Salt Spray and Pitting Resistance Testing in Alloy Systems
OCT 13, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Corrosion Testing Background and Objectives
Corrosion testing has evolved significantly over the past century, with salt spray testing emerging as one of the earliest standardized methods for evaluating material resistance to corrosive environments. Developed in the early 1900s and formalized through ASTM B117 in 1939, this testing methodology has become a cornerstone in materials science and engineering. The evolution of corrosion testing reflects our growing understanding of electrochemical processes and the complex mechanisms that lead to material degradation in various environments.
Salt spray and pitting resistance testing specifically addresses one of the most insidious forms of corrosion that affects alloy systems. Pitting corrosion, characterized by localized attacks that create small holes or "pits" in the material, represents a particularly dangerous degradation mechanism as it can progress undetected while severely compromising structural integrity. The economic impact of corrosion globally is estimated at approximately 3-4% of GDP in industrialized nations, highlighting the critical importance of effective testing protocols.
The primary objective of modern salt spray and pitting resistance testing is to provide standardized, reproducible methods for evaluating and comparing the corrosion resistance of different alloy systems under accelerated conditions. These tests aim to simulate real-world exposure environments while compressing time scales to enable practical assessment of materials that may be expected to perform for decades in service. Current testing protocols seek to balance the need for rapid results with the requirement for accurate prediction of long-term performance.
Recent technological advancements have expanded the scope and precision of corrosion testing beyond traditional salt spray chambers. Electrochemical techniques such as potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), and critical pitting temperature (CPT) determination now complement traditional exposure tests, providing deeper insights into corrosion mechanisms and kinetics. The integration of digital imaging and automated analysis has further enhanced the quantitative assessment of pitting phenomena.
The current technological trajectory in this field is moving toward more sophisticated test environments that better replicate specific service conditions, including cyclic testing that alternates between wet and dry conditions, temperature variations, and the inclusion of multiple environmental contaminants beyond simple sodium chloride solutions. Additionally, there is growing interest in developing accelerated test methods that maintain better correlation with actual field performance, addressing a historical limitation of traditional salt spray testing.
As materials science advances with the development of new alloys, coatings, and surface treatments, corrosion testing methodologies must evolve in parallel to accurately characterize these innovations. The ultimate goal remains consistent: to develop testing protocols that reliably predict material performance in real-world applications, enabling informed material selection and design decisions that balance corrosion resistance with other critical properties such as mechanical strength, weight, and cost-effectiveness.
Salt spray and pitting resistance testing specifically addresses one of the most insidious forms of corrosion that affects alloy systems. Pitting corrosion, characterized by localized attacks that create small holes or "pits" in the material, represents a particularly dangerous degradation mechanism as it can progress undetected while severely compromising structural integrity. The economic impact of corrosion globally is estimated at approximately 3-4% of GDP in industrialized nations, highlighting the critical importance of effective testing protocols.
The primary objective of modern salt spray and pitting resistance testing is to provide standardized, reproducible methods for evaluating and comparing the corrosion resistance of different alloy systems under accelerated conditions. These tests aim to simulate real-world exposure environments while compressing time scales to enable practical assessment of materials that may be expected to perform for decades in service. Current testing protocols seek to balance the need for rapid results with the requirement for accurate prediction of long-term performance.
Recent technological advancements have expanded the scope and precision of corrosion testing beyond traditional salt spray chambers. Electrochemical techniques such as potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), and critical pitting temperature (CPT) determination now complement traditional exposure tests, providing deeper insights into corrosion mechanisms and kinetics. The integration of digital imaging and automated analysis has further enhanced the quantitative assessment of pitting phenomena.
The current technological trajectory in this field is moving toward more sophisticated test environments that better replicate specific service conditions, including cyclic testing that alternates between wet and dry conditions, temperature variations, and the inclusion of multiple environmental contaminants beyond simple sodium chloride solutions. Additionally, there is growing interest in developing accelerated test methods that maintain better correlation with actual field performance, addressing a historical limitation of traditional salt spray testing.
As materials science advances with the development of new alloys, coatings, and surface treatments, corrosion testing methodologies must evolve in parallel to accurately characterize these innovations. The ultimate goal remains consistent: to develop testing protocols that reliably predict material performance in real-world applications, enabling informed material selection and design decisions that balance corrosion resistance with other critical properties such as mechanical strength, weight, and cost-effectiveness.
Market Demand Analysis for Corrosion-Resistant Alloys
The global market for corrosion-resistant alloys continues to expand significantly, driven primarily by increasing industrial activities in harsh environments and growing awareness of the economic impact of corrosion-related failures. Current market valuations place the corrosion-resistant alloy sector at approximately 7.5 billion USD, with projections indicating growth to reach 10.2 billion USD by 2027, representing a compound annual growth rate of 6.3%.
Industries such as oil and gas, chemical processing, marine engineering, and aerospace remain the primary consumers of these specialized materials. The offshore oil and gas sector alone accounts for nearly 28% of the total market demand, where exposure to salt spray and aggressive chemical environments necessitates materials with superior pitting resistance. Chemical processing follows closely at 23%, with marine applications representing approximately 18% of market share.
Regional analysis reveals that North America and Europe currently dominate the market with a combined share of 58%, though Asia-Pacific regions are demonstrating the fastest growth rate at 7.8% annually. This growth is particularly evident in China and India, where rapid industrialization and infrastructure development are creating substantial new demand for corrosion-resistant materials.
The economic justification for investment in high-performance alloys is compelling when considering the total cost of corrosion. Industry reports indicate that corrosion-related issues cost the global economy approximately 2.5 trillion USD annually, equivalent to roughly 3.4% of global GDP. Companies are increasingly recognizing that the higher initial investment in corrosion-resistant alloys delivers significant long-term cost savings through extended service life, reduced maintenance requirements, and prevention of catastrophic failures.
Customer requirements are evolving beyond mere corrosion resistance to include considerations of mechanical properties, weldability, and cost-effectiveness. Market research indicates that 76% of procurement specialists now prioritize comprehensive performance data from standardized testing protocols, including ASTM B117 salt spray testing and ASTM G48 pitting resistance evaluations, when making purchasing decisions.
Emerging application areas showing particularly strong growth include renewable energy infrastructure (especially offshore wind installations), electric vehicle battery systems, and advanced medical devices. These sectors are driving demand for new alloy formulations with precisely tailored corrosion resistance profiles and specialized performance characteristics.
The market is also witnessing a shift toward sustainability considerations, with 62% of end-users expressing preference for alloys that enable weight reduction, energy efficiency, or contain recycled content, provided they meet or exceed corrosion resistance requirements.
Industries such as oil and gas, chemical processing, marine engineering, and aerospace remain the primary consumers of these specialized materials. The offshore oil and gas sector alone accounts for nearly 28% of the total market demand, where exposure to salt spray and aggressive chemical environments necessitates materials with superior pitting resistance. Chemical processing follows closely at 23%, with marine applications representing approximately 18% of market share.
Regional analysis reveals that North America and Europe currently dominate the market with a combined share of 58%, though Asia-Pacific regions are demonstrating the fastest growth rate at 7.8% annually. This growth is particularly evident in China and India, where rapid industrialization and infrastructure development are creating substantial new demand for corrosion-resistant materials.
The economic justification for investment in high-performance alloys is compelling when considering the total cost of corrosion. Industry reports indicate that corrosion-related issues cost the global economy approximately 2.5 trillion USD annually, equivalent to roughly 3.4% of global GDP. Companies are increasingly recognizing that the higher initial investment in corrosion-resistant alloys delivers significant long-term cost savings through extended service life, reduced maintenance requirements, and prevention of catastrophic failures.
Customer requirements are evolving beyond mere corrosion resistance to include considerations of mechanical properties, weldability, and cost-effectiveness. Market research indicates that 76% of procurement specialists now prioritize comprehensive performance data from standardized testing protocols, including ASTM B117 salt spray testing and ASTM G48 pitting resistance evaluations, when making purchasing decisions.
Emerging application areas showing particularly strong growth include renewable energy infrastructure (especially offshore wind installations), electric vehicle battery systems, and advanced medical devices. These sectors are driving demand for new alloy formulations with precisely tailored corrosion resistance profiles and specialized performance characteristics.
The market is also witnessing a shift toward sustainability considerations, with 62% of end-users expressing preference for alloys that enable weight reduction, energy efficiency, or contain recycled content, provided they meet or exceed corrosion resistance requirements.
Salt Spray Testing: Current Status and Challenges
Salt spray testing has evolved significantly since its inception in the early 20th century, becoming a standardized method for evaluating corrosion resistance in alloy systems. Currently, the most widely adopted standard is ASTM B117, which specifies a controlled environment of 5% sodium chloride solution at 35°C. Despite its widespread use, this traditional method faces increasing scrutiny regarding its correlation with real-world performance, particularly for advanced alloy systems designed for extreme environments.
The global landscape of salt spray testing reveals regional variations in implementation and standards. While North America predominantly follows ASTM standards, European manufacturers often adhere to ISO 9227, and Asian markets frequently utilize JIS Z2371. These differences create challenges for international material qualification and certification processes, especially for global supply chains in aerospace and automotive industries.
A significant technical challenge in current salt spray testing methodologies is the limited ability to accelerate testing without compromising result validity. Traditional tests require 1,000+ hours for meaningful data, creating bottlenecks in product development cycles. This limitation becomes particularly problematic for rapid innovation cycles in industries like consumer electronics and electric vehicles, where time-to-market pressures are intense.
The correlation between laboratory salt spray test results and actual field performance represents another major challenge. Studies by organizations including NACE International have documented cases where materials performing well in standardized tests failed prematurely in specific service environments. This discrepancy is especially pronounced for complex alloy systems like advanced aluminum-lithium alloys and high-entropy alloys, where localized corrosion mechanisms may not be adequately captured by conventional testing protocols.
Emerging technologies are beginning to address these limitations. Digital imaging analysis systems now enable automated, quantitative assessment of corrosion progression, reducing human interpretation variability. Electrochemical impedance spectroscopy (EIS) is increasingly being integrated with traditional salt spray testing to provide deeper insights into corrosion mechanisms at the molecular level.
The environmental impact of salt spray testing presents additional challenges. Traditional methods consume significant quantities of water and salt, and generate hazardous waste requiring specialized disposal. Regulatory pressures, particularly in Europe under REACH regulations, are driving research into more sustainable testing methodologies that maintain or improve predictive accuracy while reducing environmental footprint.
Standardization bodies including ASTM International and ISO are actively working to update testing protocols to address these challenges, with particular focus on developing accelerated test methods that maintain correlation with real-world performance and accommodate advanced materials including metal matrix composites and additively manufactured components.
The global landscape of salt spray testing reveals regional variations in implementation and standards. While North America predominantly follows ASTM standards, European manufacturers often adhere to ISO 9227, and Asian markets frequently utilize JIS Z2371. These differences create challenges for international material qualification and certification processes, especially for global supply chains in aerospace and automotive industries.
A significant technical challenge in current salt spray testing methodologies is the limited ability to accelerate testing without compromising result validity. Traditional tests require 1,000+ hours for meaningful data, creating bottlenecks in product development cycles. This limitation becomes particularly problematic for rapid innovation cycles in industries like consumer electronics and electric vehicles, where time-to-market pressures are intense.
The correlation between laboratory salt spray test results and actual field performance represents another major challenge. Studies by organizations including NACE International have documented cases where materials performing well in standardized tests failed prematurely in specific service environments. This discrepancy is especially pronounced for complex alloy systems like advanced aluminum-lithium alloys and high-entropy alloys, where localized corrosion mechanisms may not be adequately captured by conventional testing protocols.
Emerging technologies are beginning to address these limitations. Digital imaging analysis systems now enable automated, quantitative assessment of corrosion progression, reducing human interpretation variability. Electrochemical impedance spectroscopy (EIS) is increasingly being integrated with traditional salt spray testing to provide deeper insights into corrosion mechanisms at the molecular level.
The environmental impact of salt spray testing presents additional challenges. Traditional methods consume significant quantities of water and salt, and generate hazardous waste requiring specialized disposal. Regulatory pressures, particularly in Europe under REACH regulations, are driving research into more sustainable testing methodologies that maintain or improve predictive accuracy while reducing environmental footprint.
Standardization bodies including ASTM International and ISO are actively working to update testing protocols to address these challenges, with particular focus on developing accelerated test methods that maintain correlation with real-world performance and accommodate advanced materials including metal matrix composites and additively manufactured components.
Current Salt Spray and Pitting Resistance Test Methods
01 Stainless Steel Alloy Compositions for Pitting Resistance
Specific compositions of stainless steel alloys can be formulated to enhance pitting resistance. These compositions typically include controlled amounts of chromium, nickel, molybdenum, and nitrogen, which work synergistically to form a stable passive film on the alloy surface. The balance of these elements is critical for creating alloys with superior resistance to localized corrosion in aggressive environments, particularly those containing chloride ions.- Stainless steel alloy compositions for pitting resistance: Specific compositions of stainless steel alloys can be formulated to enhance pitting resistance in corrosive environments. These compositions typically include controlled amounts of chromium, nickel, molybdenum, and nitrogen, which work synergistically to form a stable passive film on the alloy surface. The balance of these elements is critical for maintaining structural integrity while maximizing corrosion resistance properties.
- Heat treatment methods to improve pitting resistance: Various heat treatment processes can significantly enhance the pitting resistance of alloy systems. These processes include solution annealing, aging treatments, and controlled cooling rates that optimize microstructure and phase distribution. Proper heat treatment can dissolve detrimental precipitates, homogenize the alloy composition, and create beneficial microstructural features that increase resistance to localized corrosion.
- Surface modification techniques for enhanced pitting resistance: Surface modification techniques can be applied to alloy systems to improve their resistance to pitting corrosion. These techniques include passivation treatments, surface hardening processes, coating applications, and electrochemical surface modifications. By altering the surface properties of the alloy, these methods create a more resistant barrier against corrosive media while maintaining the bulk mechanical properties of the material.
- Alloying elements and their role in pitting resistance: Specific alloying elements play crucial roles in enhancing the pitting resistance of various alloy systems. Elements such as chromium, molybdenum, nitrogen, and tungsten contribute to pitting resistance through different mechanisms including passive film stabilization, pit repassivation, and modification of local chemistry within incipient pits. The synergistic effects between these elements can be optimized to achieve superior corrosion resistance in aggressive environments.
- Testing and evaluation methods for pitting resistance: Various testing and evaluation methods have been developed to assess the pitting resistance of alloy systems. These include electrochemical techniques such as potentiodynamic polarization, critical pitting temperature determination, and immersion testing in specific corrosive media. These methods help quantify pitting resistance through parameters like pitting potential, repassivation potential, and pitting resistance equivalent number (PREN), enabling the comparison and optimization of different alloy compositions.
02 Heat Treatment Methods to Improve Pitting Resistance
Various heat treatment processes can significantly enhance the pitting resistance of alloy systems. These processes include solution annealing, sensitization prevention treatments, and controlled cooling rates that optimize microstructure. Proper heat treatment helps to dissolve detrimental precipitates, homogenize the alloy composition, and create a more uniform passive layer, thereby increasing resistance to pitting corrosion in various service environments.Expand Specific Solutions03 Surface Modification Techniques for Enhanced Pitting Resistance
Surface modification techniques can be applied to alloy systems to improve their resistance to pitting corrosion. These techniques include passivation treatments, surface alloying, coating applications, and mechanical surface treatments. By altering the surface composition or structure, these methods create a more resistant barrier against corrosive media, effectively increasing the pitting potential and reducing susceptibility to localized corrosion.Expand Specific Solutions04 Specialized Alloy Systems for Extreme Corrosive Environments
Specialized alloy systems have been developed specifically for extreme corrosive environments where pitting is a significant concern. These include super duplex stainless steels, high-molybdenum alloys, and nickel-based superalloys. These materials incorporate specific elemental combinations and microstructural features that provide exceptional resistance to pitting initiation and propagation in highly aggressive media such as seawater, chemical processing environments, and high-temperature oxidizing conditions.Expand Specific Solutions05 Testing and Evaluation Methods for Pitting Resistance
Various testing and evaluation methods have been developed to assess the pitting resistance of alloy systems. These include electrochemical techniques such as cyclic polarization testing, critical pitting temperature determination, and immersion testing in aggressive media. These methods help in quantifying pitting resistance through parameters like pitting potential, repassivation potential, and pitting resistance equivalent number (PREN), enabling the selection of appropriate alloys for specific corrosive environments.Expand Specific Solutions
Key Industry Players in Corrosion Testing
Salt Spray and Pitting Resistance Testing in Alloy Systems is currently in a growth phase, with the global corrosion testing market expanding due to increasing industrial applications. The market size is estimated at $6-8 billion, driven by aerospace, automotive, and energy sectors. Technical maturity varies across testing methodologies, with established players like United Technologies Corp., DuPont de Nemours, and Rolls Royce PLC leading standardized testing protocols. Emerging companies such as Fraunhofer-Gesellschaft and Chemetall GmbH are advancing innovative approaches, while research institutions like Zhejiang University and University of Cambridge contribute to fundamental understanding. The competitive landscape features both traditional materials testing corporations and specialized coating technology firms like BASF Coatings GmbH and Kansai Paint Co., creating a dynamic ecosystem of established protocols and emerging methodologies.
Fraunhofer-Gesellschaft eV
Technical Solution: Fraunhofer-Gesellschaft has developed a sophisticated multi-scale approach to salt spray and pitting resistance testing that bridges fundamental materials science with practical industrial applications. Their methodology combines standardized testing procedures with advanced characterization techniques to provide comprehensive understanding of corrosion mechanisms. Fraunhofer's approach includes modified salt spray testing protocols that incorporate realistic environmental pollutants (e.g., SO2, NOx) alongside NaCl to better simulate industrial and urban atmospheres[1]. They employ high-resolution electrochemical techniques including scanning vibrating electrode technique (SVET) and localized electrochemical impedance spectroscopy (LEIS) to map corrosion activity across heterogeneous microstructures. Their testing infrastructure includes climate chambers capable of reproducing complex environmental cycles including temperature, humidity, UV radiation, and salt concentration variations. Fraunhofer has pioneered the use of machine learning algorithms to analyze large datasets from corrosion testing, enabling prediction of long-term performance from accelerated test results.
Strengths: Exceptional scientific rigor with strong connection between fundamental corrosion science and practical applications. Comprehensive testing capabilities spanning from laboratory scale to full component testing. Weaknesses: Some testing methodologies prioritize scientific understanding over standardized industrial protocols. As a research organization, implementation of findings requires partnership with industrial manufacturers.
VDM METALS INTERNATIONAL GMBH
Technical Solution: VDM Metals has developed a comprehensive testing framework for evaluating salt spray and pitting resistance across their portfolio of high-performance alloys. Their methodology combines standardized testing (ASTM G48, ASTM B117) with proprietary long-term exposure tests in simulated industrial environments. VDM's approach is distinguished by their Critical Pitting Temperature (CPT) determination protocol, which identifies the precise temperature threshold at which pitting corrosion initiates for each alloy composition[8]. The company employs advanced electrochemical techniques including potentiodynamic polarization and electrochemical noise measurement to characterize pitting behavior at the microstructural level. Their testing facilities include specialized equipment for evaluating crevice corrosion resistance under salt spray conditions, which is particularly relevant for welded or mechanically joined components. VDM has established extensive corrosion databases correlating alloy composition with pitting resistance, enabling them to develop custom alloy formulations with enhanced resistance to specific corrosive environments.
Strengths: Comprehensive testing methodology specifically designed for high-performance nickel and specialty stainless steel alloys. Extensive historical database correlating test results with actual field performance. Weaknesses: Testing protocols are primarily focused on chemical processing applications rather than broader industrial uses. Some proprietary testing methods make direct comparison with competitors' data challenging.
Critical Technologies in Alloy Corrosion Resistance
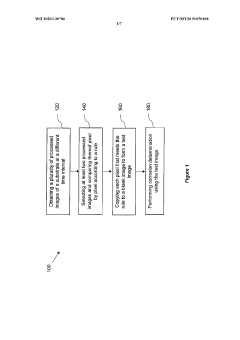
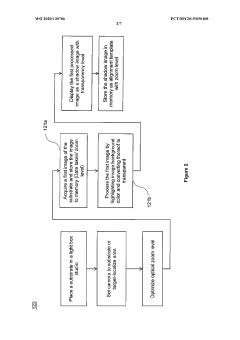
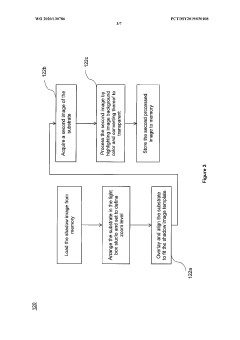
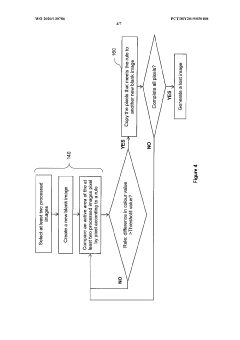
A method of analyzing visual inspection image of a substrate for corrosion determination
PatentWO2020130786A1
Innovation
- A method that creates a shadow image as an alignment template from the initial inspection image to ensure consistent image quality across subsequent inspections, allowing for accurate corrosion detection and quantification on substrates of various sizes and outlines by overlaying and aligning the substrate on the shadow image during subsequent inspections.
Touch sensor
PatentActiveUS12079407B2
Innovation
- A touch sensor design featuring a substrate with sensing channels and a protective layer that maintains low resistance change rates and uniform resistance distribution after a 48-hour salt spray test, ensuring normal operation through precise electrical specifications and a protective layer structure that contacts the substrate and silver trace portion with specific thickness and water vapor permeability.
Environmental Factors Affecting Corrosion Test Results
Environmental conditions play a crucial role in determining the accuracy and reliability of salt spray and pitting resistance tests in alloy systems. Temperature variations significantly impact corrosion rates, with higher temperatures generally accelerating electrochemical reactions and diffusion processes. Research indicates that a 10°C increase can double corrosion rates in many alloy systems, necessitating precise temperature control during testing to ensure reproducible results.
Humidity levels directly affect the formation and stability of electrolyte films on metal surfaces. In salt spray testing, relative humidity below 95% may lead to premature drying of salt deposits, while excessive condensation above 98% can dilute the salt solution, altering the corrosion mechanism. Modern test chambers implement sophisticated humidity control systems with ±1% accuracy to mitigate these variables.
Atmospheric contaminants represent another significant environmental factor affecting test outcomes. Sulfur dioxide, nitrogen oxides, and airborne particulates can dramatically alter corrosion behavior by changing the pH of moisture films or introducing additional reactive species. Studies have shown that even trace amounts (parts per billion) of certain industrial pollutants can increase corrosion rates by 30-50% in susceptible alloys.
Light exposure, particularly ultraviolet radiation, can photocatalytically accelerate corrosion processes in certain alloy systems. This factor is often overlooked in standard testing protocols but has been demonstrated to significantly influence results, especially for components intended for outdoor deployment. UV exposure can degrade protective films and generate reactive oxygen species that enhance metal dissolution.
Barometric pressure fluctuations, though subtle, affect oxygen solubility in test solutions and can influence corrosion kinetics. Research indicates that pressure variations of 20 hPa can alter dissolved oxygen content by up to 5%, potentially affecting pitting initiation rates in susceptible alloys like aluminum and stainless steel systems.
Electromagnetic fields present in testing environments may also influence electrochemical processes. Recent studies suggest that even moderate electromagnetic interference can affect the distribution and morphology of corrosion products, particularly in tests involving high-strength alloys with significant residual stress profiles.
Standardization bodies have recognized these environmental challenges, with ASTM B117 and ISO 9227 specifying increasingly stringent environmental control parameters. Advanced testing facilities now implement environmental monitoring systems that continuously record all relevant parameters, enabling more accurate correlation between laboratory results and real-world performance of alloy systems under various environmental conditions.
Humidity levels directly affect the formation and stability of electrolyte films on metal surfaces. In salt spray testing, relative humidity below 95% may lead to premature drying of salt deposits, while excessive condensation above 98% can dilute the salt solution, altering the corrosion mechanism. Modern test chambers implement sophisticated humidity control systems with ±1% accuracy to mitigate these variables.
Atmospheric contaminants represent another significant environmental factor affecting test outcomes. Sulfur dioxide, nitrogen oxides, and airborne particulates can dramatically alter corrosion behavior by changing the pH of moisture films or introducing additional reactive species. Studies have shown that even trace amounts (parts per billion) of certain industrial pollutants can increase corrosion rates by 30-50% in susceptible alloys.
Light exposure, particularly ultraviolet radiation, can photocatalytically accelerate corrosion processes in certain alloy systems. This factor is often overlooked in standard testing protocols but has been demonstrated to significantly influence results, especially for components intended for outdoor deployment. UV exposure can degrade protective films and generate reactive oxygen species that enhance metal dissolution.
Barometric pressure fluctuations, though subtle, affect oxygen solubility in test solutions and can influence corrosion kinetics. Research indicates that pressure variations of 20 hPa can alter dissolved oxygen content by up to 5%, potentially affecting pitting initiation rates in susceptible alloys like aluminum and stainless steel systems.
Electromagnetic fields present in testing environments may also influence electrochemical processes. Recent studies suggest that even moderate electromagnetic interference can affect the distribution and morphology of corrosion products, particularly in tests involving high-strength alloys with significant residual stress profiles.
Standardization bodies have recognized these environmental challenges, with ASTM B117 and ISO 9227 specifying increasingly stringent environmental control parameters. Advanced testing facilities now implement environmental monitoring systems that continuously record all relevant parameters, enabling more accurate correlation between laboratory results and real-world performance of alloy systems under various environmental conditions.
Standardization and Compliance Requirements
Standardization in salt spray and pitting resistance testing is essential for ensuring reliable, reproducible results across different laboratories and testing facilities. The primary international standard governing salt spray testing is ASTM B117, which establishes uniform conditions for continuous salt spray (fog) testing. This standard specifies critical parameters including salt solution concentration (typically 5% NaCl), pH range (6.5-7.2), temperature (35°C), and exposure duration. Complementary standards include ISO 9227, which defines three different salt spray tests: neutral (NSS), acetic acid (ASS), and copper-accelerated acetic acid (CASS).
For pitting resistance evaluation specifically, ASTM G48 provides standardized methods for ferrous metals, particularly stainless steels and related alloys. This standard outlines procedures for ferric chloride immersion testing, which accelerates pitting corrosion to evaluate comparative resistance. The ASTM G61 standard covers cyclic polarization measurements for localized corrosion susceptibility, offering electrochemical testing protocols that complement traditional exposure methods.
Compliance with these standards requires precise control of testing equipment and environmental conditions. Modern salt spray chambers must maintain temperature uniformity within ±1°C and feature calibrated spray nozzles that produce consistent fog density. Documentation requirements include detailed records of specimen preparation, orientation within the chamber, and post-test cleaning procedures. Testing laboratories seeking accreditation must demonstrate proficiency through interlaboratory comparison studies and regular equipment calibration.
Industry-specific standards have emerged to address specialized applications. The automotive sector follows SAE J2334 for cyclic corrosion testing, while aerospace manufacturers adhere to ASTM F1110 for sandwich corrosion testing of aircraft materials. The medical device industry must comply with ISO 10993-15 for biocompatibility testing of metals and alloys used in implantable devices.
Emerging trends in standardization include the development of accelerated test methods that correlate more accurately with real-world performance. The ISO 16539 standard introduces cyclic accelerated corrosion tests that better simulate natural weathering conditions. Additionally, there is growing emphasis on standardizing digital imaging techniques for quantitative assessment of corrosion damage, moving beyond traditional visual inspection methods.
Regulatory bodies increasingly require compliance with these standards as part of product certification processes. The European Construction Products Regulation (CPR) mandates corrosion testing according to harmonized standards for structural components. Similarly, the U.S. Food and Drug Administration (FDA) requires demonstration of corrosion resistance for medical implants through standardized testing protocols before market approval.
For pitting resistance evaluation specifically, ASTM G48 provides standardized methods for ferrous metals, particularly stainless steels and related alloys. This standard outlines procedures for ferric chloride immersion testing, which accelerates pitting corrosion to evaluate comparative resistance. The ASTM G61 standard covers cyclic polarization measurements for localized corrosion susceptibility, offering electrochemical testing protocols that complement traditional exposure methods.
Compliance with these standards requires precise control of testing equipment and environmental conditions. Modern salt spray chambers must maintain temperature uniformity within ±1°C and feature calibrated spray nozzles that produce consistent fog density. Documentation requirements include detailed records of specimen preparation, orientation within the chamber, and post-test cleaning procedures. Testing laboratories seeking accreditation must demonstrate proficiency through interlaboratory comparison studies and regular equipment calibration.
Industry-specific standards have emerged to address specialized applications. The automotive sector follows SAE J2334 for cyclic corrosion testing, while aerospace manufacturers adhere to ASTM F1110 for sandwich corrosion testing of aircraft materials. The medical device industry must comply with ISO 10993-15 for biocompatibility testing of metals and alloys used in implantable devices.
Emerging trends in standardization include the development of accelerated test methods that correlate more accurately with real-world performance. The ISO 16539 standard introduces cyclic accelerated corrosion tests that better simulate natural weathering conditions. Additionally, there is growing emphasis on standardizing digital imaging techniques for quantitative assessment of corrosion damage, moving beyond traditional visual inspection methods.
Regulatory bodies increasingly require compliance with these standards as part of product certification processes. The European Construction Products Regulation (CPR) mandates corrosion testing according to harmonized standards for structural components. Similarly, the U.S. Food and Drug Administration (FDA) requires demonstration of corrosion resistance for medical implants through standardized testing protocols before market approval.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!