Magnesium-Based Hydrides And Nanostructuring Strategies For High Capacity
AUG 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-Based Hydrides Background and Research Objectives
Magnesium-based hydrides have emerged as promising candidates for hydrogen storage applications due to their high theoretical hydrogen capacity, abundance, and cost-effectiveness. The journey of magnesium hydride (MgH2) research dates back to the 1950s, but significant advancements have been made in the past two decades. Initially, the focus was primarily on understanding the basic properties and synthesis methods of MgH2. However, the research landscape has evolved substantially, with current efforts directed towards enhancing kinetics, reducing desorption temperatures, and improving cycling stability.
The evolution of magnesium hydride research has been marked by several key milestones. Early investigations revealed that while MgH2 offers an impressive theoretical hydrogen capacity of 7.6 wt%, its practical application was hindered by slow kinetics and high desorption temperatures (>300°C). This recognition led to the exploration of various catalysts and additives to improve performance characteristics. Subsequently, the field witnessed a paradigm shift with the introduction of nanostructuring approaches, which demonstrated remarkable improvements in hydrogen sorption properties.
Recent technological trends indicate a growing interest in complex magnesium-based hydrides, including ternary and quaternary systems, which offer enhanced thermodynamic and kinetic properties. Additionally, there has been an increasing focus on developing sustainable and scalable synthesis methods for these materials, moving beyond laboratory-scale production to industrial viability. The integration of computational modeling and high-throughput screening techniques has also accelerated the discovery and optimization of novel magnesium-based hydride systems.
The primary objective of this research is to comprehensively investigate magnesium-based hydrides and evaluate various nanostructuring strategies to achieve high hydrogen storage capacity. Specifically, the research aims to develop magnesium-based hydride systems that can store hydrogen at capacities exceeding 6 wt% under practical operating conditions (temperature <200°C, pressure <10 bar). Additionally, the research seeks to enhance the kinetics of hydrogen absorption and desorption, targeting complete cycling within 10 minutes.
Furthermore, this investigation aims to elucidate the fundamental mechanisms governing hydrogen storage in nanostructured magnesium-based systems, particularly focusing on the role of interfaces, defects, and catalytic sites. Understanding these mechanisms is crucial for rational design of next-generation hydrogen storage materials. The research also intends to explore the synergistic effects of combining nanostructuring with catalytic doping to optimize both thermodynamic and kinetic properties simultaneously.
Ultimately, this technical research endeavors to bridge the gap between theoretical potential and practical application of magnesium-based hydrides in hydrogen storage systems, contributing to the advancement of clean energy technologies and supporting the transition towards a hydrogen-based economy.
The evolution of magnesium hydride research has been marked by several key milestones. Early investigations revealed that while MgH2 offers an impressive theoretical hydrogen capacity of 7.6 wt%, its practical application was hindered by slow kinetics and high desorption temperatures (>300°C). This recognition led to the exploration of various catalysts and additives to improve performance characteristics. Subsequently, the field witnessed a paradigm shift with the introduction of nanostructuring approaches, which demonstrated remarkable improvements in hydrogen sorption properties.
Recent technological trends indicate a growing interest in complex magnesium-based hydrides, including ternary and quaternary systems, which offer enhanced thermodynamic and kinetic properties. Additionally, there has been an increasing focus on developing sustainable and scalable synthesis methods for these materials, moving beyond laboratory-scale production to industrial viability. The integration of computational modeling and high-throughput screening techniques has also accelerated the discovery and optimization of novel magnesium-based hydride systems.
The primary objective of this research is to comprehensively investigate magnesium-based hydrides and evaluate various nanostructuring strategies to achieve high hydrogen storage capacity. Specifically, the research aims to develop magnesium-based hydride systems that can store hydrogen at capacities exceeding 6 wt% under practical operating conditions (temperature <200°C, pressure <10 bar). Additionally, the research seeks to enhance the kinetics of hydrogen absorption and desorption, targeting complete cycling within 10 minutes.
Furthermore, this investigation aims to elucidate the fundamental mechanisms governing hydrogen storage in nanostructured magnesium-based systems, particularly focusing on the role of interfaces, defects, and catalytic sites. Understanding these mechanisms is crucial for rational design of next-generation hydrogen storage materials. The research also intends to explore the synergistic effects of combining nanostructuring with catalytic doping to optimize both thermodynamic and kinetic properties simultaneously.
Ultimately, this technical research endeavors to bridge the gap between theoretical potential and practical application of magnesium-based hydrides in hydrogen storage systems, contributing to the advancement of clean energy technologies and supporting the transition towards a hydrogen-based economy.
Market Analysis for High-Capacity Hydrogen Storage
The global hydrogen storage market is experiencing significant growth, driven by the increasing focus on clean energy solutions and the transition away from fossil fuels. As of 2023, the market was valued at approximately 5.7 billion USD, with projections indicating a compound annual growth rate of 11.3% through 2030. High-capacity hydrogen storage technologies, particularly those based on magnesium hydrides and nanostructured materials, represent a crucial segment within this expanding market.
The demand for efficient hydrogen storage solutions spans multiple sectors. The automotive industry constitutes the largest market share, with major manufacturers investing heavily in hydrogen fuel cell vehicles that require advanced storage systems. Toyota, Hyundai, and Honda have already commercialized hydrogen-powered vehicles, creating immediate demand for high-capacity storage technologies that can extend driving range while reducing weight and volume constraints.
Industrial applications form the second-largest market segment, where hydrogen is increasingly used for power generation, chemical processing, and as a feedstock. The stationary power sector is showing particular interest in magnesium-based storage systems due to their potential cost advantages and safety benefits compared to compressed or liquefied hydrogen storage methods.
Regional analysis reveals that Asia-Pacific currently leads the market, with Japan, South Korea, and China making substantial investments in hydrogen infrastructure and storage technologies. Europe follows closely, driven by stringent carbon reduction policies and government incentives for hydrogen adoption. The European Hydrogen Strategy aims to install at least 40 GW of renewable hydrogen electrolyzers by 2030, creating substantial demand for storage solutions.
Market barriers for magnesium-based hydride storage systems include high production costs, technical challenges related to hydrogen absorption/desorption kinetics, and competition from alternative storage technologies. Current production costs for advanced magnesium hydride systems range from 500-800 USD per kilogram of hydrogen stored, significantly higher than the U.S. Department of Energy's target of 300 USD per kilogram for commercial viability.
Consumer adoption trends indicate growing acceptance of hydrogen technologies, particularly in regions with developed hydrogen infrastructure. Market surveys show that 67% of industrial users would consider switching to hydrogen-based systems if storage efficiency improves and costs decrease by at least 30%.
The competitive landscape features established players like Air Liquide, Linde, and Worthington Industries alongside emerging technology developers focused specifically on metal hydride storage solutions. Recent strategic partnerships between automotive manufacturers and materials science companies highlight the industry's recognition of magnesium-based hydrides as a promising pathway to meet future storage requirements.
The demand for efficient hydrogen storage solutions spans multiple sectors. The automotive industry constitutes the largest market share, with major manufacturers investing heavily in hydrogen fuel cell vehicles that require advanced storage systems. Toyota, Hyundai, and Honda have already commercialized hydrogen-powered vehicles, creating immediate demand for high-capacity storage technologies that can extend driving range while reducing weight and volume constraints.
Industrial applications form the second-largest market segment, where hydrogen is increasingly used for power generation, chemical processing, and as a feedstock. The stationary power sector is showing particular interest in magnesium-based storage systems due to their potential cost advantages and safety benefits compared to compressed or liquefied hydrogen storage methods.
Regional analysis reveals that Asia-Pacific currently leads the market, with Japan, South Korea, and China making substantial investments in hydrogen infrastructure and storage technologies. Europe follows closely, driven by stringent carbon reduction policies and government incentives for hydrogen adoption. The European Hydrogen Strategy aims to install at least 40 GW of renewable hydrogen electrolyzers by 2030, creating substantial demand for storage solutions.
Market barriers for magnesium-based hydride storage systems include high production costs, technical challenges related to hydrogen absorption/desorption kinetics, and competition from alternative storage technologies. Current production costs for advanced magnesium hydride systems range from 500-800 USD per kilogram of hydrogen stored, significantly higher than the U.S. Department of Energy's target of 300 USD per kilogram for commercial viability.
Consumer adoption trends indicate growing acceptance of hydrogen technologies, particularly in regions with developed hydrogen infrastructure. Market surveys show that 67% of industrial users would consider switching to hydrogen-based systems if storage efficiency improves and costs decrease by at least 30%.
The competitive landscape features established players like Air Liquide, Linde, and Worthington Industries alongside emerging technology developers focused specifically on metal hydride storage solutions. Recent strategic partnerships between automotive manufacturers and materials science companies highlight the industry's recognition of magnesium-based hydrides as a promising pathway to meet future storage requirements.
Current Challenges in Mg-Based Hydride Technology
Despite the promising potential of magnesium-based hydrides for hydrogen storage applications, several significant technical challenges currently impede their widespread commercial adoption. The primary obstacle remains the unfavorable thermodynamics of MgH2, which exhibits a high enthalpy of formation (approximately 75 kJ/mol H2), necessitating temperatures exceeding 300°C for hydrogen desorption under practical pressures. This high operating temperature requirement severely limits practical applications in mobile and stationary systems.
Kinetic limitations present another major challenge, with both hydrogenation and dehydrogenation reactions proceeding at unacceptably slow rates under moderate conditions. The formation of a dense MgH2 layer on magnesium particles creates a diffusion barrier that significantly hinders hydrogen transport. Even after decades of research, achieving complete hydrogen absorption typically requires several hours at elevated temperatures, falling far short of the DOE target of complete refueling within minutes.
Surface oxidation and contamination further complicate practical implementation. Magnesium's high reactivity with oxygen and moisture leads to the formation of passive surface layers (primarily MgO), which inhibit hydrogen dissociation and diffusion. These surface barriers significantly reduce cycling capacity and increase activation energy requirements for hydrogen sorption processes.
Cycling stability represents another critical challenge, as magnesium-based systems often experience substantial capacity degradation over multiple hydrogenation-dehydrogenation cycles. This degradation stems from particle sintering, phase segregation in complex hydrides, and the accumulation of irreversible phases during cycling. The volumetric expansion (approximately 32%) during hydrogenation induces mechanical stress that leads to particle decrepitation and morphological changes, further compromising long-term performance.
The gravimetric capacity paradox also warrants attention. While pure MgH2 offers an impressive theoretical capacity of 7.6 wt%, practical systems incorporating catalysts, additives, and support materials typically achieve only 4-5 wt% hydrogen storage, falling short of targets for mobile applications. This capacity dilution effect becomes more pronounced when implementing nanostructuring strategies, which often require substantial amounts of non-hydrogen-storing materials.
Heat management during operation poses additional engineering challenges. The highly exothermic hydrogenation reaction generates significant heat that must be efficiently dissipated to prevent temperature spikes and potential safety issues. Conversely, the endothermic dehydrogenation process requires substantial heat input, creating thermal management complexities in practical systems.
Kinetic limitations present another major challenge, with both hydrogenation and dehydrogenation reactions proceeding at unacceptably slow rates under moderate conditions. The formation of a dense MgH2 layer on magnesium particles creates a diffusion barrier that significantly hinders hydrogen transport. Even after decades of research, achieving complete hydrogen absorption typically requires several hours at elevated temperatures, falling far short of the DOE target of complete refueling within minutes.
Surface oxidation and contamination further complicate practical implementation. Magnesium's high reactivity with oxygen and moisture leads to the formation of passive surface layers (primarily MgO), which inhibit hydrogen dissociation and diffusion. These surface barriers significantly reduce cycling capacity and increase activation energy requirements for hydrogen sorption processes.
Cycling stability represents another critical challenge, as magnesium-based systems often experience substantial capacity degradation over multiple hydrogenation-dehydrogenation cycles. This degradation stems from particle sintering, phase segregation in complex hydrides, and the accumulation of irreversible phases during cycling. The volumetric expansion (approximately 32%) during hydrogenation induces mechanical stress that leads to particle decrepitation and morphological changes, further compromising long-term performance.
The gravimetric capacity paradox also warrants attention. While pure MgH2 offers an impressive theoretical capacity of 7.6 wt%, practical systems incorporating catalysts, additives, and support materials typically achieve only 4-5 wt% hydrogen storage, falling short of targets for mobile applications. This capacity dilution effect becomes more pronounced when implementing nanostructuring strategies, which often require substantial amounts of non-hydrogen-storing materials.
Heat management during operation poses additional engineering challenges. The highly exothermic hydrogenation reaction generates significant heat that must be efficiently dissipated to prevent temperature spikes and potential safety issues. Conversely, the endothermic dehydrogenation process requires substantial heat input, creating thermal management complexities in practical systems.
State-of-the-Art Nanostructuring Approaches
01 Hydrogen storage capacity of magnesium hydrides
Magnesium-based hydrides are known for their high theoretical hydrogen storage capacity, making them promising materials for hydrogen storage applications. These hydrides can store up to 7.6 wt% hydrogen, which is one of the highest capacities among metal hydrides. The storage capacity is influenced by the composition, structure, and preparation methods of the magnesium hydrides.- Hydrogen storage capacity of magnesium hydrides: Magnesium hydrides are known for their high theoretical hydrogen storage capacity, making them promising materials for hydrogen storage applications. The capacity of magnesium-based hydrides can reach up to 7.6 wt% hydrogen, which is one of the highest among metal hydrides. However, practical applications are limited by slow kinetics and high desorption temperatures. Research focuses on enhancing the hydrogen storage capacity through various methods including alloying and catalyst addition.
- Catalysts for improving magnesium hydride performance: Various catalysts can be incorporated into magnesium-based hydrides to improve their hydrogen absorption and desorption kinetics, thereby enhancing their effective capacity. Transition metals, metal oxides, and carbon-based materials are commonly used catalysts. These additives can significantly reduce the activation energy required for hydrogen release, allowing the hydrides to operate at lower temperatures while maintaining high capacity.
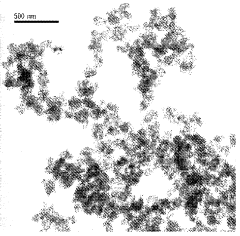
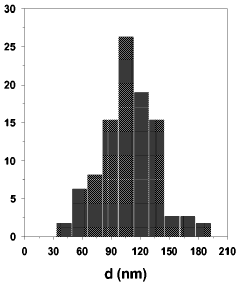
- Nanostructuring of magnesium hydrides: Nanostructuring techniques are applied to magnesium-based hydrides to enhance their hydrogen storage capacity and kinetics. By reducing particle size to nanoscale dimensions, the diffusion pathways for hydrogen are shortened, and surface area is increased. Ball milling, thin film deposition, and other methods are used to create nanostructured magnesium hydrides with improved capacity and cycling stability.
- Magnesium alloy hydrides for enhanced capacity: Alloying magnesium with other elements creates hydride systems with modified thermodynamic properties and enhanced hydrogen storage capacity. Common alloying elements include nickel, aluminum, titanium, and rare earth metals. These alloys can demonstrate improved absorption/desorption kinetics while maintaining relatively high hydrogen capacity compared to pure magnesium hydride.
- Thermal management systems for magnesium hydrides: Thermal management is crucial for optimizing the capacity of magnesium-based hydride systems. Since hydrogen absorption is exothermic and desorption is endothermic, effective heat transfer systems are required to maintain optimal operating temperatures. Various heat exchanger designs and thermal enhancement techniques are employed to improve the practical hydrogen storage capacity by managing the heat flow during cycling operations.
02 Enhancement of hydrogen absorption/desorption kinetics
Various methods have been developed to improve the hydrogen absorption and desorption kinetics of magnesium-based hydrides. These include the addition of catalysts, mechanical alloying, nanostructuring, and surface modification. These approaches help to overcome the slow kinetics that typically limit the practical application of magnesium hydrides in hydrogen storage systems.Expand Specific Solutions03 Thermal management and operating conditions
The hydrogen storage capacity of magnesium-based hydrides is significantly affected by operating temperature and pressure conditions. Typically, these hydrides require elevated temperatures (300-400°C) for efficient hydrogen release. Various approaches to thermal management and optimization of operating conditions have been developed to enhance the practical capacity and efficiency of magnesium hydride systems.Expand Specific Solutions04 Alloying and composite formation with other elements
The capacity and properties of magnesium-based hydrides can be modified through alloying with other elements or forming composite materials. Common alloying elements include nickel, aluminum, titanium, and various transition metals. These additions can improve hydrogen storage capacity, cycling stability, and kinetic properties of the hydride systems.Expand Specific Solutions05 Production methods and processing techniques
Various production methods and processing techniques have been developed to synthesize magnesium-based hydrides with optimized capacity. These include ball milling, reactive mechanical alloying, chemical vapor deposition, and solution-based methods. The processing conditions significantly influence the microstructure, particle size, and ultimately the hydrogen storage capacity of the resulting hydrides.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The magnesium-based hydrides market is currently in an early growth phase, characterized by intensive R&D activities across academic institutions and industrial players. The global hydrogen storage materials market, of which Mg-based hydrides form a significant segment, is projected to reach approximately $6.5 billion by 2027. Technologically, these materials are transitioning from laboratory research to early commercial applications, with nanostructuring strategies showing promising results for enhancing hydrogen storage capacity. Leading research institutions including Zhejiang University, Peking University, and KAIST are advancing fundamental science, while companies like Hydro-Québec, Johnson Matthey, and Saudi Aramco are developing practical applications. The collaboration between academic institutions (Rensselaer Polytechnic Institute, University of Maryland) and industrial partners (Thermoaura, Baowu Magnesium) is accelerating technology maturation, particularly in addressing key challenges of kinetics and thermal management for high-capacity hydrogen storage systems.
Hydro-Québec
Technical Solution: Hydro-Québec has developed advanced magnesium-based hydride systems for hydrogen storage applications. Their research focuses on MgH2 nanocomposites with catalytic additives to enhance hydrogen sorption kinetics. The company employs ball milling techniques to create nanostructured Mg-based materials with reduced particle sizes (typically 20-50 nm), which significantly decreases desorption temperatures from traditional 300-400°C to approximately 200-250°C. Their proprietary catalytic systems incorporate transition metals (Ni, Ti) and their compounds to facilitate hydrogen dissociation at material surfaces. Hydro-Québec has also pioneered core-shell architectures where magnesium hydride cores are encapsulated with catalytic shells, creating effective hydrogen diffusion pathways while maintaining high storage capacity (6-7 wt%). Their research extends to integration of these materials into practical energy storage systems for renewable energy applications and electric vehicle infrastructure.
Strengths: Extensive experience in energy storage systems integration; strong industrial-scale manufacturing capabilities; established infrastructure for technology deployment. Weaknesses: Higher production costs compared to conventional storage technologies; challenges in thermal management during hydrogen cycling; requires further optimization for commercial viability in mobile applications.
Zhejiang University
Technical Solution: Zhejiang University has developed advanced nanostructuring strategies for magnesium-based hydrides focusing on hierarchical composite architectures. Their research employs a combination of ball milling and chemical synthesis methods to create multi-scale structured materials with enhanced hydrogen storage properties. The university has pioneered the development of core-shell nanostructures where magnesium cores (typically 100-200 nm) are encapsulated by catalytic shells composed of transition metal compounds (TiO2, Nb2O5) with thicknesses of 5-10 nm. These structures effectively reduce hydrogen diffusion distances while protecting the magnesium from oxidation. Their innovative "nano-confinement" approach involves infiltrating magnesium into nanoporous carbon scaffolds with controlled pore sizes (3-10 nm), which constrains the growth of MgH2 particles and significantly improves dehydrogenation kinetics, reducing onset temperatures from 300°C to approximately 200°C. Zhejiang University researchers have also developed novel doping strategies using rare earth elements that create beneficial defects in the crystal structure, enhancing hydrogen mobility throughout the material matrix.
Strengths: Strong fundamental research capabilities; innovative approaches to nanostructure design; excellent characterization facilities for material analysis. Weaknesses: Scalability challenges for complex nanostructured materials; higher production costs compared to conventional methods; potential degradation of performance over extended cycling.
Critical Patents and Breakthroughs in Mg-Hydride Systems
Nanoparticle magnesium hydride, preparation method thereof and use of same
PatentWO2007003679A1
Innovation
- The development of nanoparticulate magnesium hydride with a particle size between 50 and 150 nm and a monodomain nanocrystalline microstructure is achieved through gas phase condensation, involving resistive evaporation of magnesium in an inert gas atmosphere followed by in situ hydrogen treatment, resulting in improved kinetics and reduced aggregation.
Environmental Impact and Sustainability Assessment
The environmental impact of magnesium-based hydrides and their nanostructuring strategies represents a critical dimension in evaluating their viability as hydrogen storage solutions. When compared to conventional fossil fuel technologies, these materials offer significant environmental advantages through their potential to enable clean hydrogen energy systems, thereby reducing greenhouse gas emissions and air pollutants associated with carbon-based energy sources.
The life cycle assessment of magnesium hydride systems reveals both benefits and challenges. While operational phases demonstrate minimal environmental footprint due to the reversible nature of hydrogen storage and release, the production phase presents notable concerns. The energy-intensive processes required for magnesium extraction and hydride synthesis currently contribute to substantial carbon emissions, particularly when powered by non-renewable energy sources.
Nanostructuring processes introduce additional environmental considerations. Chemical methods involving toxic solvents and high-temperature mechanical milling operations consume significant energy. However, recent advancements in green synthesis approaches utilizing bio-derived templates and ambient temperature processes show promise in reducing these environmental impacts.
Resource efficiency represents another important sustainability metric. Magnesium is abundantly available in the Earth's crust and seawater, making it less susceptible to supply constraints compared to rare earth elements used in competing technologies. Additionally, the theoretical recyclability of magnesium hydrides offers potential for closed-loop material systems, though practical recycling infrastructure remains underdeveloped.
Water consumption patterns vary significantly across different nanostructuring methods. Ball milling techniques require minimal water resources, while wet chemical approaches may have substantial water footprints. This consideration becomes particularly relevant in water-stressed regions where hydrogen technologies might be deployed.
The end-of-life management of magnesium-based hydrides presents both opportunities and challenges. While the base materials are theoretically recyclable, the presence of additives and catalysts in nanostructured systems may complicate recovery processes. Current research indicates that approximately 85% of magnesium content could be recovered through appropriate recycling protocols.
Regulatory frameworks governing these materials continue to evolve globally. The European Union's REACH regulations and similar frameworks in North America and Asia increasingly emphasize life cycle sustainability, potentially accelerating the development of environmentally optimized nanostructuring approaches for magnesium hydrides.
The life cycle assessment of magnesium hydride systems reveals both benefits and challenges. While operational phases demonstrate minimal environmental footprint due to the reversible nature of hydrogen storage and release, the production phase presents notable concerns. The energy-intensive processes required for magnesium extraction and hydride synthesis currently contribute to substantial carbon emissions, particularly when powered by non-renewable energy sources.
Nanostructuring processes introduce additional environmental considerations. Chemical methods involving toxic solvents and high-temperature mechanical milling operations consume significant energy. However, recent advancements in green synthesis approaches utilizing bio-derived templates and ambient temperature processes show promise in reducing these environmental impacts.
Resource efficiency represents another important sustainability metric. Magnesium is abundantly available in the Earth's crust and seawater, making it less susceptible to supply constraints compared to rare earth elements used in competing technologies. Additionally, the theoretical recyclability of magnesium hydrides offers potential for closed-loop material systems, though practical recycling infrastructure remains underdeveloped.
Water consumption patterns vary significantly across different nanostructuring methods. Ball milling techniques require minimal water resources, while wet chemical approaches may have substantial water footprints. This consideration becomes particularly relevant in water-stressed regions where hydrogen technologies might be deployed.
The end-of-life management of magnesium-based hydrides presents both opportunities and challenges. While the base materials are theoretically recyclable, the presence of additives and catalysts in nanostructured systems may complicate recovery processes. Current research indicates that approximately 85% of magnesium content could be recovered through appropriate recycling protocols.
Regulatory frameworks governing these materials continue to evolve globally. The European Union's REACH regulations and similar frameworks in North America and Asia increasingly emphasize life cycle sustainability, potentially accelerating the development of environmentally optimized nanostructuring approaches for magnesium hydrides.
Safety Standards and Regulatory Framework
The safety standards and regulatory framework surrounding magnesium-based hydrides research and applications have evolved significantly in response to the unique properties and potential hazards associated with these materials. Hydrogen storage systems utilizing magnesium hydrides must comply with international standards such as ISO/TS 15869 for gaseous hydrogen fuel tanks and ISO 16111 for transportable gas storage devices using hydrogen absorbed in reversible metal hydrides.
In the United States, the Department of Energy (DOE) has established specific safety guidelines for hydrogen storage materials research, with particular attention to reactive metal hydrides. These guidelines mandate rigorous risk assessment protocols, proper laboratory ventilation systems, and specialized handling procedures. The Code of Federal Regulations (CFR) Title 49 governs the transportation of hazardous materials, including magnesium hydrides, requiring appropriate packaging, labeling, and shipping documentation.
European regulations, particularly the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) framework, impose additional requirements for the registration and safety assessment of novel nanomaterials, including nanostructured magnesium hydrides. The European Chemicals Agency (ECHA) has published specific guidance for nanomaterials that researchers must follow when developing new nanostructuring strategies for magnesium-based hydrides.
Laboratory safety protocols for handling magnesium hydrides are particularly stringent due to their pyrophoric nature and potential for violent reactions with water or air. Standard operating procedures typically require inert atmosphere gloveboxes, specialized fire suppression systems, and regular safety training for research personnel. The American Chemical Society and similar organizations worldwide have published detailed guidelines for the safe handling of reactive metal hydrides in research settings.
For commercial applications, product safety certification becomes paramount. Organizations such as Underwriters Laboratories (UL) and TÜV have developed testing protocols for hydrogen storage systems, addressing concerns related to thermal management, pressure containment, and failure modes. These certifications are essential for market acceptance and insurance purposes.
Environmental regulations also impact magnesium hydride research, particularly regarding the disposal of spent materials and the sustainability of production processes. Life cycle assessment methodologies are increasingly being applied to evaluate the environmental footprint of magnesium-based hydrogen storage technologies, with regulatory bodies requiring comprehensive environmental impact studies before granting approvals for large-scale implementation.
As nanostructuring strategies advance, regulatory frameworks are evolving to address the unique considerations of engineered nanomaterials. The International Organization for Standardization (ISO) Technical Committee 229 has developed standards specifically for nanotechnologies, which apply to nanostructured magnesium hydrides and must be considered during research and development activities.
In the United States, the Department of Energy (DOE) has established specific safety guidelines for hydrogen storage materials research, with particular attention to reactive metal hydrides. These guidelines mandate rigorous risk assessment protocols, proper laboratory ventilation systems, and specialized handling procedures. The Code of Federal Regulations (CFR) Title 49 governs the transportation of hazardous materials, including magnesium hydrides, requiring appropriate packaging, labeling, and shipping documentation.
European regulations, particularly the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) framework, impose additional requirements for the registration and safety assessment of novel nanomaterials, including nanostructured magnesium hydrides. The European Chemicals Agency (ECHA) has published specific guidance for nanomaterials that researchers must follow when developing new nanostructuring strategies for magnesium-based hydrides.
Laboratory safety protocols for handling magnesium hydrides are particularly stringent due to their pyrophoric nature and potential for violent reactions with water or air. Standard operating procedures typically require inert atmosphere gloveboxes, specialized fire suppression systems, and regular safety training for research personnel. The American Chemical Society and similar organizations worldwide have published detailed guidelines for the safe handling of reactive metal hydrides in research settings.
For commercial applications, product safety certification becomes paramount. Organizations such as Underwriters Laboratories (UL) and TÜV have developed testing protocols for hydrogen storage systems, addressing concerns related to thermal management, pressure containment, and failure modes. These certifications are essential for market acceptance and insurance purposes.
Environmental regulations also impact magnesium hydride research, particularly regarding the disposal of spent materials and the sustainability of production processes. Life cycle assessment methodologies are increasingly being applied to evaluate the environmental footprint of magnesium-based hydrogen storage technologies, with regulatory bodies requiring comprehensive environmental impact studies before granting approvals for large-scale implementation.
As nanostructuring strategies advance, regulatory frameworks are evolving to address the unique considerations of engineered nanomaterials. The International Organization for Standardization (ISO) Technical Committee 229 has developed standards specifically for nanotechnologies, which apply to nanostructured magnesium hydrides and must be considered during research and development activities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!