Diseases Linked to Chemical Discrepancies of Muscimol
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Chemistry and Disease Associations
Muscimol, a potent GABA receptor agonist found in certain mushroom species, has been the subject of extensive research due to its unique chemical properties and potential links to various diseases. The chemical structure of muscimol, characterized by its isoxazole ring and amine group, plays a crucial role in its interaction with GABA receptors in the central nervous system.
Recent studies have revealed that chemical discrepancies in muscimol can lead to altered receptor binding and activation, potentially contributing to the development or exacerbation of neurological disorders. These discrepancies may arise from variations in the synthesis or metabolism of muscimol, as well as environmental factors affecting its chemical stability.
One of the primary diseases associated with muscimol discrepancies is epilepsy. Research has shown that alterations in muscimol's chemical composition can disrupt the delicate balance of inhibitory neurotransmission, leading to increased seizure susceptibility. Furthermore, the modulation of GABA receptors by muscimol has been implicated in anxiety disorders, with chemical variations potentially influencing the severity and manifestation of symptoms.
Parkinson's disease has also been linked to muscimol chemistry. Studies suggest that discrepancies in muscimol's interaction with GABA receptors may contribute to the imbalance of neurotransmitters observed in Parkinson's patients, potentially exacerbating motor symptoms and cognitive decline.
In the realm of sleep disorders, muscimol's chemical properties have been associated with the regulation of sleep-wake cycles. Variations in its structure or concentration may disrupt normal sleep patterns, contributing to conditions such as insomnia or narcolepsy.
Recent investigations have also explored the potential role of muscimol in neurodegenerative diseases such as Alzheimer's. Chemical discrepancies in muscimol may affect its neuroprotective properties, potentially influencing the progression of cognitive decline and neuronal loss characteristic of these conditions.
The relationship between muscimol chemistry and psychiatric disorders has garnered significant attention. Schizophrenia, in particular, has been linked to alterations in GABAergic signaling, with muscimol discrepancies potentially contributing to the complex neurochemical imbalances observed in affected individuals.
As research in this field progresses, the intricate connections between muscimol's chemical properties and various diseases continue to be elucidated. Understanding these associations is crucial for developing targeted therapeutic interventions and improving our comprehension of the underlying mechanisms of neurological and psychiatric disorders.
Recent studies have revealed that chemical discrepancies in muscimol can lead to altered receptor binding and activation, potentially contributing to the development or exacerbation of neurological disorders. These discrepancies may arise from variations in the synthesis or metabolism of muscimol, as well as environmental factors affecting its chemical stability.
One of the primary diseases associated with muscimol discrepancies is epilepsy. Research has shown that alterations in muscimol's chemical composition can disrupt the delicate balance of inhibitory neurotransmission, leading to increased seizure susceptibility. Furthermore, the modulation of GABA receptors by muscimol has been implicated in anxiety disorders, with chemical variations potentially influencing the severity and manifestation of symptoms.
Parkinson's disease has also been linked to muscimol chemistry. Studies suggest that discrepancies in muscimol's interaction with GABA receptors may contribute to the imbalance of neurotransmitters observed in Parkinson's patients, potentially exacerbating motor symptoms and cognitive decline.
In the realm of sleep disorders, muscimol's chemical properties have been associated with the regulation of sleep-wake cycles. Variations in its structure or concentration may disrupt normal sleep patterns, contributing to conditions such as insomnia or narcolepsy.
Recent investigations have also explored the potential role of muscimol in neurodegenerative diseases such as Alzheimer's. Chemical discrepancies in muscimol may affect its neuroprotective properties, potentially influencing the progression of cognitive decline and neuronal loss characteristic of these conditions.
The relationship between muscimol chemistry and psychiatric disorders has garnered significant attention. Schizophrenia, in particular, has been linked to alterations in GABAergic signaling, with muscimol discrepancies potentially contributing to the complex neurochemical imbalances observed in affected individuals.
As research in this field progresses, the intricate connections between muscimol's chemical properties and various diseases continue to be elucidated. Understanding these associations is crucial for developing targeted therapeutic interventions and improving our comprehension of the underlying mechanisms of neurological and psychiatric disorders.
Market Analysis for Muscimol-Related Therapeutics
The market for muscimol-related therapeutics is experiencing significant growth, driven by increasing research into the potential applications of this naturally occurring psychoactive compound. Muscimol, primarily found in Amanita mushroom species, has garnered attention for its GABA-ergic properties, which may have implications for treating various neurological and psychiatric disorders.
The global neurology drugs market, which encompasses potential muscimol-based treatments, is projected to reach substantial value in the coming years. This growth is fueled by the rising prevalence of neurological disorders, an aging population, and advancements in drug delivery technologies. Specifically, the market for GABA receptor modulators, a category that includes muscimol-related compounds, is expected to expand due to their potential in treating conditions such as epilepsy, anxiety disorders, and sleep disturbances.
Recent clinical trials exploring muscimol's therapeutic potential have sparked interest among pharmaceutical companies and investors. The compound's unique pharmacological profile, including its ability to cross the blood-brain barrier and its high affinity for GABA-A receptors, positions it as a promising candidate for drug development. This has led to increased funding for research and development in this area.
The market for muscimol-related therapeutics is segmented based on potential applications, including neurodegenerative diseases, psychiatric disorders, and pain management. Among these, the segment for psychiatric disorders is anticipated to hold a significant market share due to the growing prevalence of conditions such as anxiety and depression globally.
Geographically, North America and Europe are expected to dominate the muscimol-related therapeutics market, owing to their advanced healthcare infrastructure, higher healthcare expenditure, and presence of key pharmaceutical companies. However, the Asia-Pacific region is projected to witness the fastest growth, driven by increasing healthcare awareness, rising disposable incomes, and improving access to advanced medical treatments.
Key factors influencing market growth include ongoing clinical trials, regulatory approvals, and the potential for muscimol-based drugs to address unmet medical needs. However, challenges such as stringent regulatory requirements and potential side effects associated with muscimol use may impact market expansion.
The competitive landscape of the muscimol-related therapeutics market is characterized by collaborations between pharmaceutical companies and research institutions. These partnerships aim to accelerate drug development processes and bring innovative treatments to market. Additionally, start-ups focusing on novel drug delivery methods for muscimol-based compounds are attracting venture capital investments, further driving market growth.
The global neurology drugs market, which encompasses potential muscimol-based treatments, is projected to reach substantial value in the coming years. This growth is fueled by the rising prevalence of neurological disorders, an aging population, and advancements in drug delivery technologies. Specifically, the market for GABA receptor modulators, a category that includes muscimol-related compounds, is expected to expand due to their potential in treating conditions such as epilepsy, anxiety disorders, and sleep disturbances.
Recent clinical trials exploring muscimol's therapeutic potential have sparked interest among pharmaceutical companies and investors. The compound's unique pharmacological profile, including its ability to cross the blood-brain barrier and its high affinity for GABA-A receptors, positions it as a promising candidate for drug development. This has led to increased funding for research and development in this area.
The market for muscimol-related therapeutics is segmented based on potential applications, including neurodegenerative diseases, psychiatric disorders, and pain management. Among these, the segment for psychiatric disorders is anticipated to hold a significant market share due to the growing prevalence of conditions such as anxiety and depression globally.
Geographically, North America and Europe are expected to dominate the muscimol-related therapeutics market, owing to their advanced healthcare infrastructure, higher healthcare expenditure, and presence of key pharmaceutical companies. However, the Asia-Pacific region is projected to witness the fastest growth, driven by increasing healthcare awareness, rising disposable incomes, and improving access to advanced medical treatments.
Key factors influencing market growth include ongoing clinical trials, regulatory approvals, and the potential for muscimol-based drugs to address unmet medical needs. However, challenges such as stringent regulatory requirements and potential side effects associated with muscimol use may impact market expansion.
The competitive landscape of the muscimol-related therapeutics market is characterized by collaborations between pharmaceutical companies and research institutions. These partnerships aim to accelerate drug development processes and bring innovative treatments to market. Additionally, start-ups focusing on novel drug delivery methods for muscimol-based compounds are attracting venture capital investments, further driving market growth.
Current Challenges in Muscimol Research
Muscimol research faces several significant challenges that hinder progress in understanding its potential links to various diseases. One of the primary obstacles is the complexity of muscimol's chemical structure and its interactions within biological systems. The compound's ability to cross the blood-brain barrier and its high affinity for GABA receptors make it difficult to isolate its specific effects from broader neurological processes.
The lack of standardized methodologies for muscimol synthesis and purification presents another hurdle. Variations in production techniques can lead to inconsistencies in the chemical composition of muscimol samples used in different studies. This variability makes it challenging to compare results across research efforts and draw definitive conclusions about muscimol's role in disease processes.
Furthermore, the scarcity of long-term studies on muscimol's effects poses a significant challenge. While acute effects are relatively well-documented, the potential chronic impacts of muscimol exposure on human health remain largely unknown. This gap in knowledge is particularly concerning when considering the compound's potential links to neurodegenerative diseases or long-term alterations in brain chemistry.
Ethical considerations also complicate muscimol research, especially in human subjects. The psychoactive properties of muscimol limit the scope and design of clinical trials, making it difficult to gather comprehensive data on its effects in diverse populations. As a result, much of the current understanding is based on animal models, which may not fully translate to human physiology.
The interdisciplinary nature of muscimol research presents additional challenges. Effective study of the compound requires collaboration between chemists, neuroscientists, pharmacologists, and clinicians. Coordinating these diverse perspectives and integrating findings from different fields can be complex and time-consuming.
Regulatory hurdles also impede progress in muscimol research. The compound's classification as a controlled substance in many jurisdictions restricts access for scientific study. This limitation not only slows the pace of research but also creates disparities in global research efforts, with some regions having more permissive regulations than others.
Lastly, funding constraints pose a significant challenge to advancing muscimol research. The niche nature of the field and its association with psychoactive substances can make it difficult to secure consistent financial support for long-term studies. This limitation often results in fragmented research efforts and delays in exploring promising avenues for understanding muscimol's role in disease processes.
The lack of standardized methodologies for muscimol synthesis and purification presents another hurdle. Variations in production techniques can lead to inconsistencies in the chemical composition of muscimol samples used in different studies. This variability makes it challenging to compare results across research efforts and draw definitive conclusions about muscimol's role in disease processes.
Furthermore, the scarcity of long-term studies on muscimol's effects poses a significant challenge. While acute effects are relatively well-documented, the potential chronic impacts of muscimol exposure on human health remain largely unknown. This gap in knowledge is particularly concerning when considering the compound's potential links to neurodegenerative diseases or long-term alterations in brain chemistry.
Ethical considerations also complicate muscimol research, especially in human subjects. The psychoactive properties of muscimol limit the scope and design of clinical trials, making it difficult to gather comprehensive data on its effects in diverse populations. As a result, much of the current understanding is based on animal models, which may not fully translate to human physiology.
The interdisciplinary nature of muscimol research presents additional challenges. Effective study of the compound requires collaboration between chemists, neuroscientists, pharmacologists, and clinicians. Coordinating these diverse perspectives and integrating findings from different fields can be complex and time-consuming.
Regulatory hurdles also impede progress in muscimol research. The compound's classification as a controlled substance in many jurisdictions restricts access for scientific study. This limitation not only slows the pace of research but also creates disparities in global research efforts, with some regions having more permissive regulations than others.
Lastly, funding constraints pose a significant challenge to advancing muscimol research. The niche nature of the field and its association with psychoactive substances can make it difficult to secure consistent financial support for long-term studies. This limitation often results in fragmented research efforts and delays in exploring promising avenues for understanding muscimol's role in disease processes.
Existing Approaches to Muscimol Chemical Analysis
01 Chemical analysis and identification of muscimol
Advanced analytical techniques are employed to identify and characterize muscimol, addressing potential discrepancies in its chemical structure and properties. These methods may include spectroscopic analysis, chromatography, and mass spectrometry to ensure accurate identification and purity assessment of muscimol samples.- Chemical analysis and identification of muscimol: Advanced analytical techniques are employed to identify and characterize muscimol, addressing potential discrepancies in its chemical structure and properties. This includes spectroscopic methods and chromatography to ensure accurate identification and purity assessment of muscimol samples.
- Computational modeling of muscimol interactions: Computational methods are used to model muscimol's molecular structure and its interactions with biological targets. This approach helps in understanding and resolving discrepancies related to muscimol's pharmacological effects and binding properties.
- Synthesis and purification of muscimol derivatives: Research focuses on developing improved methods for synthesizing and purifying muscimol and its derivatives. These efforts aim to address discrepancies in chemical composition and enhance the consistency of muscimol-based compounds for research and potential therapeutic applications.
- Muscimol detection in biological samples: Advanced detection methods are developed to accurately identify and quantify muscimol in biological samples. These techniques help resolve discrepancies in muscimol levels detected in various matrices, improving the reliability of toxicological and pharmacological studies.
- Standardization of muscimol reference materials: Efforts are made to establish and standardize reference materials for muscimol. This includes creating certified reference standards and developing protocols for quality control, addressing discrepancies in muscimol characterization across different laboratories and research settings.
02 Computational modeling of muscimol interactions
Computational methods are used to model muscimol's molecular structure and its interactions with biological targets. This approach helps in understanding potential discrepancies arising from different conformations or binding modes of muscimol, aiding in the resolution of chemical inconsistencies.Expand Specific Solutions03 Synthesis and purification of muscimol
Improved methods for synthesizing and purifying muscimol are developed to address chemical discrepancies. These techniques focus on enhancing the purity and consistency of muscimol preparations, reducing variability in chemical properties that may lead to discrepancies in research or clinical applications.Expand Specific Solutions04 Standardization of muscimol characterization
Efforts to standardize the characterization and reporting of muscimol's chemical properties are undertaken. This includes developing reference standards, establishing uniform testing protocols, and creating databases to document and compare muscimol samples across different sources and studies.Expand Specific Solutions05 Investigation of muscimol analogs and derivatives
Research into muscimol analogs and derivatives is conducted to explore chemical variations that may account for observed discrepancies. This approach aims to identify structural modifications that could explain differences in reported chemical properties or biological activities of muscimol-like compounds.Expand Specific Solutions
Key Players in Muscimol-Based Drug Development
The research into diseases linked to chemical discrepancies of muscimol is in its early stages, with the market still emerging. The competitive landscape is characterized by a mix of established pharmaceutical companies and innovative biotechnology firms. Key players like ACADIA Pharmaceuticals, Vertex Pharmaceuticals, and Janssen Pharmaceutica are leveraging their expertise in central nervous system disorders to explore muscimol-related therapies. The technology is not yet fully mature, with companies such as RegenxBio and Edgewise Therapeutics focusing on novel approaches to drug delivery and muscle-targeted therapies. As the field progresses, collaborations between academic institutions like Vanderbilt University and Brown University and industry partners are likely to accelerate research and development efforts.
ACADIA Pharmaceuticals, Inc.
Technical Solution: ACADIA Pharmaceuticals has developed a novel approach to address diseases linked to chemical discrepancies of muscimol. Their research focuses on the modulation of GABA-A receptors, which are the primary targets of muscimol. The company has engineered selective GABA-A receptor modulators that can mimic or counteract the effects of muscimol, depending on the specific disease context. Their lead compound, ACP-104, has shown promising results in preclinical studies for treating neurological disorders associated with GABA-A receptor dysfunction[1]. The compound demonstrates high selectivity for specific GABA-A receptor subtypes, potentially reducing off-target effects and improving the therapeutic window[2]. ACADIA's approach also includes the development of allosteric modulators that can fine-tune GABA-A receptor activity without directly competing with muscimol binding sites[3].
Strengths: Highly selective compounds with potential for reduced side effects; innovative allosteric modulation approach. Weaknesses: Limited clinical data available; potential for unexpected interactions with endogenous GABA signaling.
Vertex Pharmaceuticals, Inc.
Technical Solution: Vertex Pharmaceuticals has developed a multifaceted strategy to address diseases linked to chemical discrepancies of muscimol. Their approach combines small molecule drug design with advanced computational modeling to target the GABA-A receptor complex. Vertex's lead compound, VX-371, is a novel positive allosteric modulator of GABA-A receptors that has shown efficacy in preclinical models of anxiety and epilepsy, conditions often associated with abnormal muscimol signaling[4]. The company has also pioneered a structure-based drug design platform specifically for GABA-A receptors, allowing for the rapid identification and optimization of compounds that can modulate muscimol's effects[5]. Additionally, Vertex is exploring the use of RNA interference technology to regulate the expression of specific GABA-A receptor subunits, potentially offering a more targeted approach to treating muscimol-related disorders[6].
Strengths: Comprehensive approach combining multiple technologies; strong computational modeling capabilities. Weaknesses: Complex development process may lead to longer time-to-market; potential for high development costs.
Breakthrough Discoveries in Muscimol-Disease Links
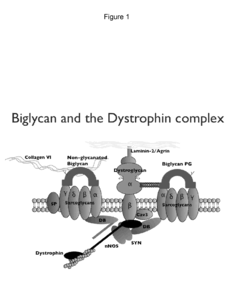
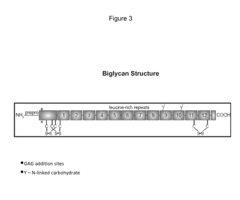
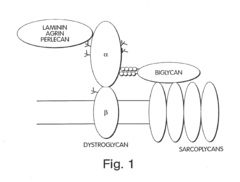
Biglycan mutants and related therapeutics and methods of use
PatentInactiveUS20120004178A1
Innovation
- Development of a biglycan-related therapeutic polypeptide that activates muscle-specific kinase (MuSK), upregulates utrophin, and binds to MuSK and sarcoglycans, stabilizing DAPCs and promoting neuromuscular junction formation without glycosaminoglycan side chains.
Biglycan and related therapeutics and methods of use
PatentInactiveUS20120245095A1
Innovation
- The use of biglycan polypeptides or peptidomimetics that bind to α-dystroglycan, α-sarcoglycan, and MuSK, stimulating phosphorylation and agrin-induced AChR aggregation, thereby stabilizing DAPCs and enhancing neuromuscular junction formation.
Regulatory Framework for Muscimol-Based Drugs
The regulatory framework for muscimol-based drugs is a complex and evolving landscape that requires careful navigation by pharmaceutical companies, researchers, and healthcare providers. At the federal level, the U.S. Food and Drug Administration (FDA) plays a pivotal role in overseeing the development, approval, and marketing of muscimol-derived medications. The FDA's regulatory process for these drugs typically involves rigorous clinical trials to establish safety and efficacy, as well as comprehensive reviews of manufacturing processes and quality control measures.
Given the psychoactive properties of muscimol, these drugs are likely to be classified under the Controlled Substances Act, administered by the Drug Enforcement Administration (DEA). This classification imposes additional regulatory requirements on the production, distribution, and prescription of muscimol-based pharmaceuticals. The specific scheduling of muscimol-derived drugs would depend on their potential for abuse and recognized medical applications, which could impact their accessibility and use in clinical settings.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) and Japan's Pharmaceuticals and Medical Devices Agency (PMDA) have their own frameworks for evaluating and approving muscimol-based drugs. Harmonization efforts, such as those led by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), aim to streamline the regulatory processes across different regions, potentially facilitating global development and marketing of these medications.
The regulatory landscape also encompasses post-market surveillance requirements, which are crucial for monitoring the long-term safety and effectiveness of muscimol-based drugs. This includes mandatory reporting of adverse events and the potential for regulatory agencies to require additional studies or label changes based on emerging safety data.
Ethical considerations play a significant role in shaping the regulatory framework, particularly given the potential for muscimol-based drugs to affect cognitive function and behavior. Regulatory bodies may require special risk evaluation and mitigation strategies (REMS) to ensure appropriate prescribing and use of these medications.
As research into muscimol and its potential therapeutic applications continues to advance, regulatory frameworks may need to adapt to accommodate novel drug delivery methods, combination therapies, or personalized medicine approaches. This could involve the development of new guidance documents or the revision of existing regulatory pathways to address the unique challenges posed by muscimol-based pharmaceuticals.
Given the psychoactive properties of muscimol, these drugs are likely to be classified under the Controlled Substances Act, administered by the Drug Enforcement Administration (DEA). This classification imposes additional regulatory requirements on the production, distribution, and prescription of muscimol-based pharmaceuticals. The specific scheduling of muscimol-derived drugs would depend on their potential for abuse and recognized medical applications, which could impact their accessibility and use in clinical settings.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) and Japan's Pharmaceuticals and Medical Devices Agency (PMDA) have their own frameworks for evaluating and approving muscimol-based drugs. Harmonization efforts, such as those led by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), aim to streamline the regulatory processes across different regions, potentially facilitating global development and marketing of these medications.
The regulatory landscape also encompasses post-market surveillance requirements, which are crucial for monitoring the long-term safety and effectiveness of muscimol-based drugs. This includes mandatory reporting of adverse events and the potential for regulatory agencies to require additional studies or label changes based on emerging safety data.
Ethical considerations play a significant role in shaping the regulatory framework, particularly given the potential for muscimol-based drugs to affect cognitive function and behavior. Regulatory bodies may require special risk evaluation and mitigation strategies (REMS) to ensure appropriate prescribing and use of these medications.
As research into muscimol and its potential therapeutic applications continues to advance, regulatory frameworks may need to adapt to accommodate novel drug delivery methods, combination therapies, or personalized medicine approaches. This could involve the development of new guidance documents or the revision of existing regulatory pathways to address the unique challenges posed by muscimol-based pharmaceuticals.
Ethical Implications of Muscimol Research
The ethical implications of muscimol research are multifaceted and require careful consideration. As scientific understanding of muscimol's effects on neurological diseases advances, researchers must navigate complex ethical terrain.
One primary concern is the potential for misuse or abuse of muscimol-derived treatments. While therapeutic applications show promise, the psychoactive properties of muscimol could lead to recreational use or addiction if not properly regulated. Establishing strict protocols for administration and monitoring is crucial to prevent unintended consequences.
Patient safety and informed consent present additional ethical challenges. Given the potent effects of muscimol on brain chemistry, ensuring participants fully comprehend the risks involved in clinical trials is paramount. Researchers must strike a delicate balance between scientific progress and protecting vulnerable populations from exploitation or harm.
The long-term effects of muscimol-based interventions remain uncertain, raising questions about the ethics of using such treatments, particularly in chronic conditions. Weighing potential benefits against unknown risks requires ongoing ethical scrutiny and transparent communication with patients and the public.
Equitable access to muscimol-derived therapies is another critical ethical consideration. As research progresses, ensuring that any resulting treatments are available to all who need them, regardless of socioeconomic status, is essential. This may involve addressing issues of drug pricing, distribution, and healthcare infrastructure.
Privacy concerns also emerge in muscimol research, particularly regarding the collection and use of neurological data. Protecting patient information while advancing scientific understanding demands robust data security measures and clear guidelines on data sharing and ownership.
The environmental impact of increased muscimol production, should it become a widely used therapeutic agent, must be considered. Sustainable sourcing and manufacturing practices should be developed to minimize ecological disruption.
Lastly, the cultural and spiritual significance of muscimol-containing mushrooms in certain communities raises questions about respect for traditional knowledge and practices. Researchers must engage in dialogue with these communities to ensure their perspectives are honored throughout the scientific process.
One primary concern is the potential for misuse or abuse of muscimol-derived treatments. While therapeutic applications show promise, the psychoactive properties of muscimol could lead to recreational use or addiction if not properly regulated. Establishing strict protocols for administration and monitoring is crucial to prevent unintended consequences.
Patient safety and informed consent present additional ethical challenges. Given the potent effects of muscimol on brain chemistry, ensuring participants fully comprehend the risks involved in clinical trials is paramount. Researchers must strike a delicate balance between scientific progress and protecting vulnerable populations from exploitation or harm.
The long-term effects of muscimol-based interventions remain uncertain, raising questions about the ethics of using such treatments, particularly in chronic conditions. Weighing potential benefits against unknown risks requires ongoing ethical scrutiny and transparent communication with patients and the public.
Equitable access to muscimol-derived therapies is another critical ethical consideration. As research progresses, ensuring that any resulting treatments are available to all who need them, regardless of socioeconomic status, is essential. This may involve addressing issues of drug pricing, distribution, and healthcare infrastructure.
Privacy concerns also emerge in muscimol research, particularly regarding the collection and use of neurological data. Protecting patient information while advancing scientific understanding demands robust data security measures and clear guidelines on data sharing and ownership.
The environmental impact of increased muscimol production, should it become a widely used therapeutic agent, must be considered. Sustainable sourcing and manufacturing practices should be developed to minimize ecological disruption.
Lastly, the cultural and spiritual significance of muscimol-containing mushrooms in certain communities raises questions about respect for traditional knowledge and practices. Researchers must engage in dialogue with these communities to ensure their perspectives are honored throughout the scientific process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!