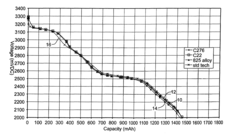
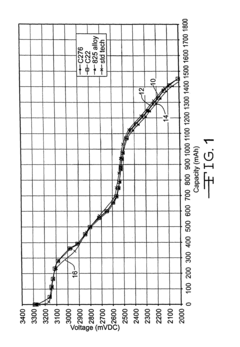
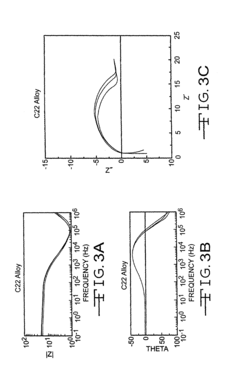
Electrochemical Corrosion Behavior of Nickel-Based Alloys
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nickel Alloy Corrosion Background and Objectives
Nickel-based alloys have emerged as critical materials in various high-performance applications due to their exceptional resistance to corrosion and oxidation, particularly in aggressive environments. The development of these alloys dates back to the early 20th century, with significant advancements occurring during the aerospace revolution of the 1950s and 1960s. Since then, the evolution of nickel-based alloys has been driven by increasing demands in industries such as aerospace, nuclear power generation, chemical processing, and marine engineering.
The electrochemical corrosion behavior of nickel-based alloys represents a complex interplay between material composition, microstructure, and environmental factors. Historical trends indicate a progressive improvement in corrosion resistance through careful alloying with elements such as chromium, molybdenum, and tungsten, which enhance passive film formation and stability. The addition of these elements has been systematically refined over decades to optimize performance in specific corrosive media.
Recent technological advancements have shifted focus toward understanding the nanoscale mechanisms governing corrosion initiation and propagation in nickel alloys. This microscopic approach has revealed critical insights into the role of grain boundaries, precipitates, and surface defects in determining corrosion susceptibility. The emergence of advanced characterization techniques, including in-situ electrochemical atomic force microscopy and synchrotron-based X-ray methods, has enabled unprecedented observation of corrosion processes in real-time.
The global trend toward more extreme operating conditions in energy production, chemical manufacturing, and aerospace applications has intensified research into nickel alloys capable of withstanding increasingly harsh environments. This includes exposure to hot corrosion in gas turbines, stress corrosion cracking in nuclear reactors, and localized corrosion in seawater applications. Each of these scenarios presents unique challenges that drive specialized alloy development.
The primary objectives of current research in this field include developing predictive models for long-term corrosion behavior, understanding the synergistic effects of mechanical stress and corrosion (corrosion fatigue), and creating novel surface modification techniques to enhance corrosion resistance. Additionally, there is growing interest in sustainable manufacturing processes for nickel alloys and in reducing dependence on critical raw materials through intelligent alloy design.
As industries continue to push operational boundaries, the development of next-generation nickel-based alloys with enhanced corrosion resistance remains a technological imperative. The ultimate goal is to establish comprehensive design principles that enable tailored alloy solutions for specific corrosive environments, thereby extending component lifetimes, improving safety margins, and reducing lifecycle costs across multiple industrial sectors.
The electrochemical corrosion behavior of nickel-based alloys represents a complex interplay between material composition, microstructure, and environmental factors. Historical trends indicate a progressive improvement in corrosion resistance through careful alloying with elements such as chromium, molybdenum, and tungsten, which enhance passive film formation and stability. The addition of these elements has been systematically refined over decades to optimize performance in specific corrosive media.
Recent technological advancements have shifted focus toward understanding the nanoscale mechanisms governing corrosion initiation and propagation in nickel alloys. This microscopic approach has revealed critical insights into the role of grain boundaries, precipitates, and surface defects in determining corrosion susceptibility. The emergence of advanced characterization techniques, including in-situ electrochemical atomic force microscopy and synchrotron-based X-ray methods, has enabled unprecedented observation of corrosion processes in real-time.
The global trend toward more extreme operating conditions in energy production, chemical manufacturing, and aerospace applications has intensified research into nickel alloys capable of withstanding increasingly harsh environments. This includes exposure to hot corrosion in gas turbines, stress corrosion cracking in nuclear reactors, and localized corrosion in seawater applications. Each of these scenarios presents unique challenges that drive specialized alloy development.
The primary objectives of current research in this field include developing predictive models for long-term corrosion behavior, understanding the synergistic effects of mechanical stress and corrosion (corrosion fatigue), and creating novel surface modification techniques to enhance corrosion resistance. Additionally, there is growing interest in sustainable manufacturing processes for nickel alloys and in reducing dependence on critical raw materials through intelligent alloy design.
As industries continue to push operational boundaries, the development of next-generation nickel-based alloys with enhanced corrosion resistance remains a technological imperative. The ultimate goal is to establish comprehensive design principles that enable tailored alloy solutions for specific corrosive environments, thereby extending component lifetimes, improving safety margins, and reducing lifecycle costs across multiple industrial sectors.
Market Demand Analysis for Corrosion-Resistant Alloys
The global market for corrosion-resistant alloys, particularly nickel-based alloys, has experienced significant growth driven by expanding applications in harsh environments across multiple industries. The oil and gas sector represents the largest market segment, with increasing deep-sea exploration and extraction activities requiring materials capable of withstanding extreme conditions including high pressure, temperature, and corrosive media. According to industry reports, this sector alone accounts for approximately 35% of the total nickel-based alloy market.
The chemical processing industry forms another substantial market segment, where equipment exposed to highly corrosive chemicals demands superior corrosion resistance. The growing emphasis on process efficiency and equipment longevity has accelerated the adoption of high-performance nickel alloys in reactors, heat exchangers, and piping systems. This sector has shown consistent annual growth rates between 4-6% over the past five years.
Power generation, particularly nuclear and advanced fossil fuel plants, represents a rapidly expanding market for nickel-based alloys. The push toward higher efficiency power cycles operating at elevated temperatures and pressures has increased demand for materials with exceptional corrosion resistance and mechanical stability. The transition to cleaner energy sources has further stimulated market growth in this segment.
Aerospace and marine applications constitute significant market opportunities, with requirements for materials that can withstand salt spray, galvanic corrosion, and oxidation at elevated temperatures. The aerospace industry's shift toward more electric aircraft and higher operating temperatures has specifically increased demand for specialized nickel alloys.
Regional analysis reveals that North America and Europe currently dominate the market for high-performance nickel alloys, though Asia-Pacific represents the fastest-growing region with expanding industrial bases in China and India. The market is projected to reach a value of $17.5 billion by 2027, with a compound annual growth rate of approximately 7.2%.
Key market drivers include increasingly stringent safety regulations across industries, growing awareness of lifecycle costs versus initial investment, and the expansion of industries operating in corrosive environments. The trend toward miniaturization and lightweighting in various applications has also created demand for alloys with improved strength-to-weight ratios while maintaining corrosion resistance.
Customer requirements are evolving toward alloys that offer multi-functional properties beyond corrosion resistance, including improved weldability, formability, and compatibility with advanced manufacturing techniques such as additive manufacturing. This presents both challenges and opportunities for materials developers and suppliers in the nickel-based alloy market.
The chemical processing industry forms another substantial market segment, where equipment exposed to highly corrosive chemicals demands superior corrosion resistance. The growing emphasis on process efficiency and equipment longevity has accelerated the adoption of high-performance nickel alloys in reactors, heat exchangers, and piping systems. This sector has shown consistent annual growth rates between 4-6% over the past five years.
Power generation, particularly nuclear and advanced fossil fuel plants, represents a rapidly expanding market for nickel-based alloys. The push toward higher efficiency power cycles operating at elevated temperatures and pressures has increased demand for materials with exceptional corrosion resistance and mechanical stability. The transition to cleaner energy sources has further stimulated market growth in this segment.
Aerospace and marine applications constitute significant market opportunities, with requirements for materials that can withstand salt spray, galvanic corrosion, and oxidation at elevated temperatures. The aerospace industry's shift toward more electric aircraft and higher operating temperatures has specifically increased demand for specialized nickel alloys.
Regional analysis reveals that North America and Europe currently dominate the market for high-performance nickel alloys, though Asia-Pacific represents the fastest-growing region with expanding industrial bases in China and India. The market is projected to reach a value of $17.5 billion by 2027, with a compound annual growth rate of approximately 7.2%.
Key market drivers include increasingly stringent safety regulations across industries, growing awareness of lifecycle costs versus initial investment, and the expansion of industries operating in corrosive environments. The trend toward miniaturization and lightweighting in various applications has also created demand for alloys with improved strength-to-weight ratios while maintaining corrosion resistance.
Customer requirements are evolving toward alloys that offer multi-functional properties beyond corrosion resistance, including improved weldability, formability, and compatibility with advanced manufacturing techniques such as additive manufacturing. This presents both challenges and opportunities for materials developers and suppliers in the nickel-based alloy market.
Current Status and Challenges in Electrochemical Corrosion Research
The field of electrochemical corrosion research for nickel-based alloys has witnessed significant advancements globally, yet continues to face substantial challenges. Current research predominantly focuses on understanding corrosion mechanisms in extreme environments, including high-temperature applications in aerospace, nuclear power generation, and chemical processing industries where these alloys are extensively utilized.
Recent studies have demonstrated that nickel-based alloys exhibit complex passivation behaviors, with the formation of protective oxide films significantly influenced by alloy composition and environmental factors. Research institutions across North America, Europe, and Asia have developed sophisticated in-situ monitoring techniques that allow real-time observation of corrosion processes at the nanoscale, providing unprecedented insights into degradation mechanisms.
Despite these advances, several critical challenges persist in the field. The synergistic effects of mechanical stress and electrochemical corrosion, known as stress corrosion cracking (SCC), remain incompletely understood, particularly in environments containing hydrogen sulfide or chlorides. This knowledge gap significantly impacts the reliability prediction of components in critical infrastructure.
Another major challenge involves accurately modeling long-term corrosion behavior. Current accelerated testing methodologies often fail to replicate the complex interactions occurring during decades of service, leading to discrepancies between laboratory results and field performance. This limitation hampers the development of truly predictive lifetime models for nickel-based alloy components.
The microstructural stability of these alloys during prolonged exposure to corrosive environments represents another frontier in research. Precipitation of secondary phases at grain boundaries can create micro-galvanic cells that accelerate localized corrosion, yet the kinetics of these transformations under varying electrochemical conditions remains poorly characterized.
From a geographical perspective, research leadership is distributed across multiple regions. Japan and Germany lead in high-temperature corrosion studies, while the United States maintains prominence in corrosion fatigue research. China has rapidly expanded its research capacity, particularly in computational modeling of corrosion processes.
Emerging research directions include the development of self-healing coating systems for nickel-based alloys and the application of machine learning algorithms to predict corrosion behavior based on environmental parameters and alloy composition. However, standardization of testing protocols and data reporting remains inconsistent across the field, hindering collaborative progress and technology transfer between research institutions and industry.
Recent studies have demonstrated that nickel-based alloys exhibit complex passivation behaviors, with the formation of protective oxide films significantly influenced by alloy composition and environmental factors. Research institutions across North America, Europe, and Asia have developed sophisticated in-situ monitoring techniques that allow real-time observation of corrosion processes at the nanoscale, providing unprecedented insights into degradation mechanisms.
Despite these advances, several critical challenges persist in the field. The synergistic effects of mechanical stress and electrochemical corrosion, known as stress corrosion cracking (SCC), remain incompletely understood, particularly in environments containing hydrogen sulfide or chlorides. This knowledge gap significantly impacts the reliability prediction of components in critical infrastructure.
Another major challenge involves accurately modeling long-term corrosion behavior. Current accelerated testing methodologies often fail to replicate the complex interactions occurring during decades of service, leading to discrepancies between laboratory results and field performance. This limitation hampers the development of truly predictive lifetime models for nickel-based alloy components.
The microstructural stability of these alloys during prolonged exposure to corrosive environments represents another frontier in research. Precipitation of secondary phases at grain boundaries can create micro-galvanic cells that accelerate localized corrosion, yet the kinetics of these transformations under varying electrochemical conditions remains poorly characterized.
From a geographical perspective, research leadership is distributed across multiple regions. Japan and Germany lead in high-temperature corrosion studies, while the United States maintains prominence in corrosion fatigue research. China has rapidly expanded its research capacity, particularly in computational modeling of corrosion processes.
Emerging research directions include the development of self-healing coating systems for nickel-based alloys and the application of machine learning algorithms to predict corrosion behavior based on environmental parameters and alloy composition. However, standardization of testing protocols and data reporting remains inconsistent across the field, hindering collaborative progress and technology transfer between research institutions and industry.
Current Electrochemical Corrosion Prevention Solutions
01 Composition of corrosion-resistant nickel-based alloys
Specific compositions of nickel-based alloys can be formulated to enhance corrosion resistance in various environments. These alloys typically contain chromium, molybdenum, and other elements in precise proportions to create a protective passive film on the surface. The addition of elements like tungsten, copper, and niobium can further improve resistance to specific types of corrosion such as pitting, crevice, and stress corrosion cracking in aggressive media.- Composition of corrosion-resistant nickel-based alloys: Specific compositions of nickel-based alloys can be formulated to enhance corrosion resistance in various electrochemical environments. These alloys typically contain chromium, molybdenum, and other elements in precise proportions to create a protective passive film on the surface. The addition of elements like tungsten, copper, and niobium can further improve resistance to specific types of corrosion such as pitting, crevice corrosion, and stress corrosion cracking in aggressive media.
- Surface treatments to improve corrosion behavior: Various surface treatments can be applied to nickel-based alloys to enhance their electrochemical corrosion resistance. These include passivation treatments, electrochemical polishing, and the application of protective coatings. Surface modification techniques alter the composition and structure of the alloy surface, creating a more stable oxide layer that provides improved barrier properties against corrosive environments. These treatments can significantly extend the service life of components in aggressive conditions.
- Electrochemical testing methods for nickel alloys: Specialized electrochemical testing methods are used to evaluate the corrosion behavior of nickel-based alloys. These include potentiodynamic polarization, electrochemical impedance spectroscopy, and cyclic voltammetry. These techniques provide insights into corrosion mechanisms, passive film stability, and long-term performance in specific environments. Testing under simulated service conditions helps predict the behavior of these alloys in real-world applications and guides material selection for critical components.
- Environmental factors affecting corrosion behavior: The electrochemical corrosion behavior of nickel-based alloys is significantly influenced by environmental factors such as temperature, pH, chloride concentration, and the presence of oxidizing species. Understanding these relationships is crucial for predicting performance in specific applications. Nickel alloys generally show excellent resistance in reducing environments but may be susceptible to localized corrosion in certain oxidizing conditions. The synergistic effects of multiple environmental factors must be considered when evaluating corrosion resistance.
- Microstructural effects on corrosion resistance: The microstructure of nickel-based alloys plays a critical role in determining their electrochemical corrosion behavior. Factors such as grain size, precipitate distribution, and phase composition significantly influence corrosion resistance. Heat treatments and processing methods can be optimized to develop microstructures that enhance corrosion performance. The presence of secondary phases, grain boundary characteristics, and crystallographic orientation can either improve corrosion resistance or create susceptible sites for preferential attack depending on the specific alloy system and environment.
02 Surface treatments for improved corrosion resistance
Various surface treatments can be applied to nickel-based alloys to enhance their electrochemical corrosion behavior. These include passivation treatments, electrochemical polishing, and the application of protective coatings. Such treatments modify the surface properties of the alloys, creating more stable oxide layers that provide better protection against corrosive environments and extend the service life of components made from these materials.Expand Specific Solutions03 Electrochemical testing methods for nickel alloys
Specialized electrochemical testing methods are used to evaluate the corrosion behavior of nickel-based alloys. These include potentiodynamic polarization, electrochemical impedance spectroscopy, and cyclic voltammetry. These techniques provide valuable data on corrosion rates, passivation behavior, and susceptibility to localized corrosion, allowing for the optimization of alloy compositions and processing methods to achieve desired corrosion resistance properties.Expand Specific Solutions04 Heat treatment effects on corrosion properties
Heat treatment processes significantly influence the electrochemical corrosion behavior of nickel-based alloys. Controlled heating and cooling cycles can optimize microstructure, reduce residual stresses, and enhance the formation of beneficial precipitates. Proper heat treatment can improve the uniformity of protective oxide layers and minimize susceptibility to intergranular corrosion, particularly in welded components or those exposed to high-temperature environments.Expand Specific Solutions05 Environmental factors affecting corrosion behavior
The electrochemical corrosion behavior of nickel-based alloys is significantly influenced by environmental factors such as temperature, pH, chloride concentration, and the presence of oxidizing species. Understanding these relationships helps in selecting appropriate alloy compositions for specific service conditions. Nickel alloys designed for particular environments, such as seawater, high-temperature steam, or acidic chemical processing, incorporate specific elements to counter the predominant corrosion mechanisms in those settings.Expand Specific Solutions
Major Industry Players in Nickel Alloy Development
The electrochemical corrosion behavior of nickel-based alloys market is currently in a growth phase, driven by increasing demand in aerospace, nuclear, and chemical processing industries. The global market size is estimated at approximately $4-5 billion, with projected annual growth of 5-7% through 2027. Technologically, the field has reached moderate maturity but continues to evolve with specialized applications. Leading players include VDM Metals GmbH, which dominates the European market with advanced nickel alloy development; Mitsubishi Materials and Toshiba in Asia focusing on nuclear applications; and ATI and Alleima offering specialized corrosion-resistant solutions. Academic institutions like University of Science & Technology Beijing and Harbin Institute of Technology are advancing fundamental research, while companies like Framatome and Safran drive innovation in extreme environment applications.
VDM METALS GMBH
Technical Solution: VDM Metals has developed proprietary nickel-based alloys specifically engineered to resist electrochemical corrosion in aggressive environments. Their flagship Alloy 625 and Alloy 59 incorporate precise amounts of chromium (20-23%), molybdenum (8-16%), and niobium to form stable passive films that significantly enhance corrosion resistance. VDM's research has demonstrated that their alloys maintain structural integrity in chloride-containing environments at temperatures exceeding 300°C, with corrosion rates below 0.1 mm/year. Their manufacturing process includes solution annealing treatments (1040-1150°C) followed by rapid cooling to prevent sensitization and optimize microstructure for maximum corrosion resistance. VDM has also pioneered electrochemical testing protocols using potentiodynamic polarization and electrochemical impedance spectroscopy to characterize pitting potential and repassivation behavior in various electrolytes, enabling tailored alloy selection for specific industrial applications.
Strengths: Superior resistance to localized corrosion in chloride environments; excellent performance in both oxidizing and reducing conditions; maintains mechanical properties at elevated temperatures. Weaknesses: Higher cost compared to standard stainless steels; limited availability in certain product forms; requires specialized welding procedures to maintain corrosion resistance in heat-affected zones.
Mitsubishi Materials Corp.
Technical Solution: Mitsubishi Materials has pioneered innovative nickel-based alloy systems specifically designed to combat electrochemical corrosion in extreme environments. Their research has focused on developing alloys with optimized chromium, molybdenum, and tungsten content to enhance passive film stability across a wide pH range. Mitsubishi's proprietary MC-Alloy series incorporates nano-dispersed oxide particles that act as anchoring sites for the protective oxide layer, significantly improving resistance to film breakdown in chloride-containing environments. Their manufacturing process employs advanced vacuum melting techniques followed by precise thermomechanical processing to control grain size and distribution of strengthening phases without compromising corrosion resistance. Electrochemical testing has demonstrated that their alloys maintain passive behavior at potentials up to 1.2V vs. SCE in 3.5% NaCl solution at 80°C, with repassivation occurring within seconds after mechanical damage to the surface. Mitsubishi has also developed specialized surface treatment protocols that enhance the chromium enrichment in the passive layer, further improving resistance to localized corrosion phenomena.
Strengths: Exceptional resistance to crevice corrosion in seawater environments; excellent performance in high-temperature oxidizing acids; maintains passive behavior across wide potential ranges. Weaknesses: Higher manufacturing costs due to complex processing requirements; limited global availability compared to more established alloy systems; requires specialized joining techniques to maintain corrosion resistance in fabricated structures.
Key Technical Innovations in Corrosion Resistance
Nickel-based alloys as positive electrode support materials in electrochemical cells containing nonaqueous electrolytes
PatentInactiveUS7465521B2
Innovation
- Nickel-based alloys with specific compositions, including at least 23% nickel, 5% molybdenum, and 20% chromium, provide high corrosion resistance and maintain interfacial conductivity, suitable for use in demanding environments and capable of being fabricated in thin forms for increased energy density and surface area.
Environmental Impact of Nickel Alloy Corrosion Products
The corrosion of nickel-based alloys in various environments results in the release of metal ions and corrosion products that can have significant environmental implications. These environmental impacts extend beyond the immediate degradation of the materials themselves to affect surrounding ecosystems, water quality, and potentially human health through various exposure pathways.
When nickel alloys corrode, they release nickel ions (Ni²⁺) and other metallic components such as chromium, molybdenum, and iron into the environment. These ions can persist in soil and water systems, with mobility dependent on environmental conditions including pH, redox potential, and the presence of complexing agents. In aquatic environments, nickel ions can be particularly problematic as they may bioaccumulate in certain organisms and disrupt aquatic ecosystems.
The toxicity of nickel corrosion products varies significantly based on their chemical form. Soluble nickel compounds generally pose greater environmental risks than insoluble forms due to their higher bioavailability. Studies have shown that elevated concentrations of nickel in water bodies can adversely affect fish populations, invertebrates, and algal communities, potentially disrupting food chains and reducing biodiversity in affected areas.
In industrial settings where nickel alloys are extensively used, such as chemical processing facilities and power plants, the management of corrosion products becomes a critical environmental consideration. Cooling water systems utilizing nickel alloys can become vectors for distributing corrosion products into natural water bodies if not properly monitored and treated. This has led to increasingly stringent environmental regulations governing the discharge of metals from industrial operations.
The environmental fate of nickel corrosion products is influenced by various geochemical processes including adsorption, precipitation, and complexation. In soil environments, nickel ions can bind to organic matter and clay minerals, affecting their mobility and bioavailability. The transformation of these corrosion products under different environmental conditions remains an active area of research, particularly regarding long-term environmental persistence.
Climate change factors, including increasing ocean acidification and changing precipitation patterns, may exacerbate the environmental impacts of nickel alloy corrosion. Lower pH values in marine environments can accelerate corrosion rates and increase the release of nickel ions, potentially affecting marine ecosystems that are already under stress from other anthropogenic pressures.
Mitigation strategies to reduce the environmental impact of nickel alloy corrosion products include the development of more corrosion-resistant alloys, improved corrosion inhibitors with lower environmental toxicity, and advanced treatment technologies for metal-containing wastewaters. Life cycle assessment approaches are increasingly being applied to evaluate the total environmental footprint of nickel alloys, considering not only their performance during use but also the environmental implications of their eventual degradation.
When nickel alloys corrode, they release nickel ions (Ni²⁺) and other metallic components such as chromium, molybdenum, and iron into the environment. These ions can persist in soil and water systems, with mobility dependent on environmental conditions including pH, redox potential, and the presence of complexing agents. In aquatic environments, nickel ions can be particularly problematic as they may bioaccumulate in certain organisms and disrupt aquatic ecosystems.
The toxicity of nickel corrosion products varies significantly based on their chemical form. Soluble nickel compounds generally pose greater environmental risks than insoluble forms due to their higher bioavailability. Studies have shown that elevated concentrations of nickel in water bodies can adversely affect fish populations, invertebrates, and algal communities, potentially disrupting food chains and reducing biodiversity in affected areas.
In industrial settings where nickel alloys are extensively used, such as chemical processing facilities and power plants, the management of corrosion products becomes a critical environmental consideration. Cooling water systems utilizing nickel alloys can become vectors for distributing corrosion products into natural water bodies if not properly monitored and treated. This has led to increasingly stringent environmental regulations governing the discharge of metals from industrial operations.
The environmental fate of nickel corrosion products is influenced by various geochemical processes including adsorption, precipitation, and complexation. In soil environments, nickel ions can bind to organic matter and clay minerals, affecting their mobility and bioavailability. The transformation of these corrosion products under different environmental conditions remains an active area of research, particularly regarding long-term environmental persistence.
Climate change factors, including increasing ocean acidification and changing precipitation patterns, may exacerbate the environmental impacts of nickel alloy corrosion. Lower pH values in marine environments can accelerate corrosion rates and increase the release of nickel ions, potentially affecting marine ecosystems that are already under stress from other anthropogenic pressures.
Mitigation strategies to reduce the environmental impact of nickel alloy corrosion products include the development of more corrosion-resistant alloys, improved corrosion inhibitors with lower environmental toxicity, and advanced treatment technologies for metal-containing wastewaters. Life cycle assessment approaches are increasingly being applied to evaluate the total environmental footprint of nickel alloys, considering not only their performance during use but also the environmental implications of their eventual degradation.
Standardization and Testing Protocols for Corrosion Behavior
The standardization of testing protocols for nickel-based alloys' electrochemical corrosion behavior is crucial for ensuring reliable and comparable results across different research institutions and industries. Currently, several international organizations have established standardized methods, with ASTM International, NACE International (now AMPP), and ISO leading these efforts. These standards provide detailed guidelines for specimen preparation, test environment control, electrochemical measurement techniques, and data analysis.
ASTM G5 and G61 are widely recognized standards for potentiodynamic polarization measurements, while ASTM G102 offers guidelines for electrochemical measurements in corrosion testing. These protocols specify critical parameters such as scan rates, potential ranges, and electrolyte compositions that significantly influence test outcomes. For nickel-based alloys specifically, ASTM G28 addresses testing methods for detecting susceptibility to intergranular attack.
Temperature control represents a critical aspect of standardized testing, as corrosion behavior of nickel-based alloys varies dramatically with temperature changes. Most protocols require temperature control within ±1°C to ensure reproducibility. Similarly, solution chemistry standardization is essential, with precise specifications for pH, dissolved oxygen content, and impurity levels.
Recent advancements in testing methodologies have introduced electrochemical impedance spectroscopy (EIS) as a powerful non-destructive technique for evaluating corrosion behavior. Standards such as ASTM B117 and G85 have been updated to incorporate these modern approaches, though complete standardization of EIS for nickel-based alloys remains under development.
A significant challenge in standardization efforts is addressing the diverse environmental conditions that nickel-based alloys encounter in service. Industry-specific standards have emerged to bridge this gap, with NACE MR0175/ISO 15156 focusing on oil and gas applications, while ASTM F2129 addresses biomedical implant materials. These specialized protocols consider unique environmental factors relevant to specific applications.
Data reporting and interpretation frameworks have also been standardized to ensure consistency across different laboratories. These frameworks specify minimum reporting requirements, including material composition, microstructure characterization, surface preparation methods, and complete electrochemical parameters used during testing. This comprehensive approach enables meaningful comparison of results from different sources.
Despite these advances, gaps remain in standardization for accelerated testing methods that reliably predict long-term corrosion behavior. Current research focuses on developing protocols that correlate short-term electrochemical measurements with long-term performance, particularly for emerging nickel-based superalloys designed for extreme environments.
ASTM G5 and G61 are widely recognized standards for potentiodynamic polarization measurements, while ASTM G102 offers guidelines for electrochemical measurements in corrosion testing. These protocols specify critical parameters such as scan rates, potential ranges, and electrolyte compositions that significantly influence test outcomes. For nickel-based alloys specifically, ASTM G28 addresses testing methods for detecting susceptibility to intergranular attack.
Temperature control represents a critical aspect of standardized testing, as corrosion behavior of nickel-based alloys varies dramatically with temperature changes. Most protocols require temperature control within ±1°C to ensure reproducibility. Similarly, solution chemistry standardization is essential, with precise specifications for pH, dissolved oxygen content, and impurity levels.
Recent advancements in testing methodologies have introduced electrochemical impedance spectroscopy (EIS) as a powerful non-destructive technique for evaluating corrosion behavior. Standards such as ASTM B117 and G85 have been updated to incorporate these modern approaches, though complete standardization of EIS for nickel-based alloys remains under development.
A significant challenge in standardization efforts is addressing the diverse environmental conditions that nickel-based alloys encounter in service. Industry-specific standards have emerged to bridge this gap, with NACE MR0175/ISO 15156 focusing on oil and gas applications, while ASTM F2129 addresses biomedical implant materials. These specialized protocols consider unique environmental factors relevant to specific applications.
Data reporting and interpretation frameworks have also been standardized to ensure consistency across different laboratories. These frameworks specify minimum reporting requirements, including material composition, microstructure characterization, surface preparation methods, and complete electrochemical parameters used during testing. This comprehensive approach enables meaningful comparison of results from different sources.
Despite these advances, gaps remain in standardization for accelerated testing methods that reliably predict long-term corrosion behavior. Current research focuses on developing protocols that correlate short-term electrochemical measurements with long-term performance, particularly for emerging nickel-based superalloys designed for extreme environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!