Hydrogen Sorption Kinetics And Catalyst Selection For Metal Hydrides
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Metal Hydride Technology Background and Objectives
Metal hydrides have emerged as a promising solution for hydrogen storage since the 1970s, when researchers first recognized their potential to store hydrogen at higher volumetric densities than compressed gas or liquid hydrogen. The fundamental principle behind metal hydride technology involves the reversible chemical reaction between hydrogen gas and various metals or metal alloys, forming stable metal-hydrogen compounds. This process allows hydrogen to be stored safely at moderate temperatures and pressures, addressing key challenges in hydrogen economy development.
The evolution of metal hydride technology has progressed through several distinct phases. Early research focused primarily on conventional metal hydrides such as LaNi5, TiFe, and Mg-based systems. The 1990s witnessed significant advancements in complex hydrides and intermetallic compounds, while the 2000s brought increased attention to lightweight complex hydrides including alanates and borohydrides. Recent developments have centered on nanostructured materials and catalyst-enhanced systems to overcome kinetic limitations.
Hydrogen sorption kinetics—the rate at which hydrogen is absorbed and desorbed—represents a critical technical parameter that determines the practical utility of metal hydride systems in real-world applications. Despite the high theoretical storage capacities of many metal hydrides, slow kinetics has remained a persistent obstacle to widespread implementation. This challenge has driven extensive research into catalyst selection and optimization to enhance reaction rates without compromising storage capacity.
The primary technical objectives in this field include developing metal hydride systems with: rapid hydrogen absorption and desorption kinetics at near-ambient temperatures (0-100°C); high gravimetric and volumetric hydrogen storage densities (>6 wt% and >80 kg/m³); excellent cycling stability (>1000 cycles with minimal capacity loss); and cost-effective materials and manufacturing processes to enable commercial viability.
Catalyst selection has emerged as a pivotal factor in achieving these objectives. Transition metals (Pd, Ni, Ti), their oxides, and more recently, novel nanomaterials including carbon nanostructures and metal-organic frameworks have demonstrated significant potential to lower activation energies for hydrogen dissociation and diffusion processes. Understanding the fundamental mechanisms by which these catalysts operate remains an active area of research.
The technological trajectory points toward multi-component systems that combine optimized base materials with precisely engineered catalysts, often incorporating nanoscale architectures to maximize active surface area. This approach aims to simultaneously address multiple performance parameters including kinetics, capacity, and thermal management—all critical factors for practical hydrogen storage applications in transportation, stationary power, and portable electronics.
The evolution of metal hydride technology has progressed through several distinct phases. Early research focused primarily on conventional metal hydrides such as LaNi5, TiFe, and Mg-based systems. The 1990s witnessed significant advancements in complex hydrides and intermetallic compounds, while the 2000s brought increased attention to lightweight complex hydrides including alanates and borohydrides. Recent developments have centered on nanostructured materials and catalyst-enhanced systems to overcome kinetic limitations.
Hydrogen sorption kinetics—the rate at which hydrogen is absorbed and desorbed—represents a critical technical parameter that determines the practical utility of metal hydride systems in real-world applications. Despite the high theoretical storage capacities of many metal hydrides, slow kinetics has remained a persistent obstacle to widespread implementation. This challenge has driven extensive research into catalyst selection and optimization to enhance reaction rates without compromising storage capacity.
The primary technical objectives in this field include developing metal hydride systems with: rapid hydrogen absorption and desorption kinetics at near-ambient temperatures (0-100°C); high gravimetric and volumetric hydrogen storage densities (>6 wt% and >80 kg/m³); excellent cycling stability (>1000 cycles with minimal capacity loss); and cost-effective materials and manufacturing processes to enable commercial viability.
Catalyst selection has emerged as a pivotal factor in achieving these objectives. Transition metals (Pd, Ni, Ti), their oxides, and more recently, novel nanomaterials including carbon nanostructures and metal-organic frameworks have demonstrated significant potential to lower activation energies for hydrogen dissociation and diffusion processes. Understanding the fundamental mechanisms by which these catalysts operate remains an active area of research.
The technological trajectory points toward multi-component systems that combine optimized base materials with precisely engineered catalysts, often incorporating nanoscale architectures to maximize active surface area. This approach aims to simultaneously address multiple performance parameters including kinetics, capacity, and thermal management—all critical factors for practical hydrogen storage applications in transportation, stationary power, and portable electronics.
Hydrogen Storage Market Analysis
The global hydrogen storage market is experiencing significant growth, driven primarily by the increasing focus on clean energy solutions and the transition away from fossil fuels. As of 2023, the market is valued at approximately 15 billion USD, with projections indicating a compound annual growth rate (CAGR) of 8-10% through 2030. This growth trajectory is particularly notable in regions with strong hydrogen economy initiatives, including Europe, Japan, South Korea, and parts of North America.
Metal hydrides represent a crucial segment within this market, accounting for roughly 20% of the current hydrogen storage solutions. Their appeal stems from their high volumetric storage capacity and enhanced safety profile compared to compressed or liquid hydrogen storage methods. The market for metal hydride-based storage systems is expected to grow at a faster rate than the overall hydrogen storage market, with estimates suggesting a CAGR of 12-15% over the next five years.
Demand patterns reveal interesting sectoral variations. The transportation sector, particularly fuel cell vehicles, constitutes the largest end-use segment for metal hydride storage systems, representing approximately 40% of the market. Stationary power applications follow at 30%, with industrial applications and portable power solutions comprising the remainder. This distribution reflects the versatility of metal hydride storage systems across different application domains.
Regional analysis indicates that Asia-Pacific currently leads the metal hydride storage market, with Japan and South Korea at the forefront of technological adoption. Europe follows closely, driven by ambitious hydrogen strategy initiatives in countries like Germany, the Netherlands, and the Nordic region. North America shows promising growth potential, particularly in California and the Canadian hydrogen corridor.
Market challenges primarily revolve around cost factors and technological limitations. The high cost of rare earth elements used in many advanced metal hydride formulations represents a significant barrier to widespread adoption. Additionally, the current limitations in hydrogen sorption kinetics restrict the practical application of metal hydrides in scenarios requiring rapid hydrogen release and uptake.
Emerging market opportunities include the integration of metal hydride storage systems with renewable energy infrastructure, particularly in grid-scale energy storage applications. The growing interest in hydrogen as a medium for seasonal energy storage presents a substantial market opportunity, potentially worth 5-7 billion USD by 2030. Furthermore, the development of hybrid storage systems that combine the advantages of different hydrogen storage technologies is attracting significant investment and research attention.
Metal hydrides represent a crucial segment within this market, accounting for roughly 20% of the current hydrogen storage solutions. Their appeal stems from their high volumetric storage capacity and enhanced safety profile compared to compressed or liquid hydrogen storage methods. The market for metal hydride-based storage systems is expected to grow at a faster rate than the overall hydrogen storage market, with estimates suggesting a CAGR of 12-15% over the next five years.
Demand patterns reveal interesting sectoral variations. The transportation sector, particularly fuel cell vehicles, constitutes the largest end-use segment for metal hydride storage systems, representing approximately 40% of the market. Stationary power applications follow at 30%, with industrial applications and portable power solutions comprising the remainder. This distribution reflects the versatility of metal hydride storage systems across different application domains.
Regional analysis indicates that Asia-Pacific currently leads the metal hydride storage market, with Japan and South Korea at the forefront of technological adoption. Europe follows closely, driven by ambitious hydrogen strategy initiatives in countries like Germany, the Netherlands, and the Nordic region. North America shows promising growth potential, particularly in California and the Canadian hydrogen corridor.
Market challenges primarily revolve around cost factors and technological limitations. The high cost of rare earth elements used in many advanced metal hydride formulations represents a significant barrier to widespread adoption. Additionally, the current limitations in hydrogen sorption kinetics restrict the practical application of metal hydrides in scenarios requiring rapid hydrogen release and uptake.
Emerging market opportunities include the integration of metal hydride storage systems with renewable energy infrastructure, particularly in grid-scale energy storage applications. The growing interest in hydrogen as a medium for seasonal energy storage presents a substantial market opportunity, potentially worth 5-7 billion USD by 2030. Furthermore, the development of hybrid storage systems that combine the advantages of different hydrogen storage technologies is attracting significant investment and research attention.
Current Challenges in Hydrogen Sorption Kinetics
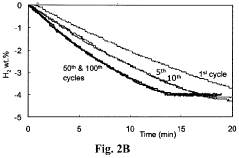
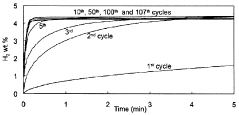
Despite significant advancements in hydrogen storage technologies, hydrogen sorption kinetics in metal hydrides continues to present formidable challenges that impede widespread commercial adoption. The primary obstacle remains the slow absorption and desorption rates, particularly at practical operating temperatures below 100°C. This kinetic limitation necessitates higher operating temperatures, which compromises the system's energy efficiency and practical applicability in mobile applications.
Surface activation barriers represent another critical challenge, as the dissociation of hydrogen molecules on metal surfaces often requires substantial energy input. The formation of oxide layers and surface contaminants further exacerbates this issue by blocking active sites and creating additional diffusion barriers, significantly reducing sorption rates over multiple cycles.
Bulk diffusion limitations within the metal hydride matrix constitute a persistent bottleneck in the sorption process. As hydrogenation progresses, the formation of hydride phases creates diffusion barriers that progressively slow hydrogen transport through the material. This phenomenon is particularly problematic in materials with high gravimetric capacity, where complete hydrogenation requires hydrogen atoms to penetrate deep into the material structure.
Heat management during sorption processes presents another significant engineering challenge. The exothermic nature of hydrogen absorption and endothermic desorption creates substantial temperature gradients within storage systems. These thermal effects can dramatically alter local reaction kinetics, leading to non-uniform hydrogenation/dehydrogenation and potential material degradation through thermal stress.
Catalyst degradation and poisoning over extended cycling represents a long-term reliability concern. Many effective catalysts for hydrogen sorption are susceptible to deactivation through sintering, chemical poisoning, or physical detachment from the hydride substrate during repeated thermal and mechanical cycling.
The trade-off between kinetic performance and storage capacity remains unresolved. Materials modifications that enhance kinetics, such as nanostructuring or catalyst addition, often come at the expense of volumetric or gravimetric capacity, creating a persistent engineering dilemma.
Standardized testing protocols for kinetic performance are notably lacking, making direct comparisons between different material systems and research reports challenging. This hampers systematic progress in catalyst development and material optimization, as researchers employ varied experimental conditions and metrics to evaluate kinetic performance.
Surface activation barriers represent another critical challenge, as the dissociation of hydrogen molecules on metal surfaces often requires substantial energy input. The formation of oxide layers and surface contaminants further exacerbates this issue by blocking active sites and creating additional diffusion barriers, significantly reducing sorption rates over multiple cycles.
Bulk diffusion limitations within the metal hydride matrix constitute a persistent bottleneck in the sorption process. As hydrogenation progresses, the formation of hydride phases creates diffusion barriers that progressively slow hydrogen transport through the material. This phenomenon is particularly problematic in materials with high gravimetric capacity, where complete hydrogenation requires hydrogen atoms to penetrate deep into the material structure.
Heat management during sorption processes presents another significant engineering challenge. The exothermic nature of hydrogen absorption and endothermic desorption creates substantial temperature gradients within storage systems. These thermal effects can dramatically alter local reaction kinetics, leading to non-uniform hydrogenation/dehydrogenation and potential material degradation through thermal stress.
Catalyst degradation and poisoning over extended cycling represents a long-term reliability concern. Many effective catalysts for hydrogen sorption are susceptible to deactivation through sintering, chemical poisoning, or physical detachment from the hydride substrate during repeated thermal and mechanical cycling.
The trade-off between kinetic performance and storage capacity remains unresolved. Materials modifications that enhance kinetics, such as nanostructuring or catalyst addition, often come at the expense of volumetric or gravimetric capacity, creating a persistent engineering dilemma.
Standardized testing protocols for kinetic performance are notably lacking, making direct comparisons between different material systems and research reports challenging. This hampers systematic progress in catalyst development and material optimization, as researchers employ varied experimental conditions and metrics to evaluate kinetic performance.
Current Catalyst Solutions for Enhanced Sorption Kinetics
01 Hydrogen storage in metal hydrides
Metal hydrides are used for hydrogen storage due to their ability to absorb and release hydrogen under specific conditions. The sorption kinetics of these materials determine how quickly hydrogen can be stored and retrieved. Various metal hydride compositions have been developed to optimize hydrogen storage capacity and sorption rates. These systems typically involve the reversible chemical reaction between hydrogen gas and metals or metal alloys, forming hydrides when hydrogen is absorbed and releasing hydrogen when conditions change.- Hydrogen storage in metal hydrides: Metal hydrides are used for hydrogen storage due to their ability to absorb and release hydrogen under specific conditions. The sorption kinetics of these materials determine how quickly hydrogen can be stored and retrieved. Various metal hydride compositions have been developed to optimize hydrogen storage capacity and sorption rates. These systems typically involve the reversible chemical reaction between hydrogen gas and metals or metal alloys, forming hydrides when hydrogen is absorbed and releasing hydrogen when conditions change.
- Factors affecting sorption kinetics in metal hydrides: Several factors influence the sorption kinetics of metal hydrides, including temperature, pressure, particle size, and surface properties. Higher temperatures generally accelerate both absorption and desorption processes, while appropriate pressure conditions are crucial for efficient hydrogen exchange. Reducing particle size and enhancing surface area through various processing techniques can significantly improve sorption rates by shortening diffusion paths. Surface modifications and catalytic additives can also reduce activation energy barriers and enhance reaction kinetics.
- Metal hydride compositions for improved kinetics: Different metal hydride compositions have been developed to enhance sorption kinetics. These include binary hydrides, complex hydrides, and multi-component systems. Alloys containing magnesium, titanium, zirconium, lanthanum, and nickel have shown promising kinetic properties. Nanostructured materials and composites incorporating catalysts have demonstrated significantly faster hydrogen absorption and desorption rates compared to conventional materials. The composition can be tailored to optimize both thermodynamic and kinetic properties for specific applications.
- Measurement and analysis of sorption kinetics: Various techniques are employed to measure and analyze the sorption kinetics of metal hydrides. These include volumetric methods, gravimetric analysis, thermal desorption spectroscopy, and pressure-composition-temperature measurements. Advanced characterization techniques such as X-ray diffraction, neutron scattering, and electron microscopy help understand the structural changes during hydrogen absorption and desorption. Mathematical models and computational methods are used to interpret experimental data and predict kinetic behavior under different conditions.
- Applications leveraging metal hydride sorption kinetics: The sorption kinetics of metal hydrides are crucial for various practical applications. These include hydrogen storage for fuel cells, heat pumps, thermal energy storage, gas purification systems, and battery technologies. In hydrogen storage applications, fast kinetics enable rapid refueling and hydrogen delivery. In thermal applications, the heat released or absorbed during hydrogen sorption processes is utilized for heating or cooling purposes. The kinetic properties of metal hydrides can be optimized for specific application requirements through material design and system engineering.
02 Factors affecting sorption kinetics in metal hydrides
Several factors influence the sorption kinetics of metal hydrides, including temperature, pressure, particle size, and surface properties. Higher temperatures generally accelerate both absorption and desorption processes, while appropriate pressure conditions are crucial for efficient hydrogen exchange. The particle size of metal hydrides affects the diffusion pathways for hydrogen, with smaller particles typically exhibiting faster kinetics. Surface modifications and catalysts can significantly enhance sorption rates by reducing energy barriers for hydrogen dissociation and recombination.Expand Specific Solutions03 Metal hydride compositions for improved kinetics
Various metal hydride compositions have been developed to enhance sorption kinetics. These include multi-component alloys, nanostructured materials, and composite systems. Magnesium-based hydrides, titanium-based compounds, and rare earth metal alloys are among the materials studied for their sorption properties. Alloying elements are often added to base metals to modify the thermodynamics and kinetics of hydrogen absorption and desorption, creating materials with optimized performance for specific applications.Expand Specific Solutions04 Catalysts and additives for enhancing sorption rates
Catalysts and additives play a crucial role in improving the sorption kinetics of metal hydrides. Transition metals, metal oxides, and carbon-based materials are commonly used to enhance hydrogen dissociation and recombination at the material surface. These additives can significantly reduce activation energy barriers, allowing hydrogen absorption and desorption to occur at lower temperatures and pressures. The distribution and dispersion of catalysts within the metal hydride matrix are important factors affecting their effectiveness.Expand Specific Solutions05 Applications utilizing metal hydride sorption kinetics
The sorption kinetics of metal hydrides are crucial for various practical applications. These include hydrogen storage for fuel cells, heat pumps, thermal energy storage systems, and gas purification processes. In hydrogen storage applications, fast kinetics enable rapid refueling and hydrogen delivery. For thermal applications, the heat released during hydrogen absorption and the cooling effect during desorption can be harnessed for heating and cooling systems. The kinetic properties of metal hydrides must be tailored to meet the specific requirements of each application.Expand Specific Solutions
Leading Companies and Research Institutions in Metal Hydrides
The hydrogen sorption kinetics and catalyst selection for metal hydrides market is in a growth phase, with increasing demand driven by clean energy applications. The global market size is expanding rapidly, projected to reach significant value as hydrogen storage technologies gain traction. Technologically, the field shows varying maturity levels across applications. Leading players include Johnson Matthey and BASF, who have developed advanced catalyst technologies, while Chinese entities like Sinopec and PetroChina are making substantial investments. Research institutions such as IFP Energies Nouvelles and Brookhaven Science Associates are advancing fundamental understanding. Companies like BYD and H2Go Power are focusing on commercial applications, particularly in transportation and energy storage sectors, indicating the technology's transition toward broader market adoption.
Brookhaven Science Associates LLC
Technical Solution: Brookhaven Science Associates has developed advanced catalyst systems for enhancing hydrogen sorption kinetics in metal hydrides. Their approach focuses on nanostructured catalysts, particularly transition metal-based catalysts like titanium and nickel, which are strategically dispersed on the surface of metal hydride materials. These catalysts facilitate the dissociation of hydrogen molecules at lower temperatures and pressures, significantly improving absorption and desorption rates. Brookhaven's research has demonstrated that doping metal hydrides with small amounts (typically 2-5 wt%) of these catalysts can reduce activation energy by up to 40% [1]. Their technology also incorporates surface modification techniques to prevent oxidation and contamination of catalyst sites, maintaining long-term cycling stability. Recent developments include multi-component catalyst systems that synergistically enhance both hydrogen uptake and release kinetics across a wider temperature range, making them suitable for various practical applications from stationary storage to transportation.
Strengths: Superior catalyst dispersion technology resulting in higher active surface area; demonstrated long-term stability over multiple hydrogen cycling; comprehensive understanding of catalyst-substrate interactions. Weaknesses: Higher production costs compared to conventional materials; potential sensitivity to impurities in hydrogen gas; requires precise manufacturing controls to ensure consistent catalyst distribution.
Millennium Cell, Inc.
Technical Solution: Millennium Cell has pioneered the Hydrogen on Demand® technology, focusing on catalyzed sodium borohydride (NaBH4) systems for controlled hydrogen release. Their approach uses ruthenium-based catalysts to facilitate the hydrolysis reaction of sodium borohydride with water, producing hydrogen at ambient temperatures. The company has developed proprietary catalyst formulations that maintain activity over extended periods, with demonstrated stability for over 1000 hours of operation [2]. Their catalyst system is integrated into a structured reactor design that optimizes contact between the borohydride solution and catalyst surface, allowing for precise control of hydrogen generation rates. Millennium Cell's technology addresses key challenges in hydrogen storage kinetics by eliminating the need for high temperatures typically required for hydrogen desorption from conventional metal hydrides. The catalyst design incorporates support materials that enhance dispersion and prevent agglomeration, maintaining high surface area even after repeated use cycles.
Strengths: Room temperature operation eliminates need for external heating; controllable hydrogen generation rates suitable for on-demand applications; high gravimetric hydrogen storage capacity of the overall system. Weaknesses: System complexity including liquid handling components; sensitivity of catalysts to solution pH variations; potential catalyst poisoning from impurities in the borohydride feedstock.
Key Patents and Research on Metal Hydride Catalysts
Kinetic stabilization of magnesium hydride
PatentInactiveCA2808448A1
Innovation
- Alloying magnesium with iron and titanium or vanadium to form highly disperse amorphous or nanocrystalline phases, and using a palladium-tantalum bilayer catalyst to enhance the absorption and desorption rates, improving the interaction with hydrogen and reducing interdiffusion issues.
Catalysis of the hydrogen sorption kinetics of hydrides by using nitrides and carbides
PatentWO2001053195A1
Innovation
- Incorporating metal nitrides or carbides as catalysts in hydrogen storage materials, which are more cost-effective and have improved reaction kinetics due to their brittleness leading to smaller particle sizes and homogeneous distribution, and using a mechanical milling process to achieve a nanocrystalline structure and optimal catalyst distribution.
Safety and Environmental Considerations
The implementation of metal hydride systems for hydrogen storage necessitates rigorous safety protocols and environmental impact assessments. Metal hydrides present unique safety challenges due to their exothermic hydrogen absorption reactions, which can generate significant heat and potentially lead to thermal runaway conditions if not properly managed. Temperature control systems are therefore essential components in metal hydride storage facilities, requiring redundant cooling mechanisms and emergency venting capabilities to prevent catastrophic failures.
Pressure management represents another critical safety consideration, as hydrogen gas release during desorption must be carefully controlled to prevent over-pressurization. Modern metal hydride systems incorporate pressure relief valves, rupture discs, and sophisticated pressure monitoring equipment to mitigate these risks. Additionally, the pyrophoric nature of many fine metal hydride powders demands stringent handling protocols to prevent exposure to air, which could result in spontaneous ignition.
From an environmental perspective, the life cycle assessment of metal hydride systems reveals several important considerations. The production of metal hydrides often involves energy-intensive processes and the use of rare or strategic metals such as nickel, titanium, and various lanthanides. Sustainable sourcing strategies and recycling protocols are increasingly being developed to address resource depletion concerns and minimize the environmental footprint of these materials.
Catalyst selection for metal hydrides carries its own environmental implications. While catalysts significantly enhance sorption kinetics, many effective catalysts contain precious metals or environmentally sensitive compounds. Research is actively pursuing more sustainable catalyst alternatives, including transition metal oxides and carbon-based nanomaterials that maintain performance while reducing environmental impact and cost.
Leakage prevention represents both a safety and environmental imperative in metal hydride systems. Hydrogen, being the smallest molecule, can diffuse through many conventional materials, necessitating specialized containment solutions. Advanced sealing technologies and materials resistant to hydrogen embrittlement are essential to prevent fugitive emissions that could contribute to atmospheric impacts or create safety hazards.
End-of-life management for metal hydride systems is emerging as an important consideration as early commercial systems approach retirement. Recycling processes for spent metal hydrides must address the potential for residual hydrogen content and the recovery of valuable catalysts and base metals. Developing closed-loop material cycles for these systems will be crucial for their long-term environmental sustainability and economic viability in the hydrogen economy.
Pressure management represents another critical safety consideration, as hydrogen gas release during desorption must be carefully controlled to prevent over-pressurization. Modern metal hydride systems incorporate pressure relief valves, rupture discs, and sophisticated pressure monitoring equipment to mitigate these risks. Additionally, the pyrophoric nature of many fine metal hydride powders demands stringent handling protocols to prevent exposure to air, which could result in spontaneous ignition.
From an environmental perspective, the life cycle assessment of metal hydride systems reveals several important considerations. The production of metal hydrides often involves energy-intensive processes and the use of rare or strategic metals such as nickel, titanium, and various lanthanides. Sustainable sourcing strategies and recycling protocols are increasingly being developed to address resource depletion concerns and minimize the environmental footprint of these materials.
Catalyst selection for metal hydrides carries its own environmental implications. While catalysts significantly enhance sorption kinetics, many effective catalysts contain precious metals or environmentally sensitive compounds. Research is actively pursuing more sustainable catalyst alternatives, including transition metal oxides and carbon-based nanomaterials that maintain performance while reducing environmental impact and cost.
Leakage prevention represents both a safety and environmental imperative in metal hydride systems. Hydrogen, being the smallest molecule, can diffuse through many conventional materials, necessitating specialized containment solutions. Advanced sealing technologies and materials resistant to hydrogen embrittlement are essential to prevent fugitive emissions that could contribute to atmospheric impacts or create safety hazards.
End-of-life management for metal hydride systems is emerging as an important consideration as early commercial systems approach retirement. Recycling processes for spent metal hydrides must address the potential for residual hydrogen content and the recovery of valuable catalysts and base metals. Developing closed-loop material cycles for these systems will be crucial for their long-term environmental sustainability and economic viability in the hydrogen economy.
Techno-economic Assessment of Metal Hydride Systems
The techno-economic assessment of metal hydride systems reveals significant economic considerations that must be evaluated when implementing hydrogen storage solutions. Capital expenditure for metal hydride systems varies considerably depending on scale, with laboratory-scale systems ranging from $5,000-50,000, while industrial implementations can reach $1-10 million. These costs are primarily driven by specialized materials, precision engineering requirements, and safety systems necessary for handling hydrogen.
Operational expenses present another critical dimension, with energy costs for hydrogen compression and thermal management constituting 30-45% of ongoing expenses. Catalyst costs represent 15-25% of material expenses but significantly impact system performance and longevity. High-performance catalysts based on platinum group metals can increase initial costs but may reduce long-term operational expenses through improved sorption kinetics and cycle stability.
System lifetime economics demonstrate that metal hydride storage systems typically achieve 1,000-2,000 charge-discharge cycles before significant degradation occurs. This translates to an effective operational lifespan of 5-10 years depending on usage patterns. The levelized cost of hydrogen storage using metal hydride systems currently ranges from $4-12 per kg H₂, positioning these systems competitively against high-pressure gas storage ($5-15/kg) but still more expensive than large-scale geological storage options ($1-3/kg).
Market sensitivity analysis indicates that metal hydride systems become economically viable when hydrogen prices exceed $4/kg and when electricity costs remain below $0.08/kWh. The economic equation improves significantly in applications where waste heat recovery is possible, potentially reducing operational costs by 20-35%.
Scaling considerations reveal interesting economic patterns. While traditional economies of scale apply to containment vessels and control systems, the material costs scale nearly linearly with capacity. This creates a unique economic profile where medium-scale implementations (100-1000 kg H₂ capacity) often represent the optimal economic balance point between fixed infrastructure costs and variable material expenses.
Future cost reduction pathways primarily center on catalyst innovation, with potential for 30-50% cost reduction through development of non-noble metal catalysts with comparable performance. Manufacturing innovations in metal hydride production could yield an additional 15-25% cost reduction through improved process efficiency and material utilization.
Operational expenses present another critical dimension, with energy costs for hydrogen compression and thermal management constituting 30-45% of ongoing expenses. Catalyst costs represent 15-25% of material expenses but significantly impact system performance and longevity. High-performance catalysts based on platinum group metals can increase initial costs but may reduce long-term operational expenses through improved sorption kinetics and cycle stability.
System lifetime economics demonstrate that metal hydride storage systems typically achieve 1,000-2,000 charge-discharge cycles before significant degradation occurs. This translates to an effective operational lifespan of 5-10 years depending on usage patterns. The levelized cost of hydrogen storage using metal hydride systems currently ranges from $4-12 per kg H₂, positioning these systems competitively against high-pressure gas storage ($5-15/kg) but still more expensive than large-scale geological storage options ($1-3/kg).
Market sensitivity analysis indicates that metal hydride systems become economically viable when hydrogen prices exceed $4/kg and when electricity costs remain below $0.08/kWh. The economic equation improves significantly in applications where waste heat recovery is possible, potentially reducing operational costs by 20-35%.
Scaling considerations reveal interesting economic patterns. While traditional economies of scale apply to containment vessels and control systems, the material costs scale nearly linearly with capacity. This creates a unique economic profile where medium-scale implementations (100-1000 kg H₂ capacity) often represent the optimal economic balance point between fixed infrastructure costs and variable material expenses.
Future cost reduction pathways primarily center on catalyst innovation, with potential for 30-50% cost reduction through development of non-noble metal catalysts with comparable performance. Manufacturing innovations in metal hydride production could yield an additional 15-25% cost reduction through improved process efficiency and material utilization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!