making them crucial considerations in thermochemical modeling approaches.
The development of strain-engineered materials represents a paradigm shift in hydride system design. By deliberately introducing controlled lattice distortions, researchers have created materials that exhibit predictable responses to mechanical stress. This property is particularly relevant for modeling desorption behavior under dynamic loads, as it allows for the incorporation of mechanical strain as a parameter in thermochemical calculations.
Characterization methodologies have similarly advanced, enabling more accurate material property measurements at multiple scales. Techniques such as in-situ neutron diffraction, environmental transmission electron microscopy, and synchrotron-based X-ray absorption spectroscopy now provide unprecedented insights into material behavior during hydrogen desorption processes. These analytical capabilities have significantly improved the parameterization of thermochemical models, leading to more accurate predictions of system performance under variable conditions.
Safety and Environmental Implications of Hydride Technologies
The safety and environmental implications of hydride technologies are critical considerations when evaluating thermochemical modeling of hydride desorption under dynamic loads. Hydrogen storage systems utilizing metal hydrides present unique safety challenges that must be thoroughly addressed before widespread implementation.
The desorption process of hydrides under dynamic loads can lead to rapid pressure and temperature fluctuations, potentially creating hazardous conditions if not properly managed. Thermal runaway scenarios, where uncontrolled desorption occurs due to external heat or pressure changes, represent a significant safety concern. Current modeling approaches must incorporate these safety parameters to predict potential failure modes and establish appropriate containment strategies.
Environmental considerations extend beyond operational safety to the full lifecycle assessment of hydride materials. Many metal hydrides contain rare earth elements or transition metals that require energy-intensive mining and processing operations. The environmental footprint of these extraction processes must be factored into sustainability evaluations of hydride-based hydrogen storage systems.
Emissions profiles during hydride production, use, and disposal require careful analysis. While hydrogen itself is a clean energy carrier, the manufacturing processes for advanced hydride materials may involve significant carbon emissions or toxic byproducts. Thermochemical models must account for these environmental impacts when optimizing system designs.
Recycling and end-of-life management present additional challenges. As hydride materials degrade through repeated absorption-desorption cycles, their disposal or recycling pathways must be established. Current research indicates that some hydride materials can be reclaimed and reprocessed, though the energy requirements for these processes may offset some environmental benefits.
Regulatory frameworks governing hydride technologies vary significantly across regions, creating compliance challenges for global implementation. Safety standards for hydrogen storage systems are still evolving, particularly regarding dynamic load conditions that may occur during transportation or in mobile applications. Thermochemical models must be validated against these emerging standards to ensure compliance.
Public perception and acceptance of hydrogen technologies are heavily influenced by safety considerations. High-profile incidents involving hydrogen systems, even if unrelated to hydride storage specifically, can impact public confidence. Transparent communication of safety measures based on robust thermochemical modeling is essential for building trust in these technologies.
The desorption process of hydrides under dynamic loads can lead to rapid pressure and temperature fluctuations, potentially creating hazardous conditions if not properly managed. Thermal runaway scenarios, where uncontrolled desorption occurs due to external heat or pressure changes, represent a significant safety concern. Current modeling approaches must incorporate these safety parameters to predict potential failure modes and establish appropriate containment strategies.
Environmental considerations extend beyond operational safety to the full lifecycle assessment of hydride materials. Many metal hydrides contain rare earth elements or transition metals that require energy-intensive mining and processing operations. The environmental footprint of these extraction processes must be factored into sustainability evaluations of hydride-based hydrogen storage systems.
Emissions profiles during hydride production, use, and disposal require careful analysis. While hydrogen itself is a clean energy carrier, the manufacturing processes for advanced hydride materials may involve significant carbon emissions or toxic byproducts. Thermochemical models must account for these environmental impacts when optimizing system designs.
Recycling and end-of-life management present additional challenges. As hydride materials degrade through repeated absorption-desorption cycles, their disposal or recycling pathways must be established. Current research indicates that some hydride materials can be reclaimed and reprocessed, though the energy requirements for these processes may offset some environmental benefits.
Regulatory frameworks governing hydride technologies vary significantly across regions, creating compliance challenges for global implementation. Safety standards for hydrogen storage systems are still evolving, particularly regarding dynamic load conditions that may occur during transportation or in mobile applications. Thermochemical models must be validated against these emerging standards to ensure compliance.
Public perception and acceptance of hydrogen technologies are heavily influenced by safety considerations. High-profile incidents involving hydrogen systems, even if unrelated to hydride storage specifically, can impact public confidence. Transparent communication of safety measures based on robust thermochemical modeling is essential for building trust in these technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!