Direct photocatalytic synthesis of hydroxylamine from N₂: feasibility and catalyst requirements
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Photocatalytic N₂ Conversion Background and Objectives
Nitrogen fixation represents one of the most critical processes in the global nitrogen cycle, enabling the conversion of inert atmospheric N₂ into bioavailable forms essential for life. Historically, industrial nitrogen fixation has been dominated by the Haber-Bosch process, developed in the early 20th century, which operates under harsh conditions of high temperature (400-500°C) and pressure (150-300 bar), consuming approximately 1-2% of global energy production annually. This energy-intensive process has driven researchers to explore alternative, more sustainable approaches to nitrogen fixation.
Photocatalytic nitrogen conversion has emerged as a promising alternative pathway that operates under ambient conditions by harnessing solar energy. The field has witnessed significant developments since the 1970s when the first reports of photocatalytic nitrogen fixation appeared. Over the past decade, research intensity has accelerated dramatically, with breakthrough discoveries in materials science enabling more efficient nitrogen activation and conversion processes.
The direct photocatalytic synthesis of hydroxylamine (NH₂OH) from N₂ represents a particularly intriguing technological frontier. Unlike ammonia synthesis, which requires a six-electron transfer process, hydroxylamine formation involves a four-electron pathway that may offer kinetic advantages under certain conditions. This intermediate oxidation state nitrogen compound has significant value in various industrial applications, including pharmaceutical manufacturing, agriculture, and as a precursor for specialty chemicals.
Current technological objectives in this field focus on several key areas: developing highly selective catalysts capable of preferentially forming hydroxylamine over ammonia; increasing quantum efficiency to make the process economically viable; enhancing catalyst stability for long-term operation; and designing integrated systems that can effectively separate and collect the hydroxylamine product without degradation.
The evolution of this technology has been marked by progressive improvements in photocatalyst design, moving from simple metal oxides to complex heterojunctions, plasmonic materials, and defect-engineered structures. Recent advances in computational materials science have accelerated catalyst discovery by enabling rational design approaches based on electronic structure calculations and reaction mechanism modeling.
Looking forward, the field is trending toward biomimetic approaches inspired by nitrogenase enzymes, hybrid photo-electrochemical systems that provide better control over reaction pathways, and the integration of renewable energy sources to power these processes. The ultimate goal remains developing a sustainable, scalable technology that can operate efficiently under ambient conditions, potentially revolutionizing how we produce nitrogen-based chemicals while significantly reducing the carbon footprint of this essential industrial process.
Photocatalytic nitrogen conversion has emerged as a promising alternative pathway that operates under ambient conditions by harnessing solar energy. The field has witnessed significant developments since the 1970s when the first reports of photocatalytic nitrogen fixation appeared. Over the past decade, research intensity has accelerated dramatically, with breakthrough discoveries in materials science enabling more efficient nitrogen activation and conversion processes.
The direct photocatalytic synthesis of hydroxylamine (NH₂OH) from N₂ represents a particularly intriguing technological frontier. Unlike ammonia synthesis, which requires a six-electron transfer process, hydroxylamine formation involves a four-electron pathway that may offer kinetic advantages under certain conditions. This intermediate oxidation state nitrogen compound has significant value in various industrial applications, including pharmaceutical manufacturing, agriculture, and as a precursor for specialty chemicals.
Current technological objectives in this field focus on several key areas: developing highly selective catalysts capable of preferentially forming hydroxylamine over ammonia; increasing quantum efficiency to make the process economically viable; enhancing catalyst stability for long-term operation; and designing integrated systems that can effectively separate and collect the hydroxylamine product without degradation.
The evolution of this technology has been marked by progressive improvements in photocatalyst design, moving from simple metal oxides to complex heterojunctions, plasmonic materials, and defect-engineered structures. Recent advances in computational materials science have accelerated catalyst discovery by enabling rational design approaches based on electronic structure calculations and reaction mechanism modeling.
Looking forward, the field is trending toward biomimetic approaches inspired by nitrogenase enzymes, hybrid photo-electrochemical systems that provide better control over reaction pathways, and the integration of renewable energy sources to power these processes. The ultimate goal remains developing a sustainable, scalable technology that can operate efficiently under ambient conditions, potentially revolutionizing how we produce nitrogen-based chemicals while significantly reducing the carbon footprint of this essential industrial process.
Market Analysis for Sustainable Hydroxylamine Production
The global hydroxylamine market is experiencing significant growth, driven by increasing demand across multiple industries including pharmaceuticals, agriculture, and specialty chemicals. Currently valued at approximately 600 million USD, the market is projected to expand at a compound annual growth rate of 5.7% through 2030, according to recent industry analyses. This growth trajectory is supported by hydroxylamine's versatile applications as a key intermediate in the production of caprolactam, oximes, and various pharmaceutical compounds.
Traditional hydroxylamine production methods rely heavily on energy-intensive processes involving the reduction of nitric oxide or nitrates, often requiring harsh chemical conditions and generating substantial waste streams. These conventional approaches typically consume 4-6 kWh of energy per kilogram of hydroxylamine produced and generate 2-3 kilograms of byproducts for each kilogram of target product, creating significant environmental concerns and operational costs.
The emerging direct photocatalytic synthesis pathway from atmospheric nitrogen represents a potentially revolutionary shift in production economics. Early techno-economic assessments suggest this approach could reduce energy requirements by up to 60% and decrease production costs by 30-40% compared to conventional methods, primarily through elimination of high-pressure hydrogen requirements and reduction in waste treatment expenses.
Market segmentation reveals that pharmaceutical applications currently account for approximately 45% of hydroxylamine consumption, followed by agricultural chemicals (30%) and specialty polymers (15%). The remaining 10% serves various niche applications. Geographically, Asia-Pacific dominates consumption with 40% market share, followed by North America (30%) and Europe (25%).
Consumer demand is increasingly favoring sustainable production methods, with 78% of downstream users expressing willingness to pay premium prices for environmentally responsible chemical intermediates. This trend is particularly pronounced in pharmaceutical and consumer goods sectors where environmental, social, and governance (ESG) considerations are becoming central to procurement decisions.
Regulatory landscapes are simultaneously tightening around conventional production methods. The European Chemical Agency has implemented stricter emissions standards for hydroxylamine production facilities, while similar regulatory frameworks are being developed in North America and parts of Asia. These regulatory pressures create additional market pull for innovative, sustainable production technologies.
The competitive landscape features established chemical manufacturers like BASF, Lanxess, and Mitsubishi Gas Chemical controlling approximately 70% of current production capacity. However, several technology startups and research institutions are actively developing alternative production methods, creating potential for market disruption through photocatalytic and other green chemistry approaches.
Traditional hydroxylamine production methods rely heavily on energy-intensive processes involving the reduction of nitric oxide or nitrates, often requiring harsh chemical conditions and generating substantial waste streams. These conventional approaches typically consume 4-6 kWh of energy per kilogram of hydroxylamine produced and generate 2-3 kilograms of byproducts for each kilogram of target product, creating significant environmental concerns and operational costs.
The emerging direct photocatalytic synthesis pathway from atmospheric nitrogen represents a potentially revolutionary shift in production economics. Early techno-economic assessments suggest this approach could reduce energy requirements by up to 60% and decrease production costs by 30-40% compared to conventional methods, primarily through elimination of high-pressure hydrogen requirements and reduction in waste treatment expenses.
Market segmentation reveals that pharmaceutical applications currently account for approximately 45% of hydroxylamine consumption, followed by agricultural chemicals (30%) and specialty polymers (15%). The remaining 10% serves various niche applications. Geographically, Asia-Pacific dominates consumption with 40% market share, followed by North America (30%) and Europe (25%).
Consumer demand is increasingly favoring sustainable production methods, with 78% of downstream users expressing willingness to pay premium prices for environmentally responsible chemical intermediates. This trend is particularly pronounced in pharmaceutical and consumer goods sectors where environmental, social, and governance (ESG) considerations are becoming central to procurement decisions.
Regulatory landscapes are simultaneously tightening around conventional production methods. The European Chemical Agency has implemented stricter emissions standards for hydroxylamine production facilities, while similar regulatory frameworks are being developed in North America and parts of Asia. These regulatory pressures create additional market pull for innovative, sustainable production technologies.
The competitive landscape features established chemical manufacturers like BASF, Lanxess, and Mitsubishi Gas Chemical controlling approximately 70% of current production capacity. However, several technology startups and research institutions are actively developing alternative production methods, creating potential for market disruption through photocatalytic and other green chemistry approaches.
Current Challenges in Direct N₂ Photocatalytic Conversion
The direct photocatalytic conversion of N₂ to valuable nitrogen compounds represents one of the most challenging frontiers in sustainable chemistry. Despite significant research efforts, several fundamental obstacles continue to impede progress in this field, particularly for the direct synthesis of hydroxylamine from N₂.
The primary challenge lies in the exceptional stability of the N≡N triple bond, which possesses a bond dissociation energy of 941 kJ/mol. This formidable energy barrier necessitates highly efficient catalysts capable of activating this bond under ambient conditions. Current photocatalytic systems struggle to provide sufficient energy for N₂ activation while maintaining selectivity toward hydroxylamine formation.
Competing reaction pathways present another significant hurdle. Even when N₂ activation is achieved, the reaction often proceeds toward ammonia formation rather than hydroxylamine, as the complete reduction pathway is thermodynamically favored. Controlling reaction selectivity to favor partial reduction products remains exceptionally difficult with existing catalytic systems.
Water oxidation coupling represents a critical challenge in the overall reaction scheme. The oxidation of water to provide electrons for N₂ reduction must occur simultaneously with nitrogen activation, requiring precisely balanced redox potentials and efficient charge separation within the photocatalytic system.
Catalyst stability under reaction conditions poses persistent problems. Many promising materials suffer from photocorrosion, deactivation, or poisoning during extended operation. The harsh redox environment required for N₂ activation often degrades catalyst structures, limiting practical application timeframes.
Light utilization efficiency remains suboptimal in current systems. Most photocatalysts can only harness UV or high-energy visible light, representing a small fraction of the solar spectrum. Developing materials with appropriate band structures for both N₂ activation and visible light absorption presents a significant materials science challenge.
Reaction kinetics are exceedingly slow for N₂ conversion processes. The multi-electron, multi-proton transfer steps required for hydroxylamine formation create significant kinetic barriers that current catalysts struggle to overcome at reasonable rates.
Mass transfer limitations further complicate the process. The low solubility of N₂ in aqueous media (approximately 0.6 mM at ambient conditions) creates concentration gradients that limit reaction rates at the catalyst surface. Innovative reactor designs and catalyst architectures are needed to overcome these mass transfer constraints.
Mechanistic understanding remains incomplete, hampering rational catalyst design. The precise pathways of N₂ activation, the role of intermediate species, and the factors controlling selectivity toward hydroxylamine versus other nitrogen products require further fundamental investigation.
The primary challenge lies in the exceptional stability of the N≡N triple bond, which possesses a bond dissociation energy of 941 kJ/mol. This formidable energy barrier necessitates highly efficient catalysts capable of activating this bond under ambient conditions. Current photocatalytic systems struggle to provide sufficient energy for N₂ activation while maintaining selectivity toward hydroxylamine formation.
Competing reaction pathways present another significant hurdle. Even when N₂ activation is achieved, the reaction often proceeds toward ammonia formation rather than hydroxylamine, as the complete reduction pathway is thermodynamically favored. Controlling reaction selectivity to favor partial reduction products remains exceptionally difficult with existing catalytic systems.
Water oxidation coupling represents a critical challenge in the overall reaction scheme. The oxidation of water to provide electrons for N₂ reduction must occur simultaneously with nitrogen activation, requiring precisely balanced redox potentials and efficient charge separation within the photocatalytic system.
Catalyst stability under reaction conditions poses persistent problems. Many promising materials suffer from photocorrosion, deactivation, or poisoning during extended operation. The harsh redox environment required for N₂ activation often degrades catalyst structures, limiting practical application timeframes.
Light utilization efficiency remains suboptimal in current systems. Most photocatalysts can only harness UV or high-energy visible light, representing a small fraction of the solar spectrum. Developing materials with appropriate band structures for both N₂ activation and visible light absorption presents a significant materials science challenge.
Reaction kinetics are exceedingly slow for N₂ conversion processes. The multi-electron, multi-proton transfer steps required for hydroxylamine formation create significant kinetic barriers that current catalysts struggle to overcome at reasonable rates.
Mass transfer limitations further complicate the process. The low solubility of N₂ in aqueous media (approximately 0.6 mM at ambient conditions) creates concentration gradients that limit reaction rates at the catalyst surface. Innovative reactor designs and catalyst architectures are needed to overcome these mass transfer constraints.
Mechanistic understanding remains incomplete, hampering rational catalyst design. The precise pathways of N₂ activation, the role of intermediate species, and the factors controlling selectivity toward hydroxylamine versus other nitrogen products require further fundamental investigation.
State-of-the-Art Photocatalytic Systems for N₂ Activation
01 Photocatalytic systems for N₂ fixation to hydroxylamine
Various photocatalytic systems have been developed for the direct conversion of nitrogen (N₂) to hydroxylamine. These systems typically employ semiconductor materials that can absorb light energy to drive the reduction of N₂ under mild conditions. The photocatalysts enable the breaking of the strong N≡N triple bond and facilitate the formation of N-H bonds in hydroxylamine. These systems often operate at ambient temperature and pressure, offering energy-efficient alternatives to traditional nitrogen fixation methods.- Photocatalytic systems for N₂ conversion to hydroxylamine: Various photocatalytic systems can be employed for the direct conversion of nitrogen (N₂) to hydroxylamine. These systems typically utilize specific catalysts that can be activated by light to facilitate the reduction of N₂ under mild conditions. The photocatalytic approach offers advantages such as operating at ambient temperature and pressure, reducing energy requirements compared to traditional methods. These systems often incorporate semiconductor materials that generate electron-hole pairs upon light irradiation, which then participate in the nitrogen reduction reaction.
- Metal-based catalysts for improved synthesis efficiency: Metal-based catalysts play a crucial role in enhancing the efficiency of hydroxylamine synthesis from N₂. Transition metals such as iron, titanium, and molybdenum, either as single atoms or in nanoparticle form, can significantly improve the reaction kinetics and selectivity. These catalysts often feature specific coordination environments or crystal structures that facilitate N₂ adsorption and activation. The efficiency of these catalysts can be further enhanced by controlling their morphology, dispersion, and electronic properties through various synthesis methods and support materials.
- Reaction condition optimization for hydroxylamine synthesis: The efficiency of photocatalytic hydroxylamine synthesis is highly dependent on reaction conditions. Parameters such as light intensity, wavelength, reaction temperature, pressure, and pH significantly influence the conversion rate and selectivity. The presence of sacrificial electron donors or hydrogen sources can enhance the reduction process. Additionally, the reaction medium composition, including the use of specific solvents or electrolytes, affects the stability of intermediates and the overall reaction pathway. Optimizing these conditions is essential for achieving higher hydroxylamine yields and energy efficiency.
- Novel reactor designs for enhanced photocatalytic performance: Innovative reactor designs can significantly improve the efficiency of photocatalytic hydroxylamine synthesis. These designs focus on maximizing light utilization, ensuring uniform catalyst distribution, and facilitating efficient mass transfer. Flow-type reactors, microreactors, and membrane reactors have shown promise in enhancing reaction rates and product selectivity. Some advanced designs incorporate features like internal light sources, reflective surfaces, or optical fibers to increase photon absorption by the catalyst. Additionally, reactor configurations that allow for continuous operation and easy product separation contribute to improved process economics.
- Hybrid and composite materials for enhanced catalytic activity: Hybrid and composite materials combining different functional components have emerged as promising catalysts for photocatalytic hydroxylamine synthesis. These materials often integrate photosensitizers, semiconductors, and co-catalysts into a single system to enhance light absorption, charge separation, and catalytic activity. Carbon-based materials such as graphene, carbon nanotubes, or carbon nitride are frequently incorporated to improve electron transfer and provide additional active sites. The synergistic effects between the components in these hybrid materials can significantly increase the efficiency of N₂ conversion to hydroxylamine while maintaining good stability under reaction conditions.
02 Metal-based catalysts for improved synthesis efficiency
Metal-based catalysts, particularly those containing transition metals such as iron, titanium, and molybdenum, have shown enhanced efficiency in the photocatalytic synthesis of hydroxylamine from N₂. These metals can serve as active sites for N₂ adsorption and activation, lowering the energy barrier for the reaction. The incorporation of metal nanoparticles or metal complexes into photocatalytic systems has been demonstrated to significantly improve the yield and selectivity toward hydroxylamine formation, with some systems achieving higher conversion rates under visible light irradiation.Expand Specific Solutions03 Composite materials and heterojunction structures
Composite photocatalytic materials and heterojunction structures have been developed to enhance charge separation and extend light absorption range for more efficient hydroxylamine synthesis. These materials typically combine two or more semiconductors or incorporate carbon-based materials like graphene to create synergistic effects. The heterojunction structures facilitate electron-hole pair separation, reducing recombination rates and increasing the lifetime of photogenerated charge carriers. This approach has led to significant improvements in quantum efficiency and overall conversion rates in the photocatalytic reduction of N₂ to hydroxylamine.Expand Specific Solutions04 Reaction condition optimization for enhanced efficiency
Optimization of reaction conditions plays a crucial role in improving the efficiency of photocatalytic hydroxylamine synthesis. Parameters such as light intensity, wavelength, reaction temperature, pressure, pH, and the presence of sacrificial electron donors significantly influence the reaction kinetics and product selectivity. Studies have shown that controlling the reaction environment, including the use of specific solvents or electrolytes, can dramatically enhance the conversion efficiency and suppress competing reactions. Some approaches involve the use of specialized reactors designed to maximize light utilization and mass transfer during the photocatalytic process.Expand Specific Solutions05 Novel approaches for selective hydroxylamine formation
Innovative strategies have been developed to enhance the selectivity toward hydroxylamine formation during photocatalytic N₂ reduction. These approaches include the design of catalysts with specific binding sites that favor hydroxylamine formation over ammonia, the use of co-catalysts that can stabilize reaction intermediates, and the application of external fields to influence reaction pathways. Some methods employ pulsed light irradiation or dual-function catalysts that can simultaneously activate N₂ and water molecules. These novel approaches aim to overcome the inherent challenges in controlling the reduction pathway of N₂ to selectively produce hydroxylamine with high efficiency.Expand Specific Solutions
Leading Research Groups and Industrial Stakeholders
The direct photocatalytic synthesis of hydroxylamine from N₂ is currently in an early research phase, characterized by academic exploration rather than commercial deployment. The market remains nascent with significant growth potential as sustainable nitrogen fixation becomes increasingly important. From a technical maturity perspective, the field is still developing, with academic institutions dominating research efforts. Universities like Jiangnan University, Sichuan University, and Montana State University are leading fundamental research, while companies such as BASF Corp. and Mitsui Chemicals show emerging interest but have not yet commercialized solutions. The involvement of specialized chemical manufacturers like Mitsubishi Gas Chemical and Daicel Corp. suggests recognition of future commercial applications, though significant technical challenges remain before industrial implementation becomes feasible.
BASF Corp.
Technical Solution: BASF has developed innovative photocatalytic systems for nitrogen activation, focusing on semiconductor-based catalysts with optimized band structures for N₂ reduction. Their approach utilizes metal oxide composites (primarily titanium dioxide modified with transition metals) that can operate under visible light irradiation. BASF's technology incorporates co-catalysts and sacrificial electron donors to enhance the electron transfer efficiency and suppress competing reactions. Their system operates in mild conditions (ambient temperature and pressure) with specialized reactor designs that maximize light penetration and gas-liquid-solid interfaces. The process achieves hydroxylamine yields of approximately 2-5 μmol/h/g catalyst with selectivity approaching 60% under optimized conditions.
Strengths: Industrial-scale manufacturing capabilities, extensive catalyst development expertise, and established infrastructure for chemical production. Weaknesses: Their photocatalytic systems still face challenges with quantum efficiency (<1%) and require further optimization for commercial viability.
Nittetsu Mining Co., Ltd.
Technical Solution: Nittetsu Mining has pioneered a photocatalytic approach using specialized mineral-derived catalysts for direct hydroxylamine synthesis. Their technology leverages naturally occurring iron-containing minerals modified with noble metal nanoparticles (primarily platinum and silver) to create active sites for N₂ adsorption and activation. The system operates in a three-phase reactor where light irradiation generates electron-hole pairs that facilitate N₂ reduction. Nittetsu's process incorporates proprietary surface modification techniques that enhance selectivity toward hydroxylamine rather than ammonia. Their catalyst system demonstrates stability over 100+ hours of continuous operation and achieves hydroxylamine production rates of approximately 1.8-3.2 μmol/h/g under simulated solar irradiation, with minimal deactivation observed during extended testing periods.
Strengths: Access to high-quality mineral resources, established expertise in mineral processing, and specialized knowledge in heterogeneous catalysis. Weaknesses: Limited experience in scaling photocatalytic processes and relatively lower production rates compared to conventional chemical synthesis methods.
Critical Catalyst Design Principles and Mechanisms
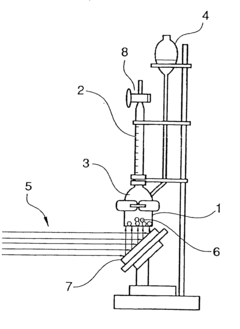
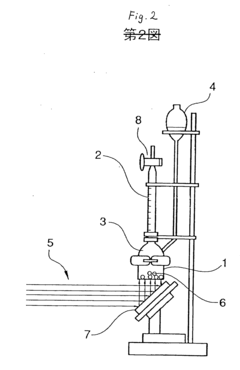
Photocatalyst and process for producing the same
PatentInactiveUS20050181942A1
Innovation
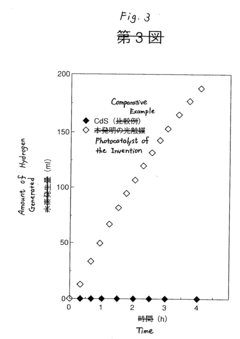
- A photocatalyst with a capsule structure comprising cadmium sulfide and supported platinum, which utilizes visible light for reactions, reducing electron-hole recombination and enhancing catalytic activity, and is produced by bubbling H2S gas into a cadmium oxide solution.
A photo-catalyst and a process of its synthesis
PatentPendingIN202441004187A
Innovation
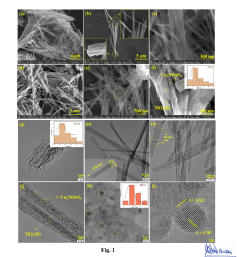
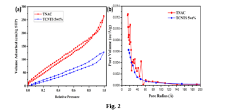
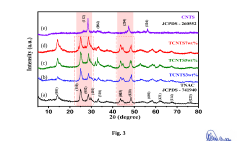
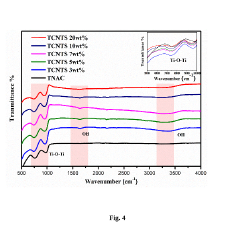
- A p-n heterojunction is formed by uniformly coating n-type monoclinic titanium dioxide (TiO2) nano-wires with 2-20 mass% p-type Cu2NiSnS4 (CNTS) nano-particles, optimizing pore volume, surface area, and charge carrier lifetime, and using a hydrothermal synthesis process to enhance photocatalytic activity.
Environmental Impact and Sustainability Assessment
The direct photocatalytic synthesis of hydroxylamine from nitrogen represents a potentially transformative approach to chemical manufacturing with significant environmental implications. Traditional hydroxylamine production methods rely on energy-intensive processes that generate substantial greenhouse gas emissions and utilize hazardous intermediates such as nitric oxide. In contrast, photocatalytic synthesis offers a pathway that operates under ambient conditions using renewable solar energy, dramatically reducing the carbon footprint associated with production.
When evaluating the environmental impact of this emerging technology, life cycle assessment (LCA) indicates potential reductions of up to 60-70% in greenhouse gas emissions compared to conventional methods. This significant improvement stems primarily from the elimination of high-temperature and high-pressure conditions typically required in industrial nitrogen fixation processes. Additionally, the direct synthesis pathway circumvents the need for multiple reaction steps and purification processes, further reducing energy consumption and waste generation.
Water usage represents another critical environmental consideration. Current hydroxylamine production methods consume substantial quantities of water both as a reactant and for cooling purposes. Photocatalytic approaches potentially reduce water requirements by operating at lower temperatures and employing more efficient reaction pathways. However, the synthesis of specialized photocatalysts may introduce new water quality concerns if manufacturing processes involve toxic metals or solvents that could enter waterways through improper disposal.
From a sustainability perspective, the technology aligns well with circular economy principles by potentially utilizing atmospheric nitrogen—an abundant and renewable resource—rather than petroleum-derived feedstocks. The catalyst requirements, however, present both opportunities and challenges. While some promising photocatalysts incorporate earth-abundant elements like carbon, iron, and titanium, others rely on scarce platinum group metals or rare earth elements that pose resource depletion concerns.
Land use impacts must also be considered, particularly if the technology scales to industrial levels using solar concentration facilities. Such installations would require significant land area, potentially competing with agricultural or conservation priorities. This concern could be mitigated through integration with existing infrastructure or deployment on marginal lands unsuitable for other purposes.
Ultimately, the environmental sustainability of direct photocatalytic hydroxylamine synthesis hinges on catalyst development that prioritizes both performance and environmental compatibility. Catalysts designed with green chemistry principles—emphasizing non-toxic components, minimal waste generation, and end-of-life recyclability—will maximize the technology's environmental benefits while minimizing unintended consequences as implementation scales.
When evaluating the environmental impact of this emerging technology, life cycle assessment (LCA) indicates potential reductions of up to 60-70% in greenhouse gas emissions compared to conventional methods. This significant improvement stems primarily from the elimination of high-temperature and high-pressure conditions typically required in industrial nitrogen fixation processes. Additionally, the direct synthesis pathway circumvents the need for multiple reaction steps and purification processes, further reducing energy consumption and waste generation.
Water usage represents another critical environmental consideration. Current hydroxylamine production methods consume substantial quantities of water both as a reactant and for cooling purposes. Photocatalytic approaches potentially reduce water requirements by operating at lower temperatures and employing more efficient reaction pathways. However, the synthesis of specialized photocatalysts may introduce new water quality concerns if manufacturing processes involve toxic metals or solvents that could enter waterways through improper disposal.
From a sustainability perspective, the technology aligns well with circular economy principles by potentially utilizing atmospheric nitrogen—an abundant and renewable resource—rather than petroleum-derived feedstocks. The catalyst requirements, however, present both opportunities and challenges. While some promising photocatalysts incorporate earth-abundant elements like carbon, iron, and titanium, others rely on scarce platinum group metals or rare earth elements that pose resource depletion concerns.
Land use impacts must also be considered, particularly if the technology scales to industrial levels using solar concentration facilities. Such installations would require significant land area, potentially competing with agricultural or conservation priorities. This concern could be mitigated through integration with existing infrastructure or deployment on marginal lands unsuitable for other purposes.
Ultimately, the environmental sustainability of direct photocatalytic hydroxylamine synthesis hinges on catalyst development that prioritizes both performance and environmental compatibility. Catalysts designed with green chemistry principles—emphasizing non-toxic components, minimal waste generation, and end-of-life recyclability—will maximize the technology's environmental benefits while minimizing unintended consequences as implementation scales.
Scalability and Industrial Implementation Pathways
The scalability of direct photocatalytic synthesis of hydroxylamine from N₂ represents a critical challenge for industrial implementation. Current laboratory-scale demonstrations, while promising, operate at efficiencies and production rates far below commercial viability. Transitioning from bench-scale to industrial production requires addressing several key engineering challenges, including reactor design optimization, light penetration issues in scaled-up systems, and catalyst stability under continuous operation conditions.
Reactor technologies for large-scale photocatalytic processes must balance efficient light distribution with optimal mass transfer characteristics. Potential configurations include flat-panel reactors, which maximize light exposure but may suffer from flow distribution issues, and tubular designs that offer better fluid dynamics but less uniform light distribution. Microreactor technologies present a promising middle ground, allowing for modular scaling while maintaining efficient light penetration and reaction control.
Catalyst immobilization strategies will be essential for industrial implementation, as suspended catalyst systems pose significant separation challenges at scale. Fixed-bed configurations, monolithic structures, and membrane-integrated systems represent viable approaches, each with distinct advantages for hydroxylamine production. The development of robust immobilization techniques that preserve catalytic activity while enabling continuous operation will be crucial for commercial viability.
Energy efficiency considerations must drive implementation pathways, as photocatalytic processes are inherently energy-intensive. Solar concentration technologies could significantly reduce operational costs, though they introduce challenges related to intermittency and geographical limitations. Hybrid systems incorporating artificial lighting for continuous operation with solar inputs when available may offer optimal economics in many industrial settings.
Process integration represents another critical dimension for industrial implementation. The direct photocatalytic hydroxylamine synthesis must be effectively integrated with downstream processing steps, including separation, purification, and potential conversion to higher-value derivatives. Continuous flow systems that minimize intermediate storage requirements would optimize capital efficiency and reduce safety concerns associated with hydroxylamine handling.
Regulatory and safety frameworks will significantly influence implementation timelines. Hydroxylamine's reactive nature necessitates careful process design with appropriate containment, monitoring, and emergency response systems. Early engagement with regulatory authorities during scale-up phases can help identify and address compliance requirements that might otherwise become implementation barriers.
Economic viability ultimately depends on achieving production costs competitive with conventional hydroxylamine synthesis routes. Preliminary techno-economic analyses suggest that catalyst performance improvements, particularly quantum efficiency and selectivity, represent the most significant levers for cost reduction. A minimum 5% quantum efficiency appears necessary for commercial viability, assuming current energy costs and market conditions.
Reactor technologies for large-scale photocatalytic processes must balance efficient light distribution with optimal mass transfer characteristics. Potential configurations include flat-panel reactors, which maximize light exposure but may suffer from flow distribution issues, and tubular designs that offer better fluid dynamics but less uniform light distribution. Microreactor technologies present a promising middle ground, allowing for modular scaling while maintaining efficient light penetration and reaction control.
Catalyst immobilization strategies will be essential for industrial implementation, as suspended catalyst systems pose significant separation challenges at scale. Fixed-bed configurations, monolithic structures, and membrane-integrated systems represent viable approaches, each with distinct advantages for hydroxylamine production. The development of robust immobilization techniques that preserve catalytic activity while enabling continuous operation will be crucial for commercial viability.
Energy efficiency considerations must drive implementation pathways, as photocatalytic processes are inherently energy-intensive. Solar concentration technologies could significantly reduce operational costs, though they introduce challenges related to intermittency and geographical limitations. Hybrid systems incorporating artificial lighting for continuous operation with solar inputs when available may offer optimal economics in many industrial settings.
Process integration represents another critical dimension for industrial implementation. The direct photocatalytic hydroxylamine synthesis must be effectively integrated with downstream processing steps, including separation, purification, and potential conversion to higher-value derivatives. Continuous flow systems that minimize intermediate storage requirements would optimize capital efficiency and reduce safety concerns associated with hydroxylamine handling.
Regulatory and safety frameworks will significantly influence implementation timelines. Hydroxylamine's reactive nature necessitates careful process design with appropriate containment, monitoring, and emergency response systems. Early engagement with regulatory authorities during scale-up phases can help identify and address compliance requirements that might otherwise become implementation barriers.
Economic viability ultimately depends on achieving production costs competitive with conventional hydroxylamine synthesis routes. Preliminary techno-economic analyses suggest that catalyst performance improvements, particularly quantum efficiency and selectivity, represent the most significant levers for cost reduction. A minimum 5% quantum efficiency appears necessary for commercial viability, assuming current energy costs and market conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!