Electrochemical Impedance Analysis of Corrosion-Resistant Alloys
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Corrosion-Resistant Alloys Technology Background and Objectives
Corrosion-resistant alloys have evolved significantly over the past century, with major technological breakthroughs occurring during the industrial revolution and accelerating through the 20th century. Initially developed for marine applications, these alloys have expanded into critical sectors including aerospace, chemical processing, oil and gas, nuclear power, and biomedical implants. The fundamental challenge these materials address is the estimated $2.5 trillion annual global cost of corrosion, representing approximately 3-4% of global GDP according to NACE International studies.
Electrochemical Impedance Spectroscopy (EIS) emerged in the 1970s as a powerful non-destructive technique for analyzing corrosion processes. This analytical method has transformed our understanding of corrosion mechanisms by providing detailed insights into the electrochemical reactions occurring at metal-environment interfaces. The technique measures the impedance response of a corroding system over a range of frequencies, revealing information about reaction kinetics, diffusion processes, and protective film properties that traditional DC techniques cannot capture.
Recent technological advancements have significantly enhanced EIS capabilities, including miniaturization of equipment, improved data processing algorithms, and integration with other analytical techniques. The development of portable EIS systems has enabled in-situ monitoring in field conditions, while machine learning approaches have improved data interpretation, particularly for complex alloy systems with multiple phases and interfaces.
The primary objective of current research in this field is to establish standardized EIS protocols for different alloy systems and corrosive environments, enabling more reliable comparison of results across different studies. Additionally, researchers aim to develop predictive models that can translate EIS data into accurate service life predictions for critical infrastructure components, potentially saving billions in maintenance and replacement costs.
Another key goal is to utilize EIS as a design tool for next-generation corrosion-resistant alloys, particularly for extreme environments such as deep-sea applications, high-temperature energy systems, and space exploration. By understanding the precise electrochemical mechanisms of corrosion resistance, metallurgists can develop tailored alloy compositions with optimized microstructures.
The integration of EIS with in-situ monitoring systems represents a significant technological frontier, with the potential to transform asset integrity management through real-time corrosion monitoring. This would enable condition-based maintenance strategies rather than time-based approaches, substantially reducing downtime and extending asset lifespans in critical infrastructure applications.
Electrochemical Impedance Spectroscopy (EIS) emerged in the 1970s as a powerful non-destructive technique for analyzing corrosion processes. This analytical method has transformed our understanding of corrosion mechanisms by providing detailed insights into the electrochemical reactions occurring at metal-environment interfaces. The technique measures the impedance response of a corroding system over a range of frequencies, revealing information about reaction kinetics, diffusion processes, and protective film properties that traditional DC techniques cannot capture.
Recent technological advancements have significantly enhanced EIS capabilities, including miniaturization of equipment, improved data processing algorithms, and integration with other analytical techniques. The development of portable EIS systems has enabled in-situ monitoring in field conditions, while machine learning approaches have improved data interpretation, particularly for complex alloy systems with multiple phases and interfaces.
The primary objective of current research in this field is to establish standardized EIS protocols for different alloy systems and corrosive environments, enabling more reliable comparison of results across different studies. Additionally, researchers aim to develop predictive models that can translate EIS data into accurate service life predictions for critical infrastructure components, potentially saving billions in maintenance and replacement costs.
Another key goal is to utilize EIS as a design tool for next-generation corrosion-resistant alloys, particularly for extreme environments such as deep-sea applications, high-temperature energy systems, and space exploration. By understanding the precise electrochemical mechanisms of corrosion resistance, metallurgists can develop tailored alloy compositions with optimized microstructures.
The integration of EIS with in-situ monitoring systems represents a significant technological frontier, with the potential to transform asset integrity management through real-time corrosion monitoring. This would enable condition-based maintenance strategies rather than time-based approaches, substantially reducing downtime and extending asset lifespans in critical infrastructure applications.
Market Demand Analysis for Advanced Corrosion Protection
The global market for advanced corrosion protection technologies has experienced significant growth in recent years, driven primarily by increasing industrial infrastructure investments and the rising costs associated with corrosion-related failures. The electrochemical impedance analysis of corrosion-resistant alloys represents a critical segment within this market, as industries seek more sophisticated methods to evaluate and enhance material performance in corrosive environments.
Current market assessments value the global corrosion protection market at approximately USD 66.5 billion, with projections indicating growth to reach USD 89.6 billion by 2026, representing a compound annual growth rate of 6.2%. Within this broader market, the segment specifically focused on corrosion-resistant alloys and their analysis technologies accounts for roughly 18% of the total market share, demonstrating the significant economic importance of this technical domain.
The oil and gas industry remains the largest consumer of advanced corrosion protection solutions, accounting for nearly 32% of market demand. This sector's operations in increasingly challenging environments—deep-sea drilling, high-pressure high-temperature wells, and exposure to highly corrosive substances—have intensified the need for more sophisticated alloys and corresponding analytical techniques. The chemical processing industry follows closely, representing approximately 24% of market demand, where process efficiency and safety considerations drive investment in superior corrosion resistance technologies.
Maritime and offshore applications constitute another significant market segment, with particular emphasis on solutions that can withstand the aggressive combination of saltwater exposure and mechanical stress. This sector has shown the fastest growth rate at 7.8% annually, reflecting the expansion of offshore energy installations and global shipping activities.
Geographically, North America and Europe currently dominate market consumption, collectively accounting for 58% of global demand. However, the Asia-Pacific region is demonstrating the most rapid market expansion, with China and India leading regional growth at rates exceeding 9% annually. This shift reflects the massive industrial development and infrastructure investments occurring throughout the region.
Market analysis reveals a growing preference for predictive and real-time monitoring solutions over traditional periodic inspection methodologies. This trend has accelerated demand for electrochemical impedance spectroscopy (EIS) and related analytical techniques that enable continuous assessment of material performance in operational environments. End-users increasingly prioritize total lifecycle cost reduction over initial investment considerations, creating market opportunities for premium solutions that offer superior long-term performance and reliability.
Current market assessments value the global corrosion protection market at approximately USD 66.5 billion, with projections indicating growth to reach USD 89.6 billion by 2026, representing a compound annual growth rate of 6.2%. Within this broader market, the segment specifically focused on corrosion-resistant alloys and their analysis technologies accounts for roughly 18% of the total market share, demonstrating the significant economic importance of this technical domain.
The oil and gas industry remains the largest consumer of advanced corrosion protection solutions, accounting for nearly 32% of market demand. This sector's operations in increasingly challenging environments—deep-sea drilling, high-pressure high-temperature wells, and exposure to highly corrosive substances—have intensified the need for more sophisticated alloys and corresponding analytical techniques. The chemical processing industry follows closely, representing approximately 24% of market demand, where process efficiency and safety considerations drive investment in superior corrosion resistance technologies.
Maritime and offshore applications constitute another significant market segment, with particular emphasis on solutions that can withstand the aggressive combination of saltwater exposure and mechanical stress. This sector has shown the fastest growth rate at 7.8% annually, reflecting the expansion of offshore energy installations and global shipping activities.
Geographically, North America and Europe currently dominate market consumption, collectively accounting for 58% of global demand. However, the Asia-Pacific region is demonstrating the most rapid market expansion, with China and India leading regional growth at rates exceeding 9% annually. This shift reflects the massive industrial development and infrastructure investments occurring throughout the region.
Market analysis reveals a growing preference for predictive and real-time monitoring solutions over traditional periodic inspection methodologies. This trend has accelerated demand for electrochemical impedance spectroscopy (EIS) and related analytical techniques that enable continuous assessment of material performance in operational environments. End-users increasingly prioritize total lifecycle cost reduction over initial investment considerations, creating market opportunities for premium solutions that offer superior long-term performance and reliability.
Current Status and Challenges in Electrochemical Impedance Analysis
Electrochemical Impedance Spectroscopy (EIS) has emerged as a powerful non-destructive technique for analyzing corrosion behavior of alloys, with significant advancements in recent decades. Currently, the technique allows for detailed characterization of electrode-electrolyte interfaces, providing insights into corrosion mechanisms, passive film properties, and degradation processes of corrosion-resistant alloys. Modern EIS systems can detect impedance changes across a wide frequency range (typically 10^-3 to 10^6 Hz), enabling comprehensive analysis of both fast and slow electrochemical processes.
Despite these capabilities, several technical challenges persist in the field. Data interpretation remains complex, often requiring sophisticated equivalent circuit models that may not uniquely represent the physical system. This ambiguity in data interpretation can lead to inconsistent conclusions among researchers analyzing identical systems. Additionally, the technique struggles with spatially heterogeneous surfaces common in practical corrosion scenarios, as traditional EIS provides averaged responses across the entire electrode surface.
Globally, research efforts are distributed across major industrial nations, with notable contributions from research institutions in the United States, Germany, China, Japan, and South Korea. European research centers have particularly focused on developing advanced modeling approaches, while Asian institutions have made significant progress in instrumentation development and miniaturization.
A significant limitation in current EIS applications is the difficulty in performing real-time monitoring under dynamic conditions. Most systems require relatively stable conditions during measurement, limiting applicability in rapidly changing environments typical of industrial settings. Furthermore, the correlation between laboratory EIS measurements and field performance remains challenging, with environmental variables often causing discrepancies between predicted and actual corrosion behavior.
Recent technological constraints include the need for improved spatial resolution, with localized EIS techniques still in early development stages. The integration of EIS with other analytical methods (such as surface analysis techniques) presents both opportunities and challenges, as data fusion methodologies are not yet standardized. Additionally, the field faces challenges in developing reliable reference electrodes for extreme environments, particularly high-temperature or highly corrosive media.
For corrosion-resistant alloys specifically, a key challenge lies in distinguishing between different degradation mechanisms that may occur simultaneously, such as pitting initiation, crevice corrosion, and stress corrosion cracking. Current EIS methodologies often struggle to deconvolute these overlapping processes, particularly in their early stages when intervention would be most effective.
Despite these capabilities, several technical challenges persist in the field. Data interpretation remains complex, often requiring sophisticated equivalent circuit models that may not uniquely represent the physical system. This ambiguity in data interpretation can lead to inconsistent conclusions among researchers analyzing identical systems. Additionally, the technique struggles with spatially heterogeneous surfaces common in practical corrosion scenarios, as traditional EIS provides averaged responses across the entire electrode surface.
Globally, research efforts are distributed across major industrial nations, with notable contributions from research institutions in the United States, Germany, China, Japan, and South Korea. European research centers have particularly focused on developing advanced modeling approaches, while Asian institutions have made significant progress in instrumentation development and miniaturization.
A significant limitation in current EIS applications is the difficulty in performing real-time monitoring under dynamic conditions. Most systems require relatively stable conditions during measurement, limiting applicability in rapidly changing environments typical of industrial settings. Furthermore, the correlation between laboratory EIS measurements and field performance remains challenging, with environmental variables often causing discrepancies between predicted and actual corrosion behavior.
Recent technological constraints include the need for improved spatial resolution, with localized EIS techniques still in early development stages. The integration of EIS with other analytical methods (such as surface analysis techniques) presents both opportunities and challenges, as data fusion methodologies are not yet standardized. Additionally, the field faces challenges in developing reliable reference electrodes for extreme environments, particularly high-temperature or highly corrosive media.
For corrosion-resistant alloys specifically, a key challenge lies in distinguishing between different degradation mechanisms that may occur simultaneously, such as pitting initiation, crevice corrosion, and stress corrosion cracking. Current EIS methodologies often struggle to deconvolute these overlapping processes, particularly in their early stages when intervention would be most effective.
Current Electrochemical Impedance Spectroscopy Methods
01 Electrochemical impedance spectroscopy for corrosion evaluation
Electrochemical impedance spectroscopy (EIS) is used to evaluate the corrosion resistance of various alloys. This technique measures the impedance of a material at different frequencies, providing information about corrosion mechanisms, protective film formation, and degradation processes. EIS allows for non-destructive testing and can detect early signs of corrosion before visible damage occurs, making it valuable for monitoring the performance of corrosion-resistant alloys in various environments.- Electrochemical impedance spectroscopy for corrosion evaluation: Electrochemical impedance spectroscopy (EIS) is used to evaluate the corrosion resistance of various alloys. This technique measures the impedance of a material at different frequencies, providing information about corrosion mechanisms, protective film formation, and degradation processes. EIS allows for non-destructive testing and can detect early stages of corrosion before visible damage occurs, making it valuable for monitoring the performance of corrosion-resistant alloys in various environments.
- Nickel-based corrosion-resistant alloys: Nickel-based alloys demonstrate superior corrosion resistance in aggressive environments due to their ability to form stable passive films. These alloys often contain chromium, molybdenum, and other elements that enhance their resistance to localized corrosion. Electrochemical impedance measurements show that these alloys exhibit high polarization resistance and low corrosion rates in acidic, alkaline, and chloride-containing environments. Their performance makes them suitable for applications in chemical processing, marine environments, and oil and gas industries.
- Surface treatments and coatings for improved corrosion resistance: Various surface treatments and coating technologies can significantly enhance the corrosion resistance of alloys. These include passivation treatments, conversion coatings, electroplating, and advanced multi-layer coating systems. Electrochemical impedance measurements demonstrate that properly applied surface treatments can increase the charge transfer resistance and decrease the corrosion current density. These treatments modify the surface properties of the alloy, creating barriers against corrosive media and improving the overall durability of the material.
- Stainless steel alloy compositions for corrosion resistance: Specific compositions of stainless steel alloys are developed to provide enhanced corrosion resistance in particular environments. The balance of chromium, nickel, molybdenum, and other alloying elements is critical for forming stable passive films that protect against corrosion. Electrochemical impedance studies reveal that higher chromium and molybdenum content generally leads to better corrosion resistance, particularly in chloride-containing environments. These specialized stainless steel compositions find applications in marine structures, chemical processing equipment, and biomedical implants.
- Corrosion monitoring and testing methodologies: Advanced methodologies for monitoring and testing corrosion resistance of alloys include various electrochemical techniques beyond basic impedance spectroscopy. These include potentiodynamic polarization, cyclic voltammetry, and electrochemical noise analysis. These methods provide complementary information about corrosion mechanisms, pitting susceptibility, and long-term performance of alloys. Standardized testing protocols ensure reliable comparison between different alloy compositions and treatments, enabling more accurate prediction of service life in corrosive environments.
02 Nickel-based corrosion-resistant alloys
Nickel-based alloys demonstrate superior corrosion resistance in aggressive environments due to their ability to form stable passive films. These alloys often contain chromium, molybdenum, and other elements that enhance their resistance to pitting, crevice corrosion, and stress corrosion cracking. Electrochemical impedance measurements show that these alloys maintain high impedance values even after extended exposure to corrosive media, indicating excellent protective properties and stability in harsh conditions such as acidic, high-temperature, or chloride-containing environments.Expand Specific Solutions03 Surface treatments to enhance corrosion resistance
Various surface treatments can significantly improve the electrochemical impedance and corrosion resistance of alloys. These treatments include passivation, nitriding, carburizing, and the application of protective coatings. Electrochemical impedance measurements reveal that treated surfaces exhibit higher charge transfer resistance and lower capacitance, indicating enhanced barrier properties against corrosive species. The effectiveness of these treatments can be monitored over time using impedance spectroscopy to assess long-term protection capabilities.Expand Specific Solutions04 Aluminum and titanium alloys with enhanced corrosion resistance
Aluminum and titanium alloys are engineered for corrosion resistance through careful composition control and processing techniques. These lightweight alloys form protective oxide layers that provide natural corrosion resistance, which can be further enhanced through alloying with elements like copper, magnesium, vanadium, or zirconium. Electrochemical impedance studies demonstrate that these alloys maintain high impedance values in various environments, making them suitable for aerospace, marine, and chemical processing applications where both weight reduction and corrosion resistance are critical.Expand Specific Solutions05 Corrosion inhibitors and their effect on electrochemical impedance
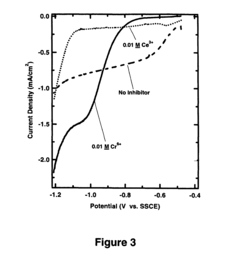
Corrosion inhibitors can be incorporated into alloy systems or applied as coatings to enhance corrosion resistance. These compounds work by adsorbing onto metal surfaces, forming protective films that increase the electrochemical impedance of the system. Impedance spectroscopy shows that effective inhibitors increase charge transfer resistance and reduce double-layer capacitance. The performance of various inhibitors can be compared through electrochemical impedance measurements, allowing for optimization of inhibitor concentration and composition for specific alloy systems and corrosive environments.Expand Specific Solutions
Key Industry Players in Corrosion-Resistant Materials
The electrochemical impedance analysis of corrosion-resistant alloys market is currently in a growth phase, with increasing demand driven by oil and gas, automotive, and infrastructure sectors. The global market is estimated at $2.5-3 billion annually, expanding at 5-7% CAGR. Leading players demonstrate varying levels of technological maturity: Schlumberger and Phillips 66 focus on oil industry applications; Kobe Steel, Alcoa, and Arconic lead in advanced alloy development; while research institutions like Central South University and Central Iron & Steel Research Institute contribute fundamental innovations. Companies like Teledyne Scientific and Hydrogenics are advancing niche applications in specialized environments, creating a competitive landscape balanced between established industrial giants and innovative research-focused entities.
Schlumberger Technologies, Inc.
Technical Solution: Schlumberger has developed advanced electrochemical impedance spectroscopy (EIS) systems specifically designed for analyzing corrosion-resistant alloys in harsh oilfield environments. Their technology combines multi-frequency impedance measurements with proprietary algorithms to characterize protective oxide films on alloy surfaces. The company's approach integrates real-time monitoring capabilities that allow for continuous assessment of corrosion processes under varying temperature and pressure conditions. Schlumberger's systems employ distributed sensor networks that can be deployed in downhole environments to monitor corrosion resistance performance of critical components. Their analytical software provides comprehensive data interpretation including equivalent circuit modeling and time-domain analysis to predict long-term corrosion behavior of specialized alloys.
Strengths: Industry-leading expertise in harsh environment applications; integrated hardware-software solutions; extensive field validation data. Weaknesses: Systems primarily optimized for oil and gas applications; relatively high implementation costs; requires specialized training for effective deployment.
Central South University
Technical Solution: Central South University has developed a sophisticated electrochemical impedance spectroscopy platform specifically for analyzing novel corrosion-resistant alloys. Their approach combines traditional EIS with innovative pulse techniques that reveal transient electrochemical behavior critical for understanding metastable pitting processes. The university's research team has pioneered the application of machine learning algorithms to interpret complex impedance data, enabling automated classification of corrosion mechanisms and prediction of long-term performance. Their methodology incorporates multi-scale modeling that links atomic-level processes to macroscopic impedance responses, providing fundamental insights into corrosion resistance mechanisms. The university has also developed specialized in-situ cells that enable impedance measurements under mechanical loading, critical for understanding stress-corrosion interactions in advanced alloys.
Strengths: Cutting-edge theoretical foundations; innovative data analysis approaches; strong integration of fundamental science and practical applications. Weaknesses: Less emphasis on standardized testing protocols; limited commercialization of technologies; primarily focused on research applications rather than industrial implementation.
Critical Patents and Literature in Impedance Analysis Techniques
Evaluation of the corrosion inhibiting activity of a coating
PatentInactiveUS20050082174A1
Innovation
- A method and apparatus that detect corrosion inhibiting species released from coatings by using a cathode with an oxygen reduction catalyst in close proximity to the coating, with laminar flow of electrolytic solution and controlled potential to measure the reduction in oxygen reduction current, providing a rapid, non-destructive, and portable evaluation of corrosion inhibiting activity.
Evaluation of the corrosion inhibiting activity of a coating
PatentInactiveUS8016987B2
Innovation
- A method and apparatus that involves placing a cathode with an oxygen reduction catalyst close to the coating, with laminar flow of electrolytic solution over both surfaces, and applying a potential to measure the reduction in oxygen reduction current, which indicates the corrosion inhibiting activity of the coating.
Regulatory Standards for Corrosion-Resistant Materials
Regulatory standards for corrosion-resistant materials play a crucial role in ensuring the safety, reliability, and longevity of structures and components across various industries. These standards are established by international, regional, and national regulatory bodies to provide guidelines for material selection, testing methodologies, and performance criteria specifically for environments where corrosion resistance is paramount.
The International Organization for Standardization (ISO) has developed several standards relevant to electrochemical impedance spectroscopy (EIS) for corrosion assessment, including ISO 16773 series which specifically addresses the application of EIS in corrosion testing. These standards provide detailed protocols for sample preparation, measurement procedures, and data interpretation, ensuring consistency and reproducibility in corrosion resistance evaluation of alloys.
ASTM International maintains comprehensive standards for corrosion testing, with ASTM G106 and G59 being particularly relevant for electrochemical impedance measurements. These standards define specific parameters for polarization resistance measurements and interpretation of impedance data for corrosion rate determination in various alloy systems.
In the European Union, the EN 13509 standard governs cathodic protection measurement techniques, including impedance-based methods for assessing the effectiveness of corrosion protection systems. This standard is particularly important for industries dealing with buried or submerged metallic structures where corrosion risks are elevated.
For specialized industries, more specific regulatory frameworks exist. The NACE (National Association of Corrosion Engineers) standards, particularly NACE TM0208, provide detailed guidelines for conducting and interpreting electrochemical impedance measurements in oil and gas applications, where high-performance corrosion-resistant alloys are frequently employed.
The aerospace industry follows standards set by organizations like SAE International, with specifications such as AMS2759 addressing heat treatment and corrosion testing requirements for high-performance alloys. These standards incorporate electrochemical testing methodologies as quality control measures for critical components.
Maritime applications are governed by classification societies such as DNV GL and Lloyd's Register, which have established specific requirements for corrosion-resistant materials used in marine environments. These standards often reference electrochemical impedance analysis as an accepted method for qualifying materials for seawater exposure.
Compliance with these regulatory standards is increasingly becoming mandatory for material suppliers and manufacturers, particularly in safety-critical applications. The trend toward more stringent regulatory requirements is driving innovation in both alloy development and testing methodologies, with electrochemical impedance analysis emerging as a preferred technique due to its non-destructive nature and ability to provide mechanistic insights into corrosion processes.
The International Organization for Standardization (ISO) has developed several standards relevant to electrochemical impedance spectroscopy (EIS) for corrosion assessment, including ISO 16773 series which specifically addresses the application of EIS in corrosion testing. These standards provide detailed protocols for sample preparation, measurement procedures, and data interpretation, ensuring consistency and reproducibility in corrosion resistance evaluation of alloys.
ASTM International maintains comprehensive standards for corrosion testing, with ASTM G106 and G59 being particularly relevant for electrochemical impedance measurements. These standards define specific parameters for polarization resistance measurements and interpretation of impedance data for corrosion rate determination in various alloy systems.
In the European Union, the EN 13509 standard governs cathodic protection measurement techniques, including impedance-based methods for assessing the effectiveness of corrosion protection systems. This standard is particularly important for industries dealing with buried or submerged metallic structures where corrosion risks are elevated.
For specialized industries, more specific regulatory frameworks exist. The NACE (National Association of Corrosion Engineers) standards, particularly NACE TM0208, provide detailed guidelines for conducting and interpreting electrochemical impedance measurements in oil and gas applications, where high-performance corrosion-resistant alloys are frequently employed.
The aerospace industry follows standards set by organizations like SAE International, with specifications such as AMS2759 addressing heat treatment and corrosion testing requirements for high-performance alloys. These standards incorporate electrochemical testing methodologies as quality control measures for critical components.
Maritime applications are governed by classification societies such as DNV GL and Lloyd's Register, which have established specific requirements for corrosion-resistant materials used in marine environments. These standards often reference electrochemical impedance analysis as an accepted method for qualifying materials for seawater exposure.
Compliance with these regulatory standards is increasingly becoming mandatory for material suppliers and manufacturers, particularly in safety-critical applications. The trend toward more stringent regulatory requirements is driving innovation in both alloy development and testing methodologies, with electrochemical impedance analysis emerging as a preferred technique due to its non-destructive nature and ability to provide mechanistic insights into corrosion processes.
Environmental Impact of Advanced Alloy Development
The development of advanced corrosion-resistant alloys represents a significant step forward in materials science, but their environmental implications warrant careful consideration. The production processes for these specialized alloys often require substantial energy inputs and generate considerable carbon emissions. Electrochemical impedance analysis techniques, while essential for evaluating corrosion resistance, typically involve chemical reagents that may pose environmental hazards if improperly managed.
Recent life cycle assessments of high-performance nickel-chromium and titanium alloys indicate that their production can generate 1.5-2.3 times more greenhouse gas emissions compared to conventional steel manufacturing. However, this initial environmental cost must be balanced against the extended service life these materials provide, which can range from 2-5 times longer than traditional alternatives in corrosive environments.
The extraction of rare elements used in advanced alloys, such as molybdenum, niobium, and tantalum, presents additional environmental challenges. Mining operations for these elements have been associated with habitat disruption, water pollution, and soil contamination in various regions globally. Electrochemical impedance spectroscopy (EIS) has emerged as a valuable tool for monitoring the environmental impact of these mining activities, providing real-time data on potential contaminant leaching.
Recycling presents a promising avenue for mitigating the environmental footprint of advanced alloys. Current recovery rates for specialized corrosion-resistant alloys range from 60-85%, significantly higher than many other engineered materials. Electrochemical techniques have proven instrumental in developing more efficient recycling processes, allowing for the separation and recovery of valuable elements with reduced chemical waste generation.
Water conservation represents another critical environmental consideration. Traditional corrosion protection methods often involve water-intensive processes and potentially harmful chemical treatments. Advanced alloys that provide inherent corrosion resistance can reduce water consumption by eliminating or minimizing these treatments. Studies indicate potential water savings of 30-50% when implementing corrosion-resistant alloys in industrial water systems.
The long-term environmental benefits of corrosion-resistant alloys extend beyond their production phase. By preventing infrastructure failure and reducing maintenance requirements, these materials contribute to resource conservation and waste reduction throughout their operational lifetime. Electrochemical impedance analysis plays a crucial role in optimizing these benefits by enabling precise monitoring of corrosion behavior under various environmental conditions, allowing for targeted application of these advanced materials where they deliver maximum environmental advantage.
Recent life cycle assessments of high-performance nickel-chromium and titanium alloys indicate that their production can generate 1.5-2.3 times more greenhouse gas emissions compared to conventional steel manufacturing. However, this initial environmental cost must be balanced against the extended service life these materials provide, which can range from 2-5 times longer than traditional alternatives in corrosive environments.
The extraction of rare elements used in advanced alloys, such as molybdenum, niobium, and tantalum, presents additional environmental challenges. Mining operations for these elements have been associated with habitat disruption, water pollution, and soil contamination in various regions globally. Electrochemical impedance spectroscopy (EIS) has emerged as a valuable tool for monitoring the environmental impact of these mining activities, providing real-time data on potential contaminant leaching.
Recycling presents a promising avenue for mitigating the environmental footprint of advanced alloys. Current recovery rates for specialized corrosion-resistant alloys range from 60-85%, significantly higher than many other engineered materials. Electrochemical techniques have proven instrumental in developing more efficient recycling processes, allowing for the separation and recovery of valuable elements with reduced chemical waste generation.
Water conservation represents another critical environmental consideration. Traditional corrosion protection methods often involve water-intensive processes and potentially harmful chemical treatments. Advanced alloys that provide inherent corrosion resistance can reduce water consumption by eliminating or minimizing these treatments. Studies indicate potential water savings of 30-50% when implementing corrosion-resistant alloys in industrial water systems.
The long-term environmental benefits of corrosion-resistant alloys extend beyond their production phase. By preventing infrastructure failure and reducing maintenance requirements, these materials contribute to resource conservation and waste reduction throughout their operational lifetime. Electrochemical impedance analysis plays a crucial role in optimizing these benefits by enabling precise monitoring of corrosion behavior under various environmental conditions, allowing for targeted application of these advanced materials where they deliver maximum environmental advantage.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!