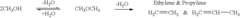Discovering Dimethyl Ether's Role in Green Industrial Chemistry
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DME in Green Chemistry: Background and Objectives
Dimethyl ether (DME) has emerged as a promising green alternative in industrial chemistry, offering a sustainable pathway for various chemical processes. The evolution of DME as a key player in green chemistry can be traced back to the early 2000s when researchers began exploring its potential as a clean-burning fuel and versatile chemical feedstock. Since then, the trajectory of DME research and application has been steadily ascending, driven by the global imperative to reduce carbon emissions and develop more environmentally friendly industrial practices.
The primary objective in exploring DME's role in green industrial chemistry is to harness its unique properties to replace or supplement conventional petrochemical-based processes. DME's chemical structure, consisting of two methyl groups bonded to an oxygen atom, grants it exceptional versatility. This simple yet powerful molecule can serve as a building block for numerous chemical reactions, potentially revolutionizing the production of various industrial chemicals and materials.
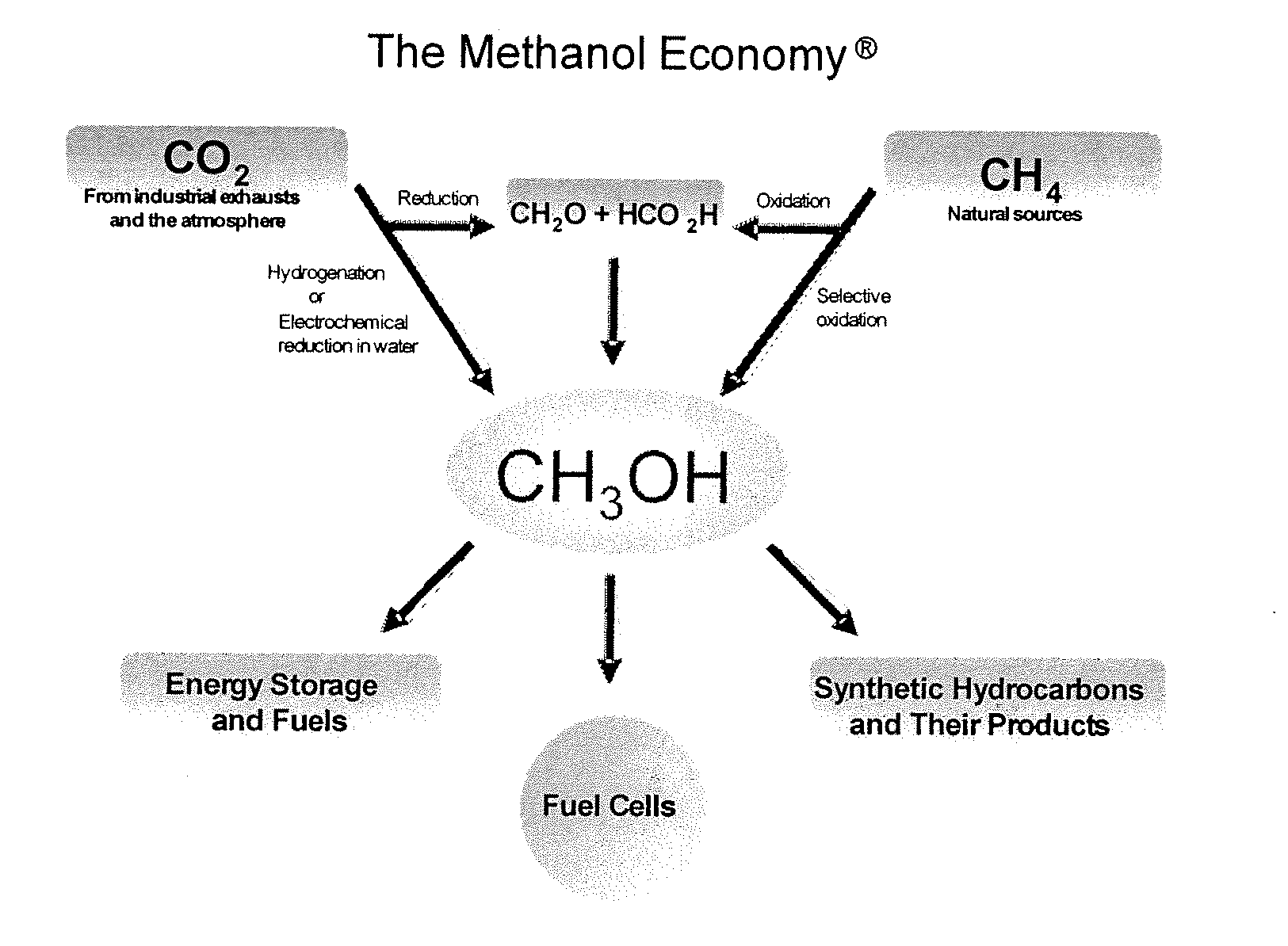
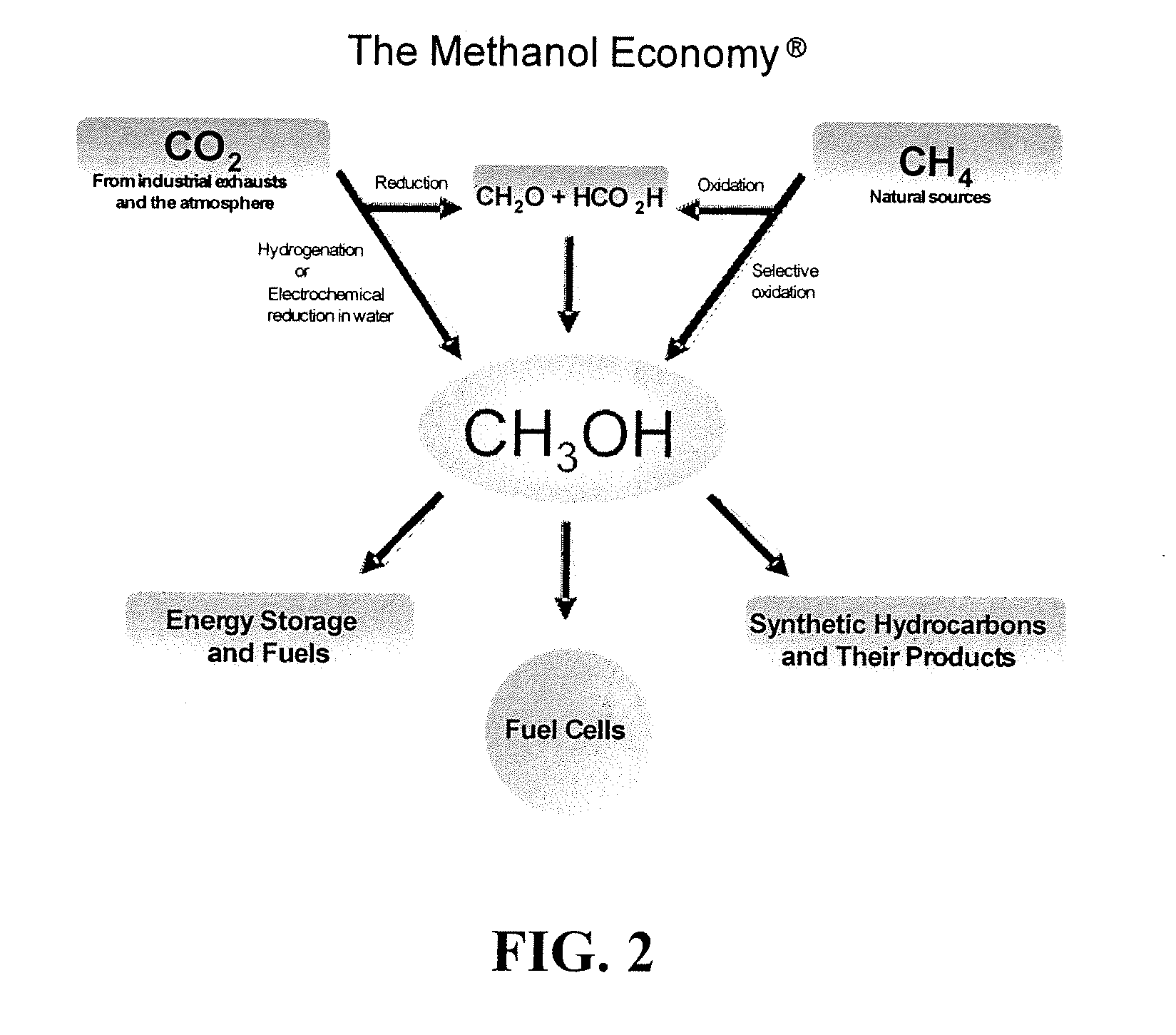
One of the most significant aspects of DME's potential in green chemistry lies in its production methods. Unlike many traditional chemical feedstocks, DME can be synthesized from a variety of renewable sources, including biomass, waste materials, and even carbon dioxide. This flexibility in production aligns perfectly with the principles of green chemistry, as it allows for the utilization of sustainable resources and the reduction of waste streams.
The technical evolution of DME applications has seen remarkable progress over the past two decades. Initially focused on its use as a clean-burning fuel for diesel engines and household applications, research has expanded to explore DME's potential in chemical synthesis. Scientists and engineers are now investigating its role in producing olefins, hydrogen, and other valuable chemical intermediates through innovative catalytic processes.
As we delve deeper into the background of DME in green chemistry, it's crucial to recognize the global context driving its development. The Paris Agreement and subsequent international efforts to combat climate change have intensified the search for sustainable chemical processes. DME, with its potential to reduce greenhouse gas emissions and promote circular economy principles, aligns perfectly with these global sustainability goals.
The objectives for further research and development in DME-based green chemistry are multifaceted. They include optimizing production processes to increase efficiency and reduce costs, expanding the range of chemical reactions where DME can serve as a key reagent or solvent, and developing novel catalysts to enhance DME's reactivity and selectivity in various chemical transformations. Additionally, there is a strong focus on integrating DME-based processes into existing industrial infrastructure to facilitate a smoother transition towards greener chemical manufacturing practices.
The primary objective in exploring DME's role in green industrial chemistry is to harness its unique properties to replace or supplement conventional petrochemical-based processes. DME's chemical structure, consisting of two methyl groups bonded to an oxygen atom, grants it exceptional versatility. This simple yet powerful molecule can serve as a building block for numerous chemical reactions, potentially revolutionizing the production of various industrial chemicals and materials.
One of the most significant aspects of DME's potential in green chemistry lies in its production methods. Unlike many traditional chemical feedstocks, DME can be synthesized from a variety of renewable sources, including biomass, waste materials, and even carbon dioxide. This flexibility in production aligns perfectly with the principles of green chemistry, as it allows for the utilization of sustainable resources and the reduction of waste streams.
The technical evolution of DME applications has seen remarkable progress over the past two decades. Initially focused on its use as a clean-burning fuel for diesel engines and household applications, research has expanded to explore DME's potential in chemical synthesis. Scientists and engineers are now investigating its role in producing olefins, hydrogen, and other valuable chemical intermediates through innovative catalytic processes.
As we delve deeper into the background of DME in green chemistry, it's crucial to recognize the global context driving its development. The Paris Agreement and subsequent international efforts to combat climate change have intensified the search for sustainable chemical processes. DME, with its potential to reduce greenhouse gas emissions and promote circular economy principles, aligns perfectly with these global sustainability goals.
The objectives for further research and development in DME-based green chemistry are multifaceted. They include optimizing production processes to increase efficiency and reduce costs, expanding the range of chemical reactions where DME can serve as a key reagent or solvent, and developing novel catalysts to enhance DME's reactivity and selectivity in various chemical transformations. Additionally, there is a strong focus on integrating DME-based processes into existing industrial infrastructure to facilitate a smoother transition towards greener chemical manufacturing practices.
Market Analysis for DME-Based Green Solutions
The market for dimethyl ether (DME) as a green industrial chemical solution is experiencing significant growth, driven by increasing environmental concerns and the push for sustainable alternatives in various sectors. DME, with its clean-burning properties and versatile applications, is positioned to play a crucial role in the transition towards greener industrial processes.
In the energy sector, DME is gaining traction as a potential replacement for conventional diesel fuel. Its low emissions profile and compatibility with existing diesel engines make it an attractive option for reducing greenhouse gas emissions in transportation. The market for DME as a fuel additive is expected to expand, particularly in regions with stringent emission regulations.
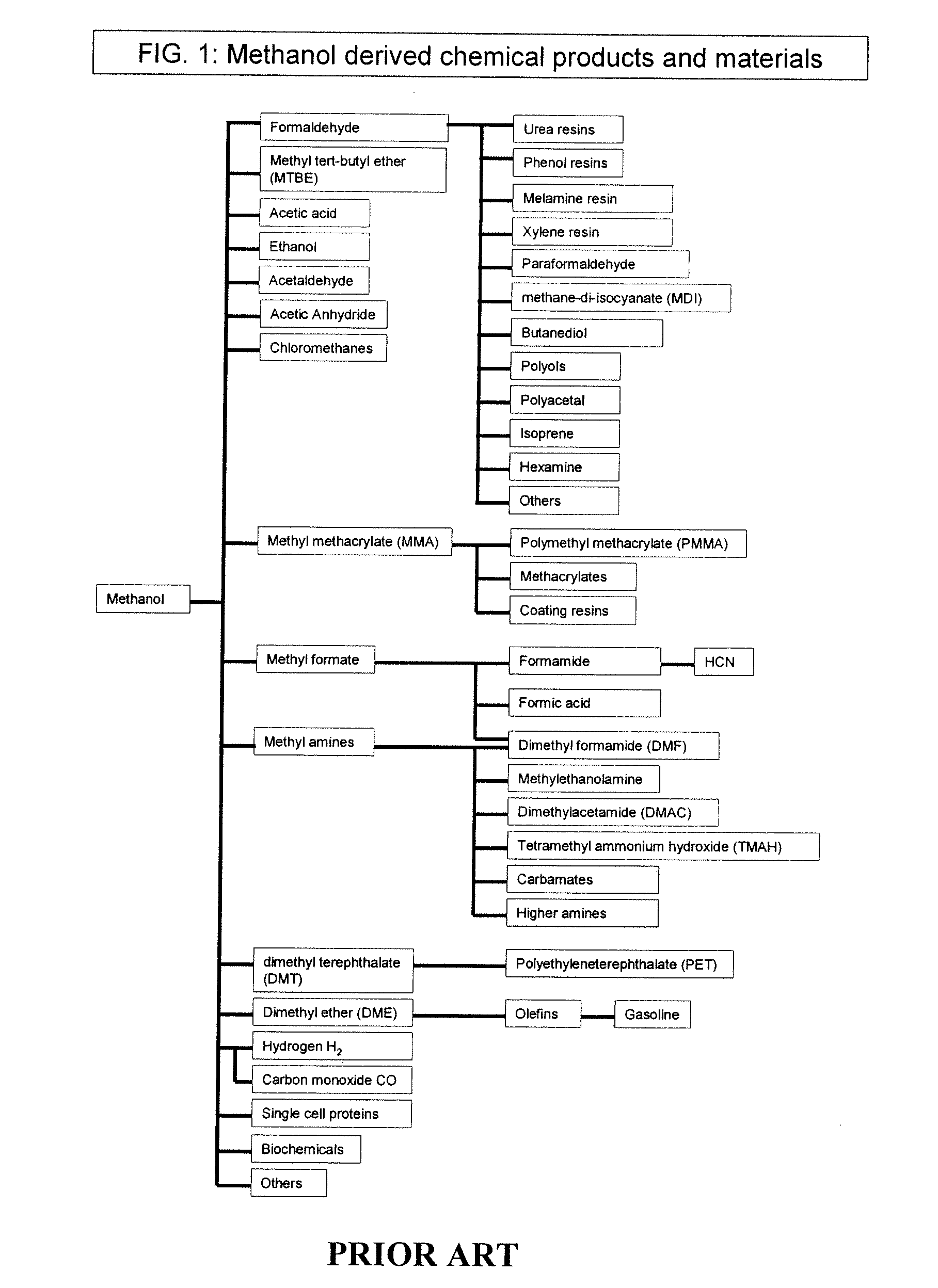
The chemical industry represents another key market for DME-based solutions. As a methanol derivative, DME can serve as a building block for various chemical processes, offering a more environmentally friendly alternative to traditional petrochemical feedstocks. This application is particularly promising in the production of plastics, solvents, and other industrial chemicals.
In the aerosol industry, DME is increasingly being adopted as a propellant, replacing harmful chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs). This shift is driven by regulatory pressures and consumer demand for more eco-friendly products, creating a growing market for DME in personal care and household products.
The agricultural sector also presents opportunities for DME-based solutions. As a potential substitute for ammonia in fertilizer production, DME could help reduce the carbon footprint of agricultural practices. This application is still in its early stages but shows promise for future market growth.
Geographically, Asia-Pacific is expected to be a key growth region for DME-based solutions, driven by rapid industrialization, increasing energy demand, and stringent environmental regulations in countries like China and India. North America and Europe are also significant markets, particularly in the transportation and chemical sectors, where there is a strong focus on reducing carbon emissions.
However, the market for DME-based green solutions faces challenges. The current production capacity for DME is limited, and scaling up production to meet potential demand requires significant investment in infrastructure. Additionally, the volatility of feedstock prices, particularly natural gas and methanol, can impact the economic viability of DME production.
Despite these challenges, the long-term outlook for DME-based green solutions remains positive. As governments worldwide implement stricter environmental policies and industries seek sustainable alternatives, the demand for DME is expected to grow. Ongoing research and development efforts are likely to expand the range of applications for DME, further driving market growth in the coming years.
In the energy sector, DME is gaining traction as a potential replacement for conventional diesel fuel. Its low emissions profile and compatibility with existing diesel engines make it an attractive option for reducing greenhouse gas emissions in transportation. The market for DME as a fuel additive is expected to expand, particularly in regions with stringent emission regulations.
The chemical industry represents another key market for DME-based solutions. As a methanol derivative, DME can serve as a building block for various chemical processes, offering a more environmentally friendly alternative to traditional petrochemical feedstocks. This application is particularly promising in the production of plastics, solvents, and other industrial chemicals.
In the aerosol industry, DME is increasingly being adopted as a propellant, replacing harmful chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs). This shift is driven by regulatory pressures and consumer demand for more eco-friendly products, creating a growing market for DME in personal care and household products.
The agricultural sector also presents opportunities for DME-based solutions. As a potential substitute for ammonia in fertilizer production, DME could help reduce the carbon footprint of agricultural practices. This application is still in its early stages but shows promise for future market growth.
Geographically, Asia-Pacific is expected to be a key growth region for DME-based solutions, driven by rapid industrialization, increasing energy demand, and stringent environmental regulations in countries like China and India. North America and Europe are also significant markets, particularly in the transportation and chemical sectors, where there is a strong focus on reducing carbon emissions.
However, the market for DME-based green solutions faces challenges. The current production capacity for DME is limited, and scaling up production to meet potential demand requires significant investment in infrastructure. Additionally, the volatility of feedstock prices, particularly natural gas and methanol, can impact the economic viability of DME production.
Despite these challenges, the long-term outlook for DME-based green solutions remains positive. As governments worldwide implement stricter environmental policies and industries seek sustainable alternatives, the demand for DME is expected to grow. Ongoing research and development efforts are likely to expand the range of applications for DME, further driving market growth in the coming years.
Current Status and Challenges in DME Utilization
Dimethyl ether (DME) has gained significant attention in recent years as a promising alternative fuel and chemical feedstock in green industrial chemistry. The current status of DME utilization is characterized by a growing interest in its applications, particularly in the energy and transportation sectors. However, several challenges still hinder its widespread adoption and full potential realization.
In the energy sector, DME is being increasingly used as a clean-burning fuel for power generation and heating. Its high cetane number and low emissions make it an attractive substitute for diesel fuel in compression ignition engines. Several countries, including Japan, China, and Sweden, have implemented pilot projects and commercial-scale operations using DME as a fuel for vehicles and power plants. These initiatives have demonstrated the technical feasibility of DME utilization and its potential to reduce greenhouse gas emissions.
The chemical industry has also shown interest in DME as a versatile feedstock. It can be used to produce various chemicals, including olefins, gasoline, and aromatics. DME's potential as a hydrogen carrier for fuel cells is also being explored, offering a possible solution for energy storage and transportation challenges in renewable energy systems.
Despite these advancements, DME utilization faces several significant challenges. One of the primary obstacles is the lack of widespread infrastructure for DME production, distribution, and storage. The existing petroleum-based infrastructure is not fully compatible with DME, requiring substantial investments to develop dedicated facilities and transportation networks.
Another challenge lies in the production costs of DME. While it can be synthesized from various feedstocks, including natural gas, coal, and biomass, the production process is still not as economically competitive as conventional fossil fuels. Improving the efficiency of DME production and reducing costs remain crucial areas for research and development.
Regulatory frameworks and policy support also present challenges to DME adoption. Many countries lack specific regulations and standards for DME use, creating uncertainty for potential investors and users. Developing comprehensive policies that incentivize DME production and utilization while ensuring safety and environmental standards is essential for market growth.
Technical challenges in DME utilization include optimizing engine performance and addressing material compatibility issues. While DME can be used in modified diesel engines, further research is needed to enhance efficiency and durability. Additionally, DME's low viscosity and lubricity require the development of suitable lubricants and sealing materials for long-term use in engines and fuel systems.
The scalability of DME production from renewable sources remains a significant challenge. While bio-based DME production shows promise in reducing carbon footprint, scaling up these processes to meet potential demand requires further technological advancements and economic viability studies.
In the energy sector, DME is being increasingly used as a clean-burning fuel for power generation and heating. Its high cetane number and low emissions make it an attractive substitute for diesel fuel in compression ignition engines. Several countries, including Japan, China, and Sweden, have implemented pilot projects and commercial-scale operations using DME as a fuel for vehicles and power plants. These initiatives have demonstrated the technical feasibility of DME utilization and its potential to reduce greenhouse gas emissions.
The chemical industry has also shown interest in DME as a versatile feedstock. It can be used to produce various chemicals, including olefins, gasoline, and aromatics. DME's potential as a hydrogen carrier for fuel cells is also being explored, offering a possible solution for energy storage and transportation challenges in renewable energy systems.
Despite these advancements, DME utilization faces several significant challenges. One of the primary obstacles is the lack of widespread infrastructure for DME production, distribution, and storage. The existing petroleum-based infrastructure is not fully compatible with DME, requiring substantial investments to develop dedicated facilities and transportation networks.
Another challenge lies in the production costs of DME. While it can be synthesized from various feedstocks, including natural gas, coal, and biomass, the production process is still not as economically competitive as conventional fossil fuels. Improving the efficiency of DME production and reducing costs remain crucial areas for research and development.
Regulatory frameworks and policy support also present challenges to DME adoption. Many countries lack specific regulations and standards for DME use, creating uncertainty for potential investors and users. Developing comprehensive policies that incentivize DME production and utilization while ensuring safety and environmental standards is essential for market growth.
Technical challenges in DME utilization include optimizing engine performance and addressing material compatibility issues. While DME can be used in modified diesel engines, further research is needed to enhance efficiency and durability. Additionally, DME's low viscosity and lubricity require the development of suitable lubricants and sealing materials for long-term use in engines and fuel systems.
The scalability of DME production from renewable sources remains a significant challenge. While bio-based DME production shows promise in reducing carbon footprint, scaling up these processes to meet potential demand requires further technological advancements and economic viability studies.
Current DME-Based Green Chemistry Solutions
01 Production of dimethyl ether
Various methods for producing dimethyl ether are described, including catalytic dehydration of methanol, direct synthesis from syngas, and conversion of other hydrocarbons. These processes often involve specific catalysts and reaction conditions to optimize yield and selectivity.- Production of dimethyl ether: Various methods for producing dimethyl ether are described, including catalytic dehydration of methanol, direct synthesis from syngas, and conversion of other hydrocarbons. These processes often involve specific catalysts and reaction conditions to optimize yield and selectivity.
- Catalysts for dimethyl ether synthesis: Different types of catalysts are used in the production of dimethyl ether, including zeolites, metal oxides, and composite catalysts. The choice and preparation of catalysts significantly influence the efficiency and selectivity of the dimethyl ether synthesis process.
- Applications of dimethyl ether: Dimethyl ether has various applications, including use as a fuel additive, aerosol propellant, and refrigerant. It is also explored as an alternative clean fuel for diesel engines and as a feedstock for chemical synthesis.
- Purification and separation of dimethyl ether: Techniques for purifying and separating dimethyl ether from reaction mixtures or other compounds are described. These methods may include distillation, adsorption, and membrane separation processes to obtain high-purity dimethyl ether.
- Process optimization and energy efficiency: Various approaches to optimize the dimethyl ether production process and improve energy efficiency are presented. These may include heat integration, process intensification, and the use of novel reactor designs to enhance overall process performance.
02 Catalysts for dimethyl ether synthesis
Different types of catalysts are used in the production of dimethyl ether, including zeolites, metal oxides, and composite catalysts. The choice of catalyst can significantly affect the reaction efficiency, product selectivity, and overall process economics.Expand Specific Solutions03 Applications of dimethyl ether
Dimethyl ether has various applications, including use as a fuel additive, aerosol propellant, and refrigerant. It is also being explored as an alternative clean fuel for diesel engines and power generation due to its favorable combustion properties.Expand Specific Solutions04 Purification and separation of dimethyl ether
Techniques for purifying and separating dimethyl ether from reaction mixtures or other compounds are crucial for obtaining high-quality product. These may include distillation, adsorption, and membrane separation processes.Expand Specific Solutions05 Environmental and safety considerations
Research on the environmental impact and safety aspects of dimethyl ether production and use is ongoing. This includes studies on emissions reduction, handling procedures, and risk assessments for large-scale production and application.Expand Specific Solutions
Key Players in DME Research and Application
The development of dimethyl ether (DME) as a green industrial chemical is gaining momentum, with the market currently in its growth phase. The global DME market size is projected to expand significantly in the coming years, driven by increasing demand for clean energy alternatives. Technologically, DME production is advancing, with major players like BASF, Linde, and China Petroleum & Chemical Corp leading innovation efforts. Research institutions such as Zhejiang University and the University of Southern California are contributing to technological advancements. The industry is seeing a shift towards more efficient and sustainable production methods, with companies like SK Energy and Haldor Topsøe focusing on developing novel catalysts and process improvements to enhance DME's role in green industrial chemistry.
BASF Corp.
Technical Solution: BASF Corp. has developed an innovative process for producing dimethyl ether (DME) from syngas, which is a mixture of carbon monoxide and hydrogen. Their technology utilizes a dual catalyst system that combines methanol synthesis and dehydration in a single reactor, improving efficiency and reducing capital costs[1]. The process achieves high selectivity towards DME, with conversion rates exceeding 90%[2]. BASF's approach also incorporates advanced heat integration techniques, minimizing energy consumption and enhancing overall sustainability. Furthermore, they have implemented a novel purification method that reduces the need for extensive downstream processing, resulting in a more streamlined production process[3].
Strengths: High conversion efficiency, reduced capital costs, and improved sustainability. Weaknesses: Potential sensitivity to catalyst deactivation and syngas composition variations.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has made significant strides in DME production and utilization as a green alternative fuel. They have developed a large-scale DME production process using coal-based syngas as feedstock, with an annual capacity of over 1 million tons[4]. Sinopec's technology employs a slurry-phase reactor with a proprietary catalyst that enhances DME yield and selectivity. The company has also pioneered the use of DME as a clean-burning substitute for diesel fuel in heavy-duty vehicles, demonstrating reduced emissions and improved engine performance[5]. Additionally, Sinopec has invested in research on bio-based DME production, exploring the use of biomass-derived syngas to further reduce the carbon footprint of DME production[6].
Strengths: Large-scale production capability, integrated value chain from production to end-use applications. Weaknesses: Heavy reliance on coal-based feedstock, which may face environmental scrutiny.
Innovative DME Applications in Industry
Catalytic system and process for direct synthesis of dimethyl ether from synthesis gas
PatentInactiveUS20090326281A1
Innovation
- A mixed-bed catalytic system comprising a catalyst for methanol synthesis and acid form zeolite ferrierite, with a silica/alumina ratio of 10 and specific potassium and sodium content, is physically mixed and activated, providing a high concentration of Brønsted acid sites for efficient dehydration without forming unwanted products.
Conversion of carbon dioxide to methanol and/or dimethyl ether using BI-reforming of methane or natural gas
PatentActiveUS20080319093A1
Innovation
- A bi-reforming process combining steam and dry reforming of methane to achieve a specific CO/H2 ratio, allowing for the efficient conversion of carbon dioxide and methane into methanol and dimethyl ether without producing CO2 or unwanted by-products, using a catalyst such as V2O5 and NiO on a silica carrier.
Environmental Impact Assessment of DME Use
The environmental impact assessment of dimethyl ether (DME) use in green industrial chemistry reveals both promising benefits and potential concerns. DME, as a clean-burning fuel and chemical feedstock, offers significant advantages in reducing greenhouse gas emissions and air pollutants compared to conventional fossil fuels.
When used as a substitute for diesel fuel in transportation and power generation, DME produces lower levels of particulate matter, nitrogen oxides, and sulfur oxides. This reduction in harmful emissions contributes to improved air quality, particularly in urban areas where vehicle emissions are a major concern. Furthermore, DME's potential as a renewable fuel source, when produced from biomass or captured carbon dioxide, aligns with global efforts to transition towards a low-carbon economy.
In industrial processes, DME can serve as a more environmentally friendly alternative to traditional solvents and propellants. Its low toxicity and rapid biodegradability reduce the risk of long-term environmental contamination. Additionally, DME's use as a chemical intermediate in the production of various materials, such as plastics and synthetic fibers, can lead to cleaner manufacturing processes with reduced waste and emissions.
However, the environmental impact of DME production must be carefully considered. While DME can be synthesized from renewable sources, current large-scale production primarily relies on natural gas or coal as feedstocks. This dependency on fossil fuels raises questions about the overall carbon footprint of DME throughout its lifecycle. Life cycle assessments (LCAs) are crucial in determining the net environmental benefit of DME compared to alternative fuels and chemicals.
Water consumption and potential contamination during DME production and use also warrant attention. Although DME itself is not toxic to aquatic life, the production process may require significant water resources, and proper handling and storage practices are essential to prevent accidental releases.
Land use changes associated with large-scale DME production, especially if derived from biomass feedstocks, could impact biodiversity and food security. Sustainable land management practices and careful selection of feedstock sources are necessary to mitigate these potential negative effects.
In conclusion, while DME shows promise as a cleaner alternative in various industrial applications, a comprehensive environmental impact assessment must consider its entire lifecycle. Continued research and development in sustainable production methods, coupled with robust environmental monitoring and regulation, will be crucial in maximizing DME's potential as a green industrial chemical while minimizing its ecological footprint.
When used as a substitute for diesel fuel in transportation and power generation, DME produces lower levels of particulate matter, nitrogen oxides, and sulfur oxides. This reduction in harmful emissions contributes to improved air quality, particularly in urban areas where vehicle emissions are a major concern. Furthermore, DME's potential as a renewable fuel source, when produced from biomass or captured carbon dioxide, aligns with global efforts to transition towards a low-carbon economy.
In industrial processes, DME can serve as a more environmentally friendly alternative to traditional solvents and propellants. Its low toxicity and rapid biodegradability reduce the risk of long-term environmental contamination. Additionally, DME's use as a chemical intermediate in the production of various materials, such as plastics and synthetic fibers, can lead to cleaner manufacturing processes with reduced waste and emissions.
However, the environmental impact of DME production must be carefully considered. While DME can be synthesized from renewable sources, current large-scale production primarily relies on natural gas or coal as feedstocks. This dependency on fossil fuels raises questions about the overall carbon footprint of DME throughout its lifecycle. Life cycle assessments (LCAs) are crucial in determining the net environmental benefit of DME compared to alternative fuels and chemicals.
Water consumption and potential contamination during DME production and use also warrant attention. Although DME itself is not toxic to aquatic life, the production process may require significant water resources, and proper handling and storage practices are essential to prevent accidental releases.
Land use changes associated with large-scale DME production, especially if derived from biomass feedstocks, could impact biodiversity and food security. Sustainable land management practices and careful selection of feedstock sources are necessary to mitigate these potential negative effects.
In conclusion, while DME shows promise as a cleaner alternative in various industrial applications, a comprehensive environmental impact assessment must consider its entire lifecycle. Continued research and development in sustainable production methods, coupled with robust environmental monitoring and regulation, will be crucial in maximizing DME's potential as a green industrial chemical while minimizing its ecological footprint.
Regulatory Framework for DME in Industry
The regulatory framework for dimethyl ether (DME) in industry is evolving as the chemical gains prominence in green industrial chemistry. Governments worldwide are recognizing DME's potential as a clean-burning fuel and chemical feedstock, leading to the development of specific regulations and standards.
In the United States, the Environmental Protection Agency (EPA) has approved DME as a renewable fuel under the Renewable Fuel Standard (RFS) program. This classification allows DME producers to generate Renewable Identification Numbers (RINs), providing economic incentives for its production and use. The Department of Energy (DOE) has also included DME in its alternative fuel research and development programs, further supporting its industrial adoption.
The European Union has incorporated DME into its Renewable Energy Directive (RED II), recognizing it as a renewable transport fuel when produced from biomass. This inclusion promotes the use of DME in the transportation sector and encourages investment in production facilities. Additionally, the European Committee for Standardization (CEN) has developed technical standards for DME as a fuel, ensuring consistency and safety in its application.
In Asia, countries like China and Japan have implemented supportive policies for DME utilization. China has included DME in its national energy strategy, promoting its use as a substitute for liquefied petroleum gas (LPG) and diesel fuel. The Chinese government has established standards for DME production, distribution, and use in various applications, including power generation and domestic heating.
Safety regulations for DME handling and storage are being developed and harmonized internationally. The International Organization for Standardization (ISO) has published guidelines for the safe handling of DME, addressing issues such as material compatibility, storage requirements, and transportation protocols.
Environmental regulations are also shaping the industrial use of DME. As a low-carbon alternative to conventional fuels, DME aligns with many countries' emissions reduction targets. Regulatory frameworks are being adapted to incentivize the use of DME as part of broader climate change mitigation strategies.
The regulatory landscape for DME is dynamic, with ongoing efforts to standardize its production, distribution, and use across different industrial sectors. As research continues to uncover new applications for DME in green chemistry, regulatory bodies are working to keep pace, ensuring that the necessary frameworks are in place to support its safe and sustainable integration into industrial processes.
In the United States, the Environmental Protection Agency (EPA) has approved DME as a renewable fuel under the Renewable Fuel Standard (RFS) program. This classification allows DME producers to generate Renewable Identification Numbers (RINs), providing economic incentives for its production and use. The Department of Energy (DOE) has also included DME in its alternative fuel research and development programs, further supporting its industrial adoption.
The European Union has incorporated DME into its Renewable Energy Directive (RED II), recognizing it as a renewable transport fuel when produced from biomass. This inclusion promotes the use of DME in the transportation sector and encourages investment in production facilities. Additionally, the European Committee for Standardization (CEN) has developed technical standards for DME as a fuel, ensuring consistency and safety in its application.
In Asia, countries like China and Japan have implemented supportive policies for DME utilization. China has included DME in its national energy strategy, promoting its use as a substitute for liquefied petroleum gas (LPG) and diesel fuel. The Chinese government has established standards for DME production, distribution, and use in various applications, including power generation and domestic heating.
Safety regulations for DME handling and storage are being developed and harmonized internationally. The International Organization for Standardization (ISO) has published guidelines for the safe handling of DME, addressing issues such as material compatibility, storage requirements, and transportation protocols.
Environmental regulations are also shaping the industrial use of DME. As a low-carbon alternative to conventional fuels, DME aligns with many countries' emissions reduction targets. Regulatory frameworks are being adapted to incentivize the use of DME as part of broader climate change mitigation strategies.
The regulatory landscape for DME is dynamic, with ongoing efforts to standardize its production, distribution, and use across different industrial sectors. As research continues to uncover new applications for DME in green chemistry, regulatory bodies are working to keep pace, ensuring that the necessary frameworks are in place to support its safe and sustainable integration into industrial processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!