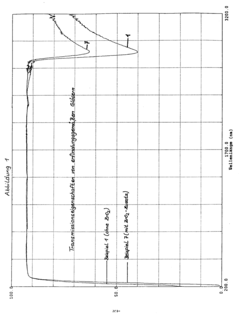
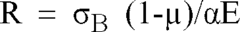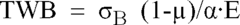Effect of Alkali Variations on Borosilicate Glass Conductivity
JUL 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Alkali-Borosilicate Glass Conductivity Background
Borosilicate glasses have been a subject of extensive research and industrial application for over a century. These glasses, characterized by their high content of boron oxide (B2O3) and silica (SiO2), were first developed in the late 19th century by German glassmaker Otto Schott. The addition of alkali oxides, such as sodium oxide (Na2O) or potassium oxide (K2O), to the borosilicate glass composition has been found to significantly influence its electrical conductivity properties.
The study of alkali variations in borosilicate glasses and their effect on conductivity has its roots in the broader field of glass science and technology. Early investigations in the mid-20th century focused on understanding the structural role of alkali ions in glass networks and their contribution to ionic conductivity. Researchers like Warren and Biscoe laid the groundwork for understanding the atomic structure of glasses, which proved crucial for later studies on conductivity mechanisms.
As the demand for specialized glass materials in various technological applications grew, so did the interest in tailoring the electrical properties of borosilicate glasses. The 1960s and 1970s saw a surge in research aimed at developing glasses with specific conductivity characteristics for applications in solid-state batteries, fuel cells, and other electrochemical devices. This period marked the beginning of systematic studies on the relationship between alkali content and ionic conductivity in borosilicate glasses.
The advent of advanced analytical techniques in the late 20th century, such as impedance spectroscopy and nuclear magnetic resonance (NMR) spectroscopy, enabled more detailed investigations into the ion transport mechanisms in these glasses. These tools allowed researchers to probe the local environment of alkali ions and their mobility within the glass network, providing crucial insights into the conductivity behavior of borosilicate glasses.
Recent years have witnessed a renewed interest in alkali-borosilicate glass conductivity, driven by emerging applications in energy storage, waste immobilization, and advanced sensing technologies. The focus has shifted towards understanding the complex interplay between glass composition, structure, and properties, with particular emphasis on how variations in alkali content and type affect the overall conductivity of the glass system.
Current research trends in this field include the exploration of mixed-alkali effects, the role of network modifiers in enhancing conductivity, and the development of predictive models for glass conductivity based on composition. Additionally, there is growing interest in the potential of nanostructured borosilicate glasses and glass-ceramics for achieving enhanced conductivity properties.
The study of alkali variations in borosilicate glasses and their effect on conductivity has its roots in the broader field of glass science and technology. Early investigations in the mid-20th century focused on understanding the structural role of alkali ions in glass networks and their contribution to ionic conductivity. Researchers like Warren and Biscoe laid the groundwork for understanding the atomic structure of glasses, which proved crucial for later studies on conductivity mechanisms.
As the demand for specialized glass materials in various technological applications grew, so did the interest in tailoring the electrical properties of borosilicate glasses. The 1960s and 1970s saw a surge in research aimed at developing glasses with specific conductivity characteristics for applications in solid-state batteries, fuel cells, and other electrochemical devices. This period marked the beginning of systematic studies on the relationship between alkali content and ionic conductivity in borosilicate glasses.
The advent of advanced analytical techniques in the late 20th century, such as impedance spectroscopy and nuclear magnetic resonance (NMR) spectroscopy, enabled more detailed investigations into the ion transport mechanisms in these glasses. These tools allowed researchers to probe the local environment of alkali ions and their mobility within the glass network, providing crucial insights into the conductivity behavior of borosilicate glasses.
Recent years have witnessed a renewed interest in alkali-borosilicate glass conductivity, driven by emerging applications in energy storage, waste immobilization, and advanced sensing technologies. The focus has shifted towards understanding the complex interplay between glass composition, structure, and properties, with particular emphasis on how variations in alkali content and type affect the overall conductivity of the glass system.
Current research trends in this field include the exploration of mixed-alkali effects, the role of network modifiers in enhancing conductivity, and the development of predictive models for glass conductivity based on composition. Additionally, there is growing interest in the potential of nanostructured borosilicate glasses and glass-ceramics for achieving enhanced conductivity properties.
Market Analysis for Conductive Glass Applications
The market for conductive glass applications has been experiencing significant growth in recent years, driven by the increasing demand for advanced electronic devices and smart technologies. Borosilicate glass, known for its excellent thermal and chemical resistance, has emerged as a key material in this sector, particularly when modified to enhance its electrical conductivity through alkali variations.
The global conductive glass market is primarily segmented into two main categories: indium tin oxide (ITO) coated glass and non-ITO coated glass. While ITO-coated glass has traditionally dominated the market, there is a growing interest in alternative conductive glass solutions, including those based on borosilicate glass with alkali modifications. This shift is partly due to the rising costs and supply constraints associated with indium.
The automotive industry represents a substantial market for conductive glass applications, particularly in the production of smart windshields and displays. The increasing adoption of electric vehicles and advanced driver assistance systems (ADAS) is expected to further boost demand in this sector. Additionally, the consumer electronics industry, including smartphones, tablets, and wearable devices, continues to be a major driver of market growth for conductive glass.
The building and construction sector is another area showing promising growth potential for conductive glass applications. Smart windows and energy-efficient building materials are gaining traction, creating new opportunities for conductive borosilicate glass products. The ability to control light transmission and heat transfer through electrically conductive glass is particularly attractive for sustainable building designs.
In the healthcare and life sciences industries, conductive glass is finding applications in advanced diagnostic equipment and laboratory instruments. The unique properties of borosilicate glass, combined with enhanced conductivity, make it suitable for various biomedical applications, including biosensors and microfluidic devices.
The Asia-Pacific region currently leads the global conductive glass market, with China and South Korea being major contributors to both production and consumption. However, North America and Europe are also significant markets, driven by technological advancements and research activities in these regions.
As the demand for more efficient and versatile conductive glass solutions grows, research into the effects of alkali variations on borosilicate glass conductivity becomes increasingly relevant. This area of study has the potential to unlock new applications and improve existing ones, potentially reshaping the market landscape for conductive glass products in the coming years.
The global conductive glass market is primarily segmented into two main categories: indium tin oxide (ITO) coated glass and non-ITO coated glass. While ITO-coated glass has traditionally dominated the market, there is a growing interest in alternative conductive glass solutions, including those based on borosilicate glass with alkali modifications. This shift is partly due to the rising costs and supply constraints associated with indium.
The automotive industry represents a substantial market for conductive glass applications, particularly in the production of smart windshields and displays. The increasing adoption of electric vehicles and advanced driver assistance systems (ADAS) is expected to further boost demand in this sector. Additionally, the consumer electronics industry, including smartphones, tablets, and wearable devices, continues to be a major driver of market growth for conductive glass.
The building and construction sector is another area showing promising growth potential for conductive glass applications. Smart windows and energy-efficient building materials are gaining traction, creating new opportunities for conductive borosilicate glass products. The ability to control light transmission and heat transfer through electrically conductive glass is particularly attractive for sustainable building designs.
In the healthcare and life sciences industries, conductive glass is finding applications in advanced diagnostic equipment and laboratory instruments. The unique properties of borosilicate glass, combined with enhanced conductivity, make it suitable for various biomedical applications, including biosensors and microfluidic devices.
The Asia-Pacific region currently leads the global conductive glass market, with China and South Korea being major contributors to both production and consumption. However, North America and Europe are also significant markets, driven by technological advancements and research activities in these regions.
As the demand for more efficient and versatile conductive glass solutions grows, research into the effects of alkali variations on borosilicate glass conductivity becomes increasingly relevant. This area of study has the potential to unlock new applications and improve existing ones, potentially reshaping the market landscape for conductive glass products in the coming years.
Current Challenges in Alkali-Borosilicate Glass Conductivity
The conductivity of alkali-borosilicate glasses presents several ongoing challenges in both research and industrial applications. One of the primary issues is the complex relationship between alkali content and electrical conductivity. As the concentration of alkali ions increases, the conductivity generally rises, but this relationship is not always linear and can be influenced by various factors.
The presence of multiple alkali species further complicates the conductivity behavior. Mixed alkali effects, where two or more alkali types are present, can lead to unexpected changes in conductivity. This phenomenon, known as the mixed alkali effect, often results in a non-additive contribution to conductivity, making it difficult to predict and control the electrical properties of multi-alkali borosilicate glasses.
Another significant challenge is the temperature dependence of conductivity in these glasses. The conductivity of alkali-borosilicate glasses typically follows an Arrhenius-type behavior, but deviations from this model are observed, especially at high temperatures or in glasses with high alkali content. Understanding and modeling these deviations remain an active area of research.
The structural role of alkali ions in the glass network also poses challenges. Alkali ions can act as network modifiers, creating non-bridging oxygens and altering the glass structure. This structural modification affects not only the conductivity but also other properties such as chemical durability and thermal expansion. Balancing these properties while optimizing conductivity is a complex task that requires careful composition design.
Ion exchange processes at the glass surface introduce additional complications. When exposed to external alkali sources, such as in electrochemical applications, the glass can undergo ion exchange, leading to changes in surface composition and conductivity over time. This dynamic behavior can affect the long-term stability and performance of devices utilizing these glasses.
The influence of glass processing conditions on conductivity is another area of concern. Factors such as melting temperature, cooling rate, and annealing conditions can significantly impact the final conductivity of the glass. Achieving consistent and reproducible conductivity values across different production batches remains a challenge in industrial settings.
Lastly, the development of accurate and reliable measurement techniques for characterizing the conductivity of alkali-borosilicate glasses, especially at high temperatures or under specific environmental conditions, continues to be an important area of research. Improving measurement precision and developing standardized testing protocols are crucial for advancing our understanding and control of conductivity in these materials.
The presence of multiple alkali species further complicates the conductivity behavior. Mixed alkali effects, where two or more alkali types are present, can lead to unexpected changes in conductivity. This phenomenon, known as the mixed alkali effect, often results in a non-additive contribution to conductivity, making it difficult to predict and control the electrical properties of multi-alkali borosilicate glasses.
Another significant challenge is the temperature dependence of conductivity in these glasses. The conductivity of alkali-borosilicate glasses typically follows an Arrhenius-type behavior, but deviations from this model are observed, especially at high temperatures or in glasses with high alkali content. Understanding and modeling these deviations remain an active area of research.
The structural role of alkali ions in the glass network also poses challenges. Alkali ions can act as network modifiers, creating non-bridging oxygens and altering the glass structure. This structural modification affects not only the conductivity but also other properties such as chemical durability and thermal expansion. Balancing these properties while optimizing conductivity is a complex task that requires careful composition design.
Ion exchange processes at the glass surface introduce additional complications. When exposed to external alkali sources, such as in electrochemical applications, the glass can undergo ion exchange, leading to changes in surface composition and conductivity over time. This dynamic behavior can affect the long-term stability and performance of devices utilizing these glasses.
The influence of glass processing conditions on conductivity is another area of concern. Factors such as melting temperature, cooling rate, and annealing conditions can significantly impact the final conductivity of the glass. Achieving consistent and reproducible conductivity values across different production batches remains a challenge in industrial settings.
Lastly, the development of accurate and reliable measurement techniques for characterizing the conductivity of alkali-borosilicate glasses, especially at high temperatures or under specific environmental conditions, continues to be an important area of research. Improving measurement precision and developing standardized testing protocols are crucial for advancing our understanding and control of conductivity in these materials.
Existing Methods for Alkali Variation in Borosilicate Glass
01 Composition of borosilicate glass for improved conductivity
Borosilicate glass compositions can be modified to enhance electrical conductivity. This typically involves incorporating specific additives or adjusting the ratio of components such as boron oxide, silica, and alkali metals. The modified composition allows for better ion mobility within the glass structure, resulting in improved electrical conductivity while maintaining the desirable properties of borosilicate glass.- Composition of borosilicate glass for improved conductivity: Borosilicate glass compositions can be modified to enhance electrical conductivity. This typically involves adding specific elements or compounds to the glass matrix, such as alkali metals or conductive oxides. The precise composition can be tailored to achieve desired conductivity levels while maintaining other essential properties of borosilicate glass.
- Surface treatment methods for increasing conductivity: Various surface treatment techniques can be applied to borosilicate glass to increase its conductivity. These may include ion implantation, coating with conductive materials, or chemical treatments that alter the surface structure. Such methods can create a conductive layer on the glass surface without significantly affecting its bulk properties.
- Nanostructure incorporation for enhanced conductivity: Incorporating nanostructures into borosilicate glass can significantly improve its conductivity. This may involve the addition of carbon nanotubes, graphene, or metallic nanoparticles during the glass formation process. These nanostructures can create conductive pathways within the glass matrix, enhancing overall electrical conductivity.
- Thermal treatment processes for conductivity modification: Specific thermal treatment processes can be employed to modify the conductivity of borosilicate glass. These may include controlled heating and cooling cycles, annealing, or tempering. Such treatments can alter the glass structure at a molecular level, potentially creating or enhancing conductive pathways within the material.
- Composite materials with borosilicate glass for tailored conductivity: Developing composite materials that combine borosilicate glass with other conductive components can yield materials with tailored conductivity. This approach allows for the retention of desirable properties of borosilicate glass while introducing controlled levels of electrical conductivity. The composite structure can be designed to meet specific application requirements.
02 Surface treatment methods for enhancing conductivity
Various surface treatment techniques can be applied to borosilicate glass to increase its electrical conductivity. These methods may include ion implantation, chemical vapor deposition, or the application of conductive coatings. Such treatments modify the surface properties of the glass, creating a conductive layer while preserving the bulk properties of the borosilicate material.Expand Specific Solutions03 Doping borosilicate glass with conductive materials
Introducing conductive dopants into the borosilicate glass matrix can significantly enhance its electrical conductivity. Common dopants include metal oxides, rare earth elements, or semiconductor materials. The doping process alters the electronic structure of the glass, creating charge carriers that facilitate electrical conduction while maintaining the thermal and chemical resistance of borosilicate glass.Expand Specific Solutions04 Nanostructure incorporation for conductivity enhancement
Integrating nanostructures such as carbon nanotubes, graphene, or metallic nanoparticles into borosilicate glass can dramatically improve its electrical conductivity. These nanostructures create conductive pathways within the glass matrix, allowing for efficient electron transport. The resulting composite material combines the conductivity of the nanostructures with the durability and chemical resistance of borosilicate glass.Expand Specific Solutions05 Heat treatment processes for conductivity modification
Specific heat treatment processes can be employed to alter the conductivity of borosilicate glass. These may include controlled cooling rates, annealing, or thermal cycling. Such treatments can induce structural changes in the glass, such as the formation of conductive crystalline phases or the redistribution of ions, leading to enhanced electrical conductivity without compromising the glass's fundamental properties.Expand Specific Solutions
Key Players in Conductive Glass Industry
The market for borosilicate glass conductivity research is in a growth phase, driven by increasing demand in various industries such as electronics, pharmaceuticals, and solar energy. The global market size for specialty glass, including borosilicate, is projected to reach significant figures in the coming years. Major players like SCHOTT AG, Corning, Inc., and AGC, Inc. are at the forefront of technological advancements in this field. These companies, along with emerging players such as Hunan Kibing Pharmaceutical Material Technology Co., Ltd., are investing heavily in R&D to improve glass conductivity and expand applications. The technology is maturing, with established companies refining existing products while new entrants focus on innovative solutions to meet evolving industry needs.
SCHOTT AG
Technical Solution: SCHOTT AG has developed advanced borosilicate glass compositions with optimized alkali content to enhance electrical conductivity. Their research focuses on tailoring the ratio of network formers (e.g., SiO2, B2O3) to network modifiers (e.g., Na2O, K2O) to achieve desired conductivity properties[1]. SCHOTT's approach involves precise control of alkali ion mobility within the glass structure, utilizing advanced melting and forming techniques to ensure homogeneous distribution of alkali species[2]. They have successfully created borosilicate glasses with ionic conductivities ranging from 10^-2 to 10^-6 S/cm at room temperature, suitable for various applications including solid-state batteries and smart windows[3].
Strengths: Extensive experience in glass manufacturing, strong R&D capabilities, and a wide range of tailored solutions. Weaknesses: Higher production costs due to specialized manufacturing processes and potential limitations in scaling up production for certain compositions.
Corning, Inc.
Technical Solution: Corning has developed a proprietary alkali-doped borosilicate glass system that exhibits enhanced ionic conductivity. Their approach involves carefully controlling the alkali content and distribution within the glass network to optimize ion transport pathways[4]. Corning's research has shown that by incorporating a combination of different alkali species (e.g., Li, Na, K) in specific ratios, they can achieve synergistic effects that enhance overall conductivity[5]. Their advanced manufacturing processes, including ion-exchange techniques, allow for the creation of graded alkali concentration profiles within the glass, further improving conductivity characteristics[6]. Corning's borosilicate glasses have demonstrated ionic conductivities up to 10^-3 S/cm at room temperature, making them suitable for applications in solid-state batteries and electrochromic devices.
Strengths: Strong patent portfolio, advanced manufacturing capabilities, and a history of successful commercialization of glass technologies. Weaknesses: Potential challenges in balancing conductivity improvements with other desirable glass properties such as chemical durability and thermal stability.
Innovations in Alkali-Borosilicate Glass Conductivity
Low boric acid borosilicate glass and its use
PatentInactiveEP0699636A1
Innovation
- Developing new borosilicate glass compositions with specific ratios of SiO2, B2O3, Al2O3, Li2O, Na2O, K2O, MgO, CaO, BaO, ZnO, ZrO2, and other oxides, ensuring high alkali resistance (LBK 1), low thermal expansion (4.0-5.3 x 10^-6 K^-1), and high UV transmission, while maintaining mechanical and thermal strength through chemical and thermal toughening.
Borosilicate glass compositions and uses thereof
PatentWO2004050575A1
Innovation
- A borosilicate glass composition with silicon dioxide, boric oxide, aluminum oxide, and at least one alkali oxide, which resists devitrification without the addition of inhibitor oxides, maintaining a suitable coefficient of thermal expansion and mechanical strength.
Environmental Impact of Alkali-Borosilicate Glass Production
The production of alkali-borosilicate glass has significant environmental implications that warrant careful consideration. The manufacturing process involves high-temperature melting of raw materials, including silica, boron oxide, and alkali metals, which consumes substantial energy and releases greenhouse gases. The primary environmental concerns stem from energy consumption, emissions, and resource extraction.
Energy-intensive production is a major contributor to the environmental footprint of alkali-borosilicate glass. Furnaces operate at temperatures exceeding 1500°C, requiring large amounts of fossil fuels or electricity. This energy demand leads to considerable CO2 emissions, contributing to climate change. Efforts to improve energy efficiency and transition to renewable energy sources are crucial for mitigating these impacts.
Emissions from the glass melting process include particulate matter, sulfur oxides, nitrogen oxides, and volatile organic compounds. These pollutants can affect air quality and human health in surrounding areas. Advanced emission control technologies, such as electrostatic precipitators and scrubbers, are essential for reducing these harmful emissions.
Raw material extraction for alkali-borosilicate glass production also poses environmental challenges. Mining operations for silica, boron, and alkali metals can lead to habitat destruction, soil erosion, and water pollution. Sustainable sourcing practices and increased use of recycled materials can help alleviate these impacts.
Water usage in the production process, particularly for cooling and cleaning, is another environmental concern. Proper water management systems and recycling techniques are necessary to minimize water consumption and prevent contamination of local water sources.
The disposal of waste materials generated during production, including off-spec glass and furnace residues, requires careful management to prevent soil and groundwater contamination. Implementing effective waste reduction strategies and exploring opportunities for byproduct utilization can significantly reduce the environmental burden.
On a positive note, the durability and chemical resistance of alkali-borosilicate glass contribute to its long lifespan and potential for recycling. This can offset some of the environmental impacts associated with production by reducing the need for frequent replacement and minimizing waste generation over time.
Addressing these environmental challenges requires a multifaceted approach, including technological innovations, process optimizations, and policy interventions. Continued research into more sustainable production methods, such as lower-temperature melting techniques and alternative raw materials, is essential for reducing the environmental footprint of alkali-borosilicate glass manufacturing.
Energy-intensive production is a major contributor to the environmental footprint of alkali-borosilicate glass. Furnaces operate at temperatures exceeding 1500°C, requiring large amounts of fossil fuels or electricity. This energy demand leads to considerable CO2 emissions, contributing to climate change. Efforts to improve energy efficiency and transition to renewable energy sources are crucial for mitigating these impacts.
Emissions from the glass melting process include particulate matter, sulfur oxides, nitrogen oxides, and volatile organic compounds. These pollutants can affect air quality and human health in surrounding areas. Advanced emission control technologies, such as electrostatic precipitators and scrubbers, are essential for reducing these harmful emissions.
Raw material extraction for alkali-borosilicate glass production also poses environmental challenges. Mining operations for silica, boron, and alkali metals can lead to habitat destruction, soil erosion, and water pollution. Sustainable sourcing practices and increased use of recycled materials can help alleviate these impacts.
Water usage in the production process, particularly for cooling and cleaning, is another environmental concern. Proper water management systems and recycling techniques are necessary to minimize water consumption and prevent contamination of local water sources.
The disposal of waste materials generated during production, including off-spec glass and furnace residues, requires careful management to prevent soil and groundwater contamination. Implementing effective waste reduction strategies and exploring opportunities for byproduct utilization can significantly reduce the environmental burden.
On a positive note, the durability and chemical resistance of alkali-borosilicate glass contribute to its long lifespan and potential for recycling. This can offset some of the environmental impacts associated with production by reducing the need for frequent replacement and minimizing waste generation over time.
Addressing these environmental challenges requires a multifaceted approach, including technological innovations, process optimizations, and policy interventions. Continued research into more sustainable production methods, such as lower-temperature melting techniques and alternative raw materials, is essential for reducing the environmental footprint of alkali-borosilicate glass manufacturing.
Standardization of Conductive Glass Testing Methods
The standardization of conductive glass testing methods is crucial for ensuring consistent and reliable measurements of borosilicate glass conductivity across different laboratories and research institutions. This standardization process involves establishing uniform procedures, equipment specifications, and data analysis techniques to accurately assess the effect of alkali variations on glass conductivity.
One of the primary challenges in standardizing testing methods is the wide range of variables that can influence conductivity measurements. These include sample preparation techniques, electrode materials and configurations, measurement frequencies, and environmental conditions such as temperature and humidity. To address these challenges, a comprehensive set of guidelines must be developed, outlining best practices for each stage of the testing process.
Sample preparation is a critical aspect of standardization. Uniform procedures for cutting, polishing, and cleaning glass samples must be established to minimize surface irregularities and contamination that could affect conductivity measurements. Additionally, standardized methods for applying electrodes to the glass surface, including specifications for electrode materials and geometries, are essential for ensuring consistent contact and reducing measurement errors.
The selection and calibration of measurement equipment play a vital role in standardization efforts. Impedance analyzers and potentiostats used for conductivity measurements should meet specific performance criteria and undergo regular calibration to maintain accuracy. Standardized protocols for equipment setup, including frequency ranges and voltage levels, must be defined to enable direct comparison of results across different laboratories.
Environmental control is another crucial factor in standardizing testing methods. Temperature and humidity can significantly impact glass conductivity, necessitating the use of climate-controlled chambers or precise environmental monitoring during measurements. Standardized procedures should specify acceptable ranges for these parameters and outline methods for compensating for environmental variations when necessary.
Data analysis and reporting techniques must also be standardized to ensure consistent interpretation of results. This includes defining methods for calculating conductivity from raw measurement data, specifying statistical analysis procedures for assessing measurement uncertainty, and establishing a standardized format for reporting results and experimental conditions.
Interlaboratory comparison studies are essential for validating and refining standardized testing methods. These studies involve multiple laboratories performing conductivity measurements on identical glass samples using the proposed standardized procedures. The results of these comparisons can highlight areas where further refinement is needed and help establish the reproducibility and reliability of the standardized methods.
One of the primary challenges in standardizing testing methods is the wide range of variables that can influence conductivity measurements. These include sample preparation techniques, electrode materials and configurations, measurement frequencies, and environmental conditions such as temperature and humidity. To address these challenges, a comprehensive set of guidelines must be developed, outlining best practices for each stage of the testing process.
Sample preparation is a critical aspect of standardization. Uniform procedures for cutting, polishing, and cleaning glass samples must be established to minimize surface irregularities and contamination that could affect conductivity measurements. Additionally, standardized methods for applying electrodes to the glass surface, including specifications for electrode materials and geometries, are essential for ensuring consistent contact and reducing measurement errors.
The selection and calibration of measurement equipment play a vital role in standardization efforts. Impedance analyzers and potentiostats used for conductivity measurements should meet specific performance criteria and undergo regular calibration to maintain accuracy. Standardized protocols for equipment setup, including frequency ranges and voltage levels, must be defined to enable direct comparison of results across different laboratories.
Environmental control is another crucial factor in standardizing testing methods. Temperature and humidity can significantly impact glass conductivity, necessitating the use of climate-controlled chambers or precise environmental monitoring during measurements. Standardized procedures should specify acceptable ranges for these parameters and outline methods for compensating for environmental variations when necessary.
Data analysis and reporting techniques must also be standardized to ensure consistent interpretation of results. This includes defining methods for calculating conductivity from raw measurement data, specifying statistical analysis procedures for assessing measurement uncertainty, and establishing a standardized format for reporting results and experimental conditions.
Interlaboratory comparison studies are essential for validating and refining standardized testing methods. These studies involve multiple laboratories performing conductivity measurements on identical glass samples using the proposed standardized procedures. The results of these comparisons can highlight areas where further refinement is needed and help establish the reproducibility and reliability of the standardized methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!