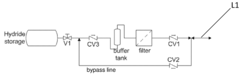
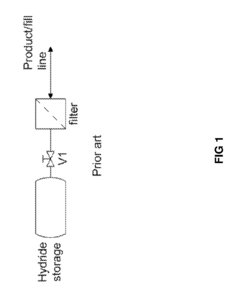
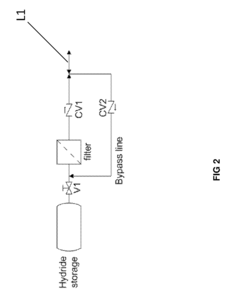
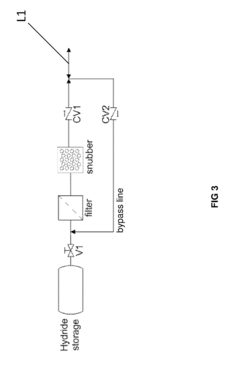
Hydrogen Purity And Contaminant Tolerance Of Metal Hydride Systems
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Metal Hydride Hydrogen Storage Background and Objectives
Metal hydride systems represent one of the most promising technologies for hydrogen storage, offering significant advantages in terms of safety, volumetric capacity, and operational flexibility. The evolution of metal hydride technology dates back to the 1970s when researchers first discovered the remarkable ability of certain metals and alloys to absorb hydrogen reversibly. Since then, the field has witnessed substantial advancements, particularly in improving storage capacity, kinetics, and thermal management.
The technological trajectory of metal hydride systems has been characterized by a progressive shift from conventional AB5-type alloys (such as LaNi5) to more advanced materials including AB2 (Ti-Zr based), body-centered cubic (BCC) alloys, and complex hydrides. Each generation has addressed specific limitations of its predecessors, gradually enhancing performance metrics critical for practical applications.
Current research focuses on optimizing hydrogen purity requirements and improving contaminant tolerance in metal hydride systems. This direction is crucial as hydrogen produced from various sources often contains impurities that can significantly degrade the performance and longevity of storage systems. Common contaminants include carbon monoxide, carbon dioxide, sulfur compounds, ammonia, and water vapor, each affecting metal hydrides through different poisoning mechanisms.
The primary technical objectives in this domain include developing metal hydride materials with enhanced resistance to common contaminants, establishing standardized protocols for evaluating contaminant effects, and designing effective purification strategies tailored to specific applications. Additionally, there is growing interest in understanding the fundamental mechanisms of contaminant interaction with metal hydride surfaces to enable rational material design.
From an industrial perspective, the goals extend to reducing the overall system costs associated with hydrogen purification, extending the operational lifetime of metal hydride storage systems in real-world conditions, and establishing reliable performance benchmarks across different operating environments. These objectives align with broader hydrogen economy initiatives that seek to establish hydrogen as a viable energy carrier.
The intersection of hydrogen purity requirements with metal hydride technology represents a critical research frontier, as it directly impacts the economic viability and technical feasibility of hydrogen storage solutions across various applications, from stationary power systems to transportation. Addressing these challenges requires interdisciplinary approaches combining materials science, surface chemistry, and system engineering to develop robust solutions capable of functioning effectively with hydrogen of varying purity levels.
The technological trajectory of metal hydride systems has been characterized by a progressive shift from conventional AB5-type alloys (such as LaNi5) to more advanced materials including AB2 (Ti-Zr based), body-centered cubic (BCC) alloys, and complex hydrides. Each generation has addressed specific limitations of its predecessors, gradually enhancing performance metrics critical for practical applications.
Current research focuses on optimizing hydrogen purity requirements and improving contaminant tolerance in metal hydride systems. This direction is crucial as hydrogen produced from various sources often contains impurities that can significantly degrade the performance and longevity of storage systems. Common contaminants include carbon monoxide, carbon dioxide, sulfur compounds, ammonia, and water vapor, each affecting metal hydrides through different poisoning mechanisms.
The primary technical objectives in this domain include developing metal hydride materials with enhanced resistance to common contaminants, establishing standardized protocols for evaluating contaminant effects, and designing effective purification strategies tailored to specific applications. Additionally, there is growing interest in understanding the fundamental mechanisms of contaminant interaction with metal hydride surfaces to enable rational material design.
From an industrial perspective, the goals extend to reducing the overall system costs associated with hydrogen purification, extending the operational lifetime of metal hydride storage systems in real-world conditions, and establishing reliable performance benchmarks across different operating environments. These objectives align with broader hydrogen economy initiatives that seek to establish hydrogen as a viable energy carrier.
The intersection of hydrogen purity requirements with metal hydride technology represents a critical research frontier, as it directly impacts the economic viability and technical feasibility of hydrogen storage solutions across various applications, from stationary power systems to transportation. Addressing these challenges requires interdisciplinary approaches combining materials science, surface chemistry, and system engineering to develop robust solutions capable of functioning effectively with hydrogen of varying purity levels.
Market Analysis for High-Purity Hydrogen Storage Solutions
The global market for high-purity hydrogen storage solutions is experiencing significant growth, driven by the increasing adoption of hydrogen as a clean energy carrier across various industries. The market size for metal hydride hydrogen storage systems was valued at approximately $420 million in 2022 and is projected to reach $1.2 billion by 2030, representing a compound annual growth rate of 14.3% during the forecast period.
The demand for high-purity hydrogen storage solutions is particularly strong in regions with advanced hydrogen infrastructure development, including North America, Europe, and parts of Asia-Pacific. Japan, Germany, and South Korea lead in terms of market maturity, with China showing the fastest growth trajectory due to aggressive government support for hydrogen technologies.
Key market segments for metal hydride storage systems include stationary power applications, transportation, industrial processes, and emerging portable electronics. The transportation sector, encompassing fuel cell vehicles and hydrogen refueling infrastructure, currently represents the largest market share at 38%, followed by industrial applications at 29%.
Customer requirements are increasingly focused on storage systems that can maintain hydrogen purity levels above 99.999% while demonstrating resilience to common contaminants such as carbon monoxide, sulfur compounds, and moisture. This trend is particularly evident in the semiconductor manufacturing sector, where ultra-high-purity hydrogen is essential for production processes.
Market analysis indicates a price premium of 25-40% for storage solutions offering enhanced contaminant tolerance, reflecting the critical importance of gas purity in high-value applications. This premium is expected to decrease to 15-20% by 2028 as manufacturing scales and technologies mature.
The competitive landscape features established industrial gas companies expanding their hydrogen storage portfolios alongside specialized technology providers focused exclusively on advanced metal hydride systems. Recent market consolidation through strategic acquisitions suggests that industry players are positioning for anticipated market expansion.
Customer adoption patterns reveal a growing preference for integrated solutions that combine storage capabilities with purification technologies, creating opportunities for comprehensive system providers. This trend is particularly pronounced in the fuel cell vehicle sector, where on-board hydrogen quality directly impacts system performance and longevity.
Market forecasts indicate that regions implementing hydrogen-focused regulatory frameworks and incentive programs will experience accelerated growth rates of 16-18% annually, compared to the global average of 14.3%. The European Union's Hydrogen Strategy and Japan's Strategic Roadmap for Hydrogen and Fuel Cells are notable examples of policy frameworks driving market development.
The demand for high-purity hydrogen storage solutions is particularly strong in regions with advanced hydrogen infrastructure development, including North America, Europe, and parts of Asia-Pacific. Japan, Germany, and South Korea lead in terms of market maturity, with China showing the fastest growth trajectory due to aggressive government support for hydrogen technologies.
Key market segments for metal hydride storage systems include stationary power applications, transportation, industrial processes, and emerging portable electronics. The transportation sector, encompassing fuel cell vehicles and hydrogen refueling infrastructure, currently represents the largest market share at 38%, followed by industrial applications at 29%.
Customer requirements are increasingly focused on storage systems that can maintain hydrogen purity levels above 99.999% while demonstrating resilience to common contaminants such as carbon monoxide, sulfur compounds, and moisture. This trend is particularly evident in the semiconductor manufacturing sector, where ultra-high-purity hydrogen is essential for production processes.
Market analysis indicates a price premium of 25-40% for storage solutions offering enhanced contaminant tolerance, reflecting the critical importance of gas purity in high-value applications. This premium is expected to decrease to 15-20% by 2028 as manufacturing scales and technologies mature.
The competitive landscape features established industrial gas companies expanding their hydrogen storage portfolios alongside specialized technology providers focused exclusively on advanced metal hydride systems. Recent market consolidation through strategic acquisitions suggests that industry players are positioning for anticipated market expansion.
Customer adoption patterns reveal a growing preference for integrated solutions that combine storage capabilities with purification technologies, creating opportunities for comprehensive system providers. This trend is particularly pronounced in the fuel cell vehicle sector, where on-board hydrogen quality directly impacts system performance and longevity.
Market forecasts indicate that regions implementing hydrogen-focused regulatory frameworks and incentive programs will experience accelerated growth rates of 16-18% annually, compared to the global average of 14.3%. The European Union's Hydrogen Strategy and Japan's Strategic Roadmap for Hydrogen and Fuel Cells are notable examples of policy frameworks driving market development.
Current Challenges in Hydrogen Purity for Metal Hydride Systems
Metal hydride systems face significant challenges related to hydrogen purity requirements and contaminant tolerance. The primary issue stems from the high sensitivity of metal hydrides to various impurities present in hydrogen gas streams. Oxygen, water vapor, carbon monoxide, and sulfur compounds are particularly problematic contaminants that can severely degrade the performance and longevity of metal hydride materials.
Oxygen and water vapor react with metal hydrides to form stable metal oxides or hydroxides, effectively reducing the active material available for hydrogen storage. This process, known as passivation, creates a barrier layer that impedes hydrogen diffusion into the material. Studies have shown that exposure to even parts-per-million levels of oxygen can reduce hydrogen storage capacity by up to 30% after multiple absorption-desorption cycles.
Carbon monoxide presents another significant challenge as it strongly adsorbs onto active metal sites, blocking hydrogen access to these crucial reaction centers. This poisoning effect is especially pronounced in transition metal-based hydrides, where CO can reduce hydrogen uptake rates by over 90% at concentrations as low as 10 ppm.
Sulfur compounds, including H2S and SO2, form irreversible chemical bonds with many metal hydride materials, permanently deactivating storage sites. Research indicates that sulfur poisoning can occur at concentrations below 1 ppm, making it one of the most detrimental contaminants for long-term system viability.
Temperature sensitivity compounds these purity challenges. At lower operating temperatures (below 100°C), metal hydrides exhibit decreased kinetics, making them more susceptible to contaminant effects. Conversely, higher temperatures that might mitigate some poisoning effects often accelerate degradation mechanisms or reduce overall storage capacity.
Current purification technologies add significant cost and complexity to metal hydride systems. Pressure Swing Adsorption (PSA) and membrane separation technologies can achieve 99.999% hydrogen purity but increase system costs by 20-40% and reduce overall energy efficiency. The trade-off between purification requirements and system economics represents a major barrier to widespread adoption.
Scale-up challenges further complicate purity management. Laboratory-scale systems can maintain controlled environments, but industrial-scale applications face variable feedstock quality and fluctuating contaminant profiles. This variability necessitates robust purification strategies that can adapt to changing input conditions while maintaining consistent performance.
Regeneration of poisoned metal hydride materials remains technically challenging and economically questionable. Current regeneration processes typically recover only 70-85% of original capacity, with diminishing returns after multiple regeneration cycles.
Oxygen and water vapor react with metal hydrides to form stable metal oxides or hydroxides, effectively reducing the active material available for hydrogen storage. This process, known as passivation, creates a barrier layer that impedes hydrogen diffusion into the material. Studies have shown that exposure to even parts-per-million levels of oxygen can reduce hydrogen storage capacity by up to 30% after multiple absorption-desorption cycles.
Carbon monoxide presents another significant challenge as it strongly adsorbs onto active metal sites, blocking hydrogen access to these crucial reaction centers. This poisoning effect is especially pronounced in transition metal-based hydrides, where CO can reduce hydrogen uptake rates by over 90% at concentrations as low as 10 ppm.
Sulfur compounds, including H2S and SO2, form irreversible chemical bonds with many metal hydride materials, permanently deactivating storage sites. Research indicates that sulfur poisoning can occur at concentrations below 1 ppm, making it one of the most detrimental contaminants for long-term system viability.
Temperature sensitivity compounds these purity challenges. At lower operating temperatures (below 100°C), metal hydrides exhibit decreased kinetics, making them more susceptible to contaminant effects. Conversely, higher temperatures that might mitigate some poisoning effects often accelerate degradation mechanisms or reduce overall storage capacity.
Current purification technologies add significant cost and complexity to metal hydride systems. Pressure Swing Adsorption (PSA) and membrane separation technologies can achieve 99.999% hydrogen purity but increase system costs by 20-40% and reduce overall energy efficiency. The trade-off between purification requirements and system economics represents a major barrier to widespread adoption.
Scale-up challenges further complicate purity management. Laboratory-scale systems can maintain controlled environments, but industrial-scale applications face variable feedstock quality and fluctuating contaminant profiles. This variability necessitates robust purification strategies that can adapt to changing input conditions while maintaining consistent performance.
Regeneration of poisoned metal hydride materials remains technically challenging and economically questionable. Current regeneration processes typically recover only 70-85% of original capacity, with diminishing returns after multiple regeneration cycles.
Existing Contaminant Mitigation Strategies for Metal Hydride Systems
01 Contaminant tolerance mechanisms in metal hydride systems
Metal hydride systems can be designed with specific mechanisms to tolerate contaminants in hydrogen gas. These mechanisms include selective adsorption layers, filtering components, and specialized alloy compositions that can maintain hydrogen storage capacity even in the presence of impurities. Some systems incorporate protective layers that prevent contaminants from reaching the core metal hydride material, while others use catalyst materials that can neutralize certain impurities before they cause degradation.- Contaminant tolerance mechanisms in metal hydride systems: Metal hydride systems can be designed with specific mechanisms to tolerate contaminants in hydrogen gas. These mechanisms include selective adsorption layers, filtering components, and specialized alloy compositions that can maintain hydrogen storage capacity even in the presence of impurities. Some systems incorporate protective layers that prevent contaminants from reaching the core metal hydride material, while others use catalyst materials that can mitigate the negative effects of specific impurities.
- Purification methods for hydrogen in metal hydride storage: Various purification methods can be integrated with metal hydride storage systems to ensure high hydrogen purity. These include pressure swing adsorption, membrane separation, and temperature-controlled desorption techniques that selectively release pure hydrogen while trapping contaminants. Some systems employ multi-stage purification processes where hydrogen passes through sequential purification steps before storage in the metal hydride material, ensuring that only high-purity hydrogen interacts with the storage medium.
- Impact of specific contaminants on metal hydride performance: Different contaminants affect metal hydride systems in various ways. Carbon monoxide, sulfur compounds, and moisture are particularly problematic as they can poison active sites on the metal hydride surface, reducing hydrogen absorption capacity and kinetics. Oxygen can lead to oxidation of the metal surface, while nitrogen compounds may form stable nitrides. Understanding these specific interactions allows for the development of metal hydride materials with enhanced tolerance to the most common contaminants in practical hydrogen applications.
- Advanced alloy compositions for improved contaminant tolerance: Specialized alloy compositions can significantly improve the contaminant tolerance of metal hydride systems. Multi-component alloys incorporating elements such as titanium, zirconium, vanadium, and rare earth metals can maintain hydrogen storage properties even when exposed to impure hydrogen streams. Some advanced compositions include catalytic elements that facilitate the decomposition of contaminants or prevent their binding to critical storage sites, while others form protective surface layers that allow hydrogen permeation while blocking larger contaminant molecules.
- Monitoring and regeneration systems for contaminated metal hydrides: Systems for monitoring hydrogen purity and regenerating contaminated metal hydrides are essential for long-term operation. These include sensors that detect decreases in performance or the presence of specific contaminants, coupled with regeneration protocols such as thermal cycling, vacuum treatment, or hydrogen flushing. Some advanced systems incorporate automated regeneration cycles that can restore performance without complete system shutdown, extending the operational lifetime of metal hydride storage systems even when regularly exposed to contaminated hydrogen sources.
02 Purification methods for hydrogen in metal hydride storage
Various purification methods can be integrated into metal hydride hydrogen storage systems to ensure high hydrogen purity. These include pressure swing adsorption, membrane separation, and catalytic purification techniques specifically designed for metal hydride applications. Some systems employ multi-stage purification processes where contaminants are progressively removed before hydrogen interacts with the metal hydride material, ensuring optimal absorption and desorption characteristics and extending the operational life of the storage system.Expand Specific Solutions03 Impact of specific contaminants on metal hydride performance
Different contaminants affect metal hydride systems in various ways. Carbon monoxide, sulfur compounds, and moisture are particularly problematic as they can poison active sites on the metal hydride surface, reducing hydrogen absorption capacity and kinetics. Oxygen can lead to oxidation of the metal surface, while nitrogen compounds may form stable nitrides. Understanding these specific contaminant effects allows for the development of targeted protection strategies and the selection of appropriate metal hydride compositions for different operating environments.Expand Specific Solutions04 Advanced alloy compositions for improved contaminant resistance
Specialized metal hydride alloy compositions can be engineered to exhibit enhanced tolerance to common contaminants. These advanced materials often incorporate multiple elements in specific ratios to create synergistic effects that maintain hydrogen storage properties despite exposure to impurities. Some alloys include catalytic elements that can mitigate the effects of certain contaminants, while others feature surface modifications or dopants that improve resistance to poisoning. These compositions are particularly valuable in applications where hydrogen purity cannot be guaranteed.Expand Specific Solutions05 Regeneration and recovery techniques for contaminated systems
When metal hydride systems become contaminated and performance degrades, various regeneration techniques can restore functionality. These include thermal cycling, vacuum treatment, and exposure to clean hydrogen at specific temperature and pressure conditions. Some systems incorporate built-in regeneration capabilities that can be activated periodically to remove accumulated contaminants. Advanced recovery methods may use reactive gases or plasma treatments to remove stubborn contaminants from the metal hydride surface, extending the useful life of the storage system.Expand Specific Solutions
Leading Companies and Research Institutions in Metal Hydride Development
The hydrogen purity and contaminant tolerance landscape for metal hydride systems is currently in a growth phase, with an estimated market size of $2-3 billion and expanding at 8-10% annually. The technology is maturing from early commercial to established applications, particularly in energy storage and transportation sectors. Leading players demonstrate varying technological approaches: GRZ Technologies and Hydro-Québec focus on dense storage solutions; Air Products & Chemicals and Air Liquide leverage industrial gas expertise; while academic institutions like California Institute of Technology and Xi'an Jiaotong University drive fundamental research. Specialized companies such as Tokamak Energy and Vodik Labs are developing niche applications, while industrial giants Samsung Electronics and POSCO Holdings integrate metal hydride systems into broader energy strategies.
GRZ Technologies SA
Technical Solution: GRZ Technologies has developed advanced metal hydride systems with exceptional hydrogen purity management capabilities. Their proprietary HYCO technology utilizes specialized alloys that can store hydrogen at moderate pressures (30-50 bar) while acting as effective purification media. The system incorporates multi-stage filtration processes where specific metal hydrides selectively absorb hydrogen while rejecting common contaminants like CO, CO2, and sulfur compounds. Their innovative approach includes regenerable metal hydride beds that can be thermally cycled to release trapped contaminants, extending operational lifetime. GRZ's systems achieve hydrogen purity levels exceeding 99.999% even when input gas contains significant impurities, making them suitable for fuel cell applications requiring high-purity hydrogen.
Strengths: Superior contaminant rejection capabilities while maintaining high storage density; regenerable systems that reduce operational costs; operates at moderate temperatures and pressures. Weaknesses: Higher initial capital costs compared to conventional purification systems; requires precise thermal management; limited tolerance to certain halogen contaminants.
Air Products & Chemicals, Inc.
Technical Solution: Air Products has pioneered advanced metal hydride systems with sophisticated contaminant management technologies. Their PRISM Hydrogen Purifiers utilize specialized metal alloys (typically Ti-Fe based compounds) that can selectively absorb hydrogen while rejecting impurities. The system employs a multi-stage purification process where hydrogen passes through beds of different metal hydride compositions, each targeting specific contaminants. Their proprietary alloy formulations demonstrate exceptional tolerance to common contaminants like CO (up to 100 ppm), CO2 (up to 500 ppm), and water vapor without significant degradation of storage capacity. Air Products' systems incorporate intelligent regeneration cycles that can restore performance after exposure to contaminants by controlled heating and purging processes, extending the operational lifetime of the metal hydride materials significantly beyond conventional systems.
Strengths: Exceptional tolerance to multiple contaminant types simultaneously; long operational lifetime due to effective regeneration capabilities; scalable from small to industrial applications. Weaknesses: Higher energy requirements for regeneration cycles; performance degradation in presence of sulfur compounds above certain thresholds; requires sophisticated control systems for optimal operation.
Critical Patents and Research on Hydrogen Purification Technologies
System for purifying hydrogen from a metal hydride storage system
PatentInactiveUS9878279B2
Innovation
- A system incorporating a particle removal unit with filters and flow control mechanisms to prevent particle dislodgement, combined with a methane removal unit using adsorbent vessels, particularly activated carbon in a pressure-swing adsorption system, to achieve low methane concentrations in the hydrogen stream.
Integrated system and method for hydrogen purification, storage and pressurization
PatentActiveUS20230416086A1
Innovation
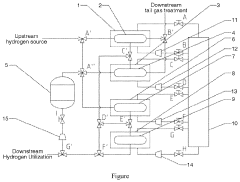
- An integrated system and method for hydrogen purification, storage, and pressurization using a multi-stage metal hydride reactor system with adjustable heat and cold sources, circulation pumps, and gas pumps, allowing for reversible hydrogen absorption and desorption reactions based on pressure-composition-temperature characteristics to achieve high-purity hydrogen at various pressure levels.
Safety Standards and Risk Assessment for Hydrogen Storage Systems
Safety standards for hydrogen storage systems, particularly those utilizing metal hydride technologies, have evolved significantly to address the unique challenges posed by hydrogen's physical properties. The ISO/TS 15869 and ISO 16111 standards specifically address portable hydrogen storage systems using metal hydrides, establishing critical parameters for pressure vessel design, material compatibility, and safety testing protocols. These standards mandate rigorous burst testing, cycle testing, and fire resistance evaluations to ensure system integrity under extreme conditions.
The SAE J2579 standard, focused on vehicular applications, provides comprehensive guidelines for fuel system integrity in hydrogen-powered vehicles, including specific provisions for metal hydride storage systems. This standard emphasizes the importance of contaminant management, recognizing that even trace impurities can significantly impact storage capacity and system longevity.
Risk assessment methodologies for metal hydride storage systems have become increasingly sophisticated, incorporating both deterministic and probabilistic approaches. HAZOP (Hazard and Operability) studies and FMEA (Failure Mode and Effects Analysis) are routinely employed to identify potential failure modes related to contaminant exposure. These assessments particularly focus on the risks associated with carbon monoxide, sulfur compounds, and moisture contamination, which can irreversibly damage metal hydride materials.
Regulatory frameworks across major markets have established hydrogen purity requirements specifically for storage applications. The European Union's regulations require 99.97% hydrogen purity for fuel cell applications, with strict limits on carbon monoxide (0.2 ppm), sulfur compounds (0.004 ppm), and total hydrocarbons (2 ppm). Similar standards have been adopted in Japan and the United States, though with some regional variations in acceptable contaminant levels.
Emerging safety protocols are increasingly addressing the dynamic relationship between hydrogen purity and system performance degradation over time. The development of in-situ monitoring systems capable of detecting contaminant breakthrough represents a significant advancement in risk mitigation strategies. These systems typically employ electrochemical sensors or optical spectroscopy to provide real-time assessment of hydrogen quality before it enters the metal hydride matrix.
International harmonization efforts, led by organizations such as the International Partnership for Hydrogen and Fuel Cells in the Economy (IPHE), are working to standardize safety requirements across global markets. These initiatives aim to establish universal protocols for contaminant tolerance testing and certification, facilitating broader commercial deployment of metal hydride storage technologies while maintaining rigorous safety standards.
The SAE J2579 standard, focused on vehicular applications, provides comprehensive guidelines for fuel system integrity in hydrogen-powered vehicles, including specific provisions for metal hydride storage systems. This standard emphasizes the importance of contaminant management, recognizing that even trace impurities can significantly impact storage capacity and system longevity.
Risk assessment methodologies for metal hydride storage systems have become increasingly sophisticated, incorporating both deterministic and probabilistic approaches. HAZOP (Hazard and Operability) studies and FMEA (Failure Mode and Effects Analysis) are routinely employed to identify potential failure modes related to contaminant exposure. These assessments particularly focus on the risks associated with carbon monoxide, sulfur compounds, and moisture contamination, which can irreversibly damage metal hydride materials.
Regulatory frameworks across major markets have established hydrogen purity requirements specifically for storage applications. The European Union's regulations require 99.97% hydrogen purity for fuel cell applications, with strict limits on carbon monoxide (0.2 ppm), sulfur compounds (0.004 ppm), and total hydrocarbons (2 ppm). Similar standards have been adopted in Japan and the United States, though with some regional variations in acceptable contaminant levels.
Emerging safety protocols are increasingly addressing the dynamic relationship between hydrogen purity and system performance degradation over time. The development of in-situ monitoring systems capable of detecting contaminant breakthrough represents a significant advancement in risk mitigation strategies. These systems typically employ electrochemical sensors or optical spectroscopy to provide real-time assessment of hydrogen quality before it enters the metal hydride matrix.
International harmonization efforts, led by organizations such as the International Partnership for Hydrogen and Fuel Cells in the Economy (IPHE), are working to standardize safety requirements across global markets. These initiatives aim to establish universal protocols for contaminant tolerance testing and certification, facilitating broader commercial deployment of metal hydride storage technologies while maintaining rigorous safety standards.
Environmental Impact and Sustainability of Metal Hydride Technologies
Metal hydride systems represent a promising technology for hydrogen storage and utilization, yet their environmental footprint and sustainability credentials warrant careful examination. The production of metal hydrides typically involves energy-intensive processes for metal extraction, refinement, and alloy formation, contributing to significant carbon emissions when powered by conventional energy sources. However, when renewable energy is employed throughout the manufacturing chain, the environmental impact can be substantially reduced.
Water consumption presents another environmental consideration, as certain metal hydride production processes require substantial water resources for cooling, cleaning, and chemical reactions. Advanced manufacturing techniques are being developed to minimize water usage and implement closed-loop systems that recycle process water, thereby reducing the overall environmental footprint.
The recyclability of metal hydride materials offers a significant sustainability advantage. Most metal hydride systems can be reclaimed and reprocessed at the end of their operational life, creating a circular economy approach that minimizes waste and reduces the need for virgin material extraction. Research indicates that up to 95% of certain metal hydride compositions can be effectively recovered and reused, substantially extending the lifecycle value of these materials.
Land use impacts associated with metal hydride technologies are generally favorable compared to alternative hydrogen storage methods. The high volumetric density of hydrogen in metal hydrides means smaller storage footprints, reducing land requirements for hydrogen infrastructure. This advantage becomes particularly significant in urban environments where space constraints are critical considerations.
From a lifecycle perspective, metal hydride systems demonstrate promising sustainability metrics. While initial production energy requirements may be high, the extended operational lifespan and recyclability of these materials often result in favorable long-term environmental performance. Lifecycle assessments indicate that metal hydride systems can achieve carbon payback periods of 3-7 years depending on application and energy source, with total lifecycle emissions potentially 40-60% lower than conventional hydrogen storage alternatives when powered by renewable energy.
The contaminant tolerance aspect of metal hydride systems also contributes to their sustainability profile. Systems with higher tolerance for impurities can utilize lower-grade hydrogen, potentially enabling the use of hydrogen produced from renewable but less pure sources such as biomass gasification or certain electrolysis processes. This flexibility could accelerate the transition to green hydrogen economies by reducing purification energy requirements and associated environmental impacts.
Future sustainability improvements in metal hydride technologies are focusing on developing alloys with reduced critical material content, lowering processing temperatures, and enhancing system durability to extend operational lifespans beyond current 10-15 year averages to potentially 20+ years, further improving lifecycle environmental performance.
Water consumption presents another environmental consideration, as certain metal hydride production processes require substantial water resources for cooling, cleaning, and chemical reactions. Advanced manufacturing techniques are being developed to minimize water usage and implement closed-loop systems that recycle process water, thereby reducing the overall environmental footprint.
The recyclability of metal hydride materials offers a significant sustainability advantage. Most metal hydride systems can be reclaimed and reprocessed at the end of their operational life, creating a circular economy approach that minimizes waste and reduces the need for virgin material extraction. Research indicates that up to 95% of certain metal hydride compositions can be effectively recovered and reused, substantially extending the lifecycle value of these materials.
Land use impacts associated with metal hydride technologies are generally favorable compared to alternative hydrogen storage methods. The high volumetric density of hydrogen in metal hydrides means smaller storage footprints, reducing land requirements for hydrogen infrastructure. This advantage becomes particularly significant in urban environments where space constraints are critical considerations.
From a lifecycle perspective, metal hydride systems demonstrate promising sustainability metrics. While initial production energy requirements may be high, the extended operational lifespan and recyclability of these materials often result in favorable long-term environmental performance. Lifecycle assessments indicate that metal hydride systems can achieve carbon payback periods of 3-7 years depending on application and energy source, with total lifecycle emissions potentially 40-60% lower than conventional hydrogen storage alternatives when powered by renewable energy.
The contaminant tolerance aspect of metal hydride systems also contributes to their sustainability profile. Systems with higher tolerance for impurities can utilize lower-grade hydrogen, potentially enabling the use of hydrogen produced from renewable but less pure sources such as biomass gasification or certain electrolysis processes. This flexibility could accelerate the transition to green hydrogen economies by reducing purification energy requirements and associated environmental impacts.
Future sustainability improvements in metal hydride technologies are focusing on developing alloys with reduced critical material content, lowering processing temperatures, and enhancing system durability to extend operational lifespans beyond current 10-15 year averages to potentially 20+ years, further improving lifecycle environmental performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!