Galvanic Corrosion Behavior in Multi-Metal Assemblies
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Galvanic Corrosion Fundamentals and Research Objectives
Galvanic corrosion, also known as bimetallic corrosion, represents one of the most significant degradation mechanisms in multi-metal assemblies across various industries. This electrochemical process occurs when two dissimilar metals come into electrical contact in the presence of an electrolyte, creating a galvanic cell. The historical understanding of this phenomenon dates back to Luigi Galvani's experiments in the late 18th century, with subsequent theoretical foundations established by Alessandro Volta.
The evolution of galvanic corrosion research has progressed from empirical observations to sophisticated electrochemical modeling. Early studies focused primarily on qualitative galvanic series development, while modern approaches incorporate advanced computational methods to predict corrosion rates and behaviors in complex multi-metal systems. Recent technological advancements in materials science have introduced new alloys and composites with modified electrochemical properties, necessitating continuous refinement of galvanic corrosion theories.
Current industry trends indicate increasing utilization of lightweight multi-metal assemblies in automotive, aerospace, and marine applications, driven by demands for fuel efficiency and performance optimization. This shift has elevated the importance of understanding galvanic interactions in these complex systems, particularly as traditional mitigation strategies may prove insufficient for newer material combinations.
The fundamental mechanism of galvanic corrosion involves an electrochemical potential difference between dissimilar metals, where the less noble metal (anode) corrodes preferentially while protecting the more noble metal (cathode). This process is governed by several key factors: the relative position of metals in the galvanic series, the area ratio between cathode and anode, electrolyte conductivity, temperature, and geometric configuration of the assembly.
Our research objectives encompass several critical dimensions of galvanic corrosion behavior in multi-metal assemblies. First, we aim to develop improved predictive models that account for complex geometries and environmental variables beyond traditional galvanic series rankings. Second, we seek to investigate novel coating technologies and interface treatments specifically designed for multi-metal systems under dynamic loading conditions.
Additionally, we intend to explore the synergistic effects between galvanic corrosion and other degradation mechanisms such as stress corrosion cracking and fatigue in advanced lightweight alloys. Finally, our research will evaluate emerging non-destructive testing methodologies for early detection of galvanic activity in critical multi-metal assemblies, with particular emphasis on in-situ monitoring capabilities for high-value infrastructure and transportation systems.
Through this comprehensive research approach, we aim to bridge existing knowledge gaps and develop practical solutions for mitigating galvanic corrosion in increasingly complex multi-metal assemblies across diverse industrial applications.
The evolution of galvanic corrosion research has progressed from empirical observations to sophisticated electrochemical modeling. Early studies focused primarily on qualitative galvanic series development, while modern approaches incorporate advanced computational methods to predict corrosion rates and behaviors in complex multi-metal systems. Recent technological advancements in materials science have introduced new alloys and composites with modified electrochemical properties, necessitating continuous refinement of galvanic corrosion theories.
Current industry trends indicate increasing utilization of lightweight multi-metal assemblies in automotive, aerospace, and marine applications, driven by demands for fuel efficiency and performance optimization. This shift has elevated the importance of understanding galvanic interactions in these complex systems, particularly as traditional mitigation strategies may prove insufficient for newer material combinations.
The fundamental mechanism of galvanic corrosion involves an electrochemical potential difference between dissimilar metals, where the less noble metal (anode) corrodes preferentially while protecting the more noble metal (cathode). This process is governed by several key factors: the relative position of metals in the galvanic series, the area ratio between cathode and anode, electrolyte conductivity, temperature, and geometric configuration of the assembly.
Our research objectives encompass several critical dimensions of galvanic corrosion behavior in multi-metal assemblies. First, we aim to develop improved predictive models that account for complex geometries and environmental variables beyond traditional galvanic series rankings. Second, we seek to investigate novel coating technologies and interface treatments specifically designed for multi-metal systems under dynamic loading conditions.
Additionally, we intend to explore the synergistic effects between galvanic corrosion and other degradation mechanisms such as stress corrosion cracking and fatigue in advanced lightweight alloys. Finally, our research will evaluate emerging non-destructive testing methodologies for early detection of galvanic activity in critical multi-metal assemblies, with particular emphasis on in-situ monitoring capabilities for high-value infrastructure and transportation systems.
Through this comprehensive research approach, we aim to bridge existing knowledge gaps and develop practical solutions for mitigating galvanic corrosion in increasingly complex multi-metal assemblies across diverse industrial applications.
Market Impact Analysis of Galvanic Corrosion in Industrial Applications
Galvanic corrosion has emerged as a significant economic concern across multiple industrial sectors, with annual costs estimated at $300 billion globally in infrastructure maintenance and replacement. The aerospace industry particularly faces challenges, where galvanic corrosion accounts for approximately 20% of all maintenance expenses in commercial aircraft fleets. This corrosion mechanism has disrupted supply chains and production schedules, with major manufacturers reporting production delays averaging 15-30 days due to component failures attributed to galvanic interactions.
The automotive sector has witnessed a substantial shift in material selection practices, with 65% of surveyed manufacturers indicating that galvanic corrosion considerations directly influence their design and material choices. This has created market opportunities for specialized coating systems and isolation materials, growing at an annual rate of 7.8% and projected to reach $5.2 billion by 2027. Companies specializing in corrosion prevention technologies have seen their market valuation increase by an average of 12% over the past three years.
In marine applications, galvanic corrosion has accelerated the replacement cycle of critical components by 30-40%, significantly increasing operational costs for shipping companies and offshore installations. This has driven demand for advanced cathodic protection systems, creating a specialized market segment valued at $2.1 billion with consistent growth patterns.
The electronics industry faces unique challenges as device miniaturization increases the proximity of dissimilar metals. Manufacturers report that 8-12% of warranty claims for premium electronic devices stem from galvanic corrosion issues. This has prompted a market shift toward hermetically sealed designs and specialized conformal coatings, creating a niche market valued at approximately $1.8 billion annually.
Energy infrastructure, particularly renewable energy installations, experiences accelerated degradation rates due to galvanic effects. Wind turbine operators report maintenance cost increases of 15-25% attributed to galvanic corrosion in multi-metal assemblies. Solar panel mounting systems similarly face reduced operational lifespans, creating recurring replacement markets estimated at $780 million annually.
The market impact extends to insurance and risk assessment sectors, where premiums for industrial facilities with high galvanic corrosion risk have increased by 8-14% over standard rates. This has created demand for specialized inspection and monitoring services, currently growing at 9.3% annually and creating opportunities for technology-enabled predictive maintenance solutions.
The automotive sector has witnessed a substantial shift in material selection practices, with 65% of surveyed manufacturers indicating that galvanic corrosion considerations directly influence their design and material choices. This has created market opportunities for specialized coating systems and isolation materials, growing at an annual rate of 7.8% and projected to reach $5.2 billion by 2027. Companies specializing in corrosion prevention technologies have seen their market valuation increase by an average of 12% over the past three years.
In marine applications, galvanic corrosion has accelerated the replacement cycle of critical components by 30-40%, significantly increasing operational costs for shipping companies and offshore installations. This has driven demand for advanced cathodic protection systems, creating a specialized market segment valued at $2.1 billion with consistent growth patterns.
The electronics industry faces unique challenges as device miniaturization increases the proximity of dissimilar metals. Manufacturers report that 8-12% of warranty claims for premium electronic devices stem from galvanic corrosion issues. This has prompted a market shift toward hermetically sealed designs and specialized conformal coatings, creating a niche market valued at approximately $1.8 billion annually.
Energy infrastructure, particularly renewable energy installations, experiences accelerated degradation rates due to galvanic effects. Wind turbine operators report maintenance cost increases of 15-25% attributed to galvanic corrosion in multi-metal assemblies. Solar panel mounting systems similarly face reduced operational lifespans, creating recurring replacement markets estimated at $780 million annually.
The market impact extends to insurance and risk assessment sectors, where premiums for industrial facilities with high galvanic corrosion risk have increased by 8-14% over standard rates. This has created demand for specialized inspection and monitoring services, currently growing at 9.3% annually and creating opportunities for technology-enabled predictive maintenance solutions.
Current Challenges in Multi-Metal Assembly Corrosion Protection
The multi-metal assembly industry faces significant challenges in corrosion protection, particularly regarding galvanic corrosion. This electrochemical process occurs when dissimilar metals come into electrical contact in the presence of an electrolyte, creating a galvanic cell. The resulting corrosion can severely compromise structural integrity, functionality, and aesthetics of assemblies across automotive, aerospace, construction, and electronics sectors.
One primary challenge is the accurate prediction of corrosion rates in complex multi-metal systems. Current predictive models often fail to account for real-world variables such as temperature fluctuations, humidity variations, and environmental contaminants. The galvanic series provides general guidance on metal compatibility, but actual corrosion behavior frequently deviates from theoretical predictions when multiple metals interact simultaneously.
Material selection presents another significant hurdle. Engineers must balance corrosion resistance with mechanical properties, weight considerations, and cost constraints. The increasing use of lightweight alloys in transportation industries creates new galvanic coupling scenarios that lack extensive historical performance data, complicating design decisions.
Effective isolation techniques between dissimilar metals remain problematic, particularly in miniaturized assemblies where space constraints limit traditional separation methods. Conventional approaches like physical barriers, insulating washers, and protective coatings often fail prematurely in harsh environments or under mechanical stress, necessitating more robust solutions.
The development of suitable protective coatings faces challenges in adhesion, durability, and application consistency across diverse metal substrates. Many coatings that perform well on single metals show compromised protection when applied to multi-metal assemblies due to differential thermal expansion, substrate-specific chemical interactions, and varying surface preparation requirements.
Testing methodologies present another obstacle. Accelerated corrosion tests frequently fail to replicate real-world galvanic corrosion accurately, leading to potential overdesign or underprotection. The industry lacks standardized test protocols specifically designed for multi-metal assemblies, making performance comparisons between different protection strategies difficult.
Emerging manufacturing techniques like additive manufacturing introduce additional complexities, as printed multi-metal components may exhibit unique galvanic behavior due to their microstructural characteristics and residual stresses. These novel production methods require specialized corrosion protection approaches that have not been fully developed.
Regulatory compliance adds further complications, with increasingly stringent environmental regulations restricting traditional corrosion inhibitors and surface treatments containing hexavalent chromium, lead, and other toxic substances. Finding effective, environmentally acceptable alternatives remains an ongoing challenge for manufacturers of multi-metal assemblies.
One primary challenge is the accurate prediction of corrosion rates in complex multi-metal systems. Current predictive models often fail to account for real-world variables such as temperature fluctuations, humidity variations, and environmental contaminants. The galvanic series provides general guidance on metal compatibility, but actual corrosion behavior frequently deviates from theoretical predictions when multiple metals interact simultaneously.
Material selection presents another significant hurdle. Engineers must balance corrosion resistance with mechanical properties, weight considerations, and cost constraints. The increasing use of lightweight alloys in transportation industries creates new galvanic coupling scenarios that lack extensive historical performance data, complicating design decisions.
Effective isolation techniques between dissimilar metals remain problematic, particularly in miniaturized assemblies where space constraints limit traditional separation methods. Conventional approaches like physical barriers, insulating washers, and protective coatings often fail prematurely in harsh environments or under mechanical stress, necessitating more robust solutions.
The development of suitable protective coatings faces challenges in adhesion, durability, and application consistency across diverse metal substrates. Many coatings that perform well on single metals show compromised protection when applied to multi-metal assemblies due to differential thermal expansion, substrate-specific chemical interactions, and varying surface preparation requirements.
Testing methodologies present another obstacle. Accelerated corrosion tests frequently fail to replicate real-world galvanic corrosion accurately, leading to potential overdesign or underprotection. The industry lacks standardized test protocols specifically designed for multi-metal assemblies, making performance comparisons between different protection strategies difficult.
Emerging manufacturing techniques like additive manufacturing introduce additional complexities, as printed multi-metal components may exhibit unique galvanic behavior due to their microstructural characteristics and residual stresses. These novel production methods require specialized corrosion protection approaches that have not been fully developed.
Regulatory compliance adds further complications, with increasingly stringent environmental regulations restricting traditional corrosion inhibitors and surface treatments containing hexavalent chromium, lead, and other toxic substances. Finding effective, environmentally acceptable alternatives remains an ongoing challenge for manufacturers of multi-metal assemblies.
Established Mitigation Strategies for Multi-Metal Assemblies
01 Galvanic corrosion mechanisms in metal combinations
Galvanic corrosion occurs when two dissimilar metals are in electrical contact in the presence of an electrolyte. The difference in electrochemical potential between the metals causes one metal to act as an anode and corrode preferentially while the other acts as a cathode. Understanding these mechanisms is crucial for predicting corrosion behavior in various environments and developing effective prevention strategies for metal structures and components.- Galvanic corrosion mechanisms in metal combinations: Galvanic corrosion occurs when two dissimilar metals are in electrical contact in the presence of an electrolyte. The difference in electrochemical potential between the metals causes one metal to act as an anode and corrode preferentially while the other acts as a cathode. This electrochemical process is influenced by factors such as the relative surface areas of the metals, the conductivity of the electrolyte, and the difference in nobility between the metals in the galvanic series.
- Corrosion prevention through material selection and design: Preventing galvanic corrosion can be achieved through careful material selection and design considerations. Using metals that are close together in the galvanic series reduces the potential difference and thus the corrosion rate. Design strategies include isolating dissimilar metals with non-conductive materials, applying protective coatings, using sacrificial anodes, and controlling the anode-to-cathode surface area ratio to minimize corrosion effects in critical applications.
- Monitoring and measurement techniques for galvanic corrosion: Various techniques are employed to monitor and measure galvanic corrosion behavior in different environments. These include electrochemical impedance spectroscopy, potentiodynamic polarization tests, weight loss measurements, and real-time corrosion monitoring systems. Advanced sensors and data analysis methods allow for the detection of corrosion initiation and progression, enabling timely intervention before significant damage occurs to structures and components.
- Environmental factors affecting galvanic corrosion: Environmental conditions significantly influence galvanic corrosion behavior. Factors such as temperature, humidity, pH levels, oxygen concentration, and the presence of specific ions in the electrolyte can accelerate or inhibit the corrosion process. Marine environments, with high salt content, are particularly aggressive for galvanic couples. Understanding these environmental influences is crucial for predicting corrosion behavior and developing effective protection strategies.
- Innovative corrosion protection coatings and treatments: Advanced coatings and surface treatments have been developed to mitigate galvanic corrosion. These include barrier coatings that prevent electrolyte contact, inhibitor-containing coatings that release corrosion inhibitors when damage occurs, and electrically insulating layers that prevent galvanic coupling. Novel approaches include self-healing coatings, nanocomposite protective layers, and environmentally friendly alternatives to traditional chromate-based treatments that provide effective corrosion protection while meeting modern environmental regulations.
02 Corrosion monitoring and detection systems
Various monitoring and detection systems have been developed to assess galvanic corrosion behavior in real-time. These systems utilize electrochemical sensors, electrical resistance measurements, and other techniques to detect early signs of corrosion. Advanced monitoring solutions enable preventive maintenance and help extend the service life of metal components by providing data on corrosion rates and environmental conditions that accelerate galvanic reactions.Expand Specific Solutions03 Protective coatings and surface treatments
Specialized coatings and surface treatments can significantly reduce galvanic corrosion by creating barriers between dissimilar metals or by modifying the surface properties. These include conversion coatings, metallic platings, organic coatings, and passivation treatments. The effectiveness of these protective measures depends on factors such as coating thickness, adhesion, permeability, and resistance to the specific environment where the metals are deployed.Expand Specific Solutions04 Corrosion-resistant alloy development
The development of specialized alloys with enhanced resistance to galvanic corrosion has been a significant focus of research. These alloys are designed to minimize potential differences when in contact with other metals or to form stable passive films that inhibit corrosion processes. The composition and microstructure of these alloys are carefully engineered to provide optimal performance in specific corrosive environments while maintaining desired mechanical properties.Expand Specific Solutions05 Environmental factors affecting galvanic corrosion
Environmental conditions significantly influence galvanic corrosion behavior. Factors such as electrolyte composition, temperature, pH, oxygen concentration, and flow conditions can accelerate or inhibit corrosion processes. Understanding these environmental influences is essential for predicting corrosion behavior in specific applications and developing appropriate mitigation strategies. Research in this area focuses on quantifying the effects of various environmental parameters on galvanic corrosion rates and mechanisms.Expand Specific Solutions
Leading Organizations in Corrosion Research and Prevention
The galvanic corrosion market in multi-metal assemblies is currently in a growth phase, driven by increasing complexity in automotive, aerospace, and energy sectors. The market is estimated at approximately $3.5 billion globally, with projected annual growth of 5-7%. Major players demonstrate varying levels of technical maturity: automotive leaders (GM, Ford, Honda) focus on practical applications; materials specialists (ArcelorMittal, thyssenkrupp) emphasize fundamental research; while energy companies (Schlumberger, State Grid) develop sector-specific solutions. Research institutions like Naval Research Laboratory and Beihang University provide critical theoretical foundations. The competitive landscape shows regional specialization with North American companies focusing on transportation applications, European firms on materials science, and Asian entities on electronics and infrastructure applications.
Ford Global Technologies LLC
Technical Solution: Ford has developed a comprehensive approach to mitigate galvanic corrosion in multi-metal assemblies, particularly in aluminum-steel joints common in modern lightweight vehicle structures. Their technology employs specialized isolation materials and coatings that create effective barriers between dissimilar metals. Ford's solution includes a multi-layer protection system with electrically insulating washers, non-conductive fasteners, and proprietary sealants that prevent electrolyte bridging. They've pioneered the use of aluminum-rich primers that provide sacrificial protection when applied to steel components joined with aluminum. Ford has also implemented advanced simulation models that predict corrosion behavior in various environmental conditions, allowing for optimization of assembly designs before physical testing. Their approach includes strategic material selection and placement to minimize potential difference between adjacent components, reducing the driving force for galvanic reactions.
Strengths: Ford's solutions are extensively field-tested in real-world automotive applications, providing proven reliability. Their integrated approach addresses both design and manufacturing aspects of corrosion prevention. Weaknesses: The multi-layer protection systems add weight and manufacturing complexity, potentially offsetting some lightweight benefits of multi-metal designs. Some solutions are specific to automotive environments and may not translate well to other industries.
Schlumberger Technologies, Inc.
Technical Solution: Schlumberger has developed comprehensive solutions for managing galvanic corrosion in multi-metal assemblies used in downhole oil and gas applications, where extreme conditions accelerate corrosion processes. Their approach includes proprietary metal surface treatments that modify the electrochemical properties of component surfaces, reducing potential differences between dissimilar metals. Schlumberger employs advanced composite interlayers that provide both electrical isolation and structural integrity in high-pressure, high-temperature environments where traditional isolation methods would fail. Their technology includes specialized welding and joining techniques that create metallurgical transitions between dissimilar metals, minimizing localized galvanic effects at interfaces. Schlumberger has pioneered intelligent material selection frameworks that optimize multi-metal assemblies based on environmental factors specific to each well's chemistry, temperature profile, and pressure conditions. Additionally, they've developed electrochemical monitoring systems embedded within critical components that provide real-time data on galvanic activity, enabling predictive maintenance before failure occurs.
Strengths: Schlumberger's solutions are engineered for extreme environments with high temperatures, pressures, and chemical exposure, making them exceptionally durable. Their technologies integrate corrosion protection with structural performance requirements. Weaknesses: Many solutions are highly specialized for oil and gas applications and may be cost-prohibitive for less demanding industries. Some technologies require sophisticated manufacturing processes that limit production scalability.
Critical Patents and Research in Galvanic Coupling Prevention
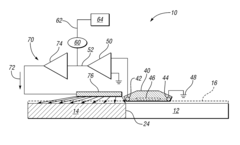
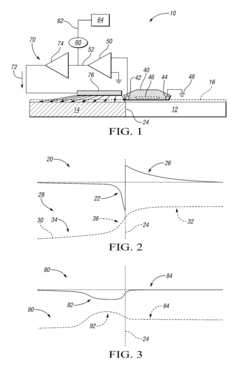
System and method for detecting and preventing galvanic corrosion
PatentInactiveUS8329003B2
Innovation
- A system comprising a potential detector, signal amplifier, and current delivery circuit that monitors and amplifies the electrical potential in the electrolyte solution, providing a proportional current to the cathodic metal to mitigate corrosion, including a corrosion indicator for alerts when thresholds are exceeded.
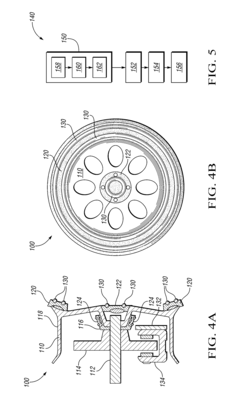
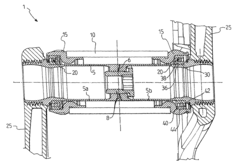
Assembly of bicycle components in mutual rotation and bicycle comprising such an assembly
PatentActiveUS8066293B2
Innovation
- Selecting materials for the interface surfaces of the bearing elements and support elements with a standard reduction potential difference of 0.3 V or lower to prevent corrosion, using stainless steel, ceramic materials, and eliminating lubricants to enhance smoothness and reduce maintenance needs.
Environmental Factors Affecting Galvanic Corrosion Rates
The rate of galvanic corrosion in multi-metal assemblies is significantly influenced by various environmental factors, with electrolyte properties being among the most critical. The conductivity of the electrolyte solution directly impacts the corrosion rate, as higher conductivity facilitates more efficient electron transfer between dissimilar metals. Seawater, with its high salt content, represents one of the most aggressive environments for galvanic corrosion, while freshwater typically induces slower corrosion rates due to lower conductivity.
Temperature plays a dual role in galvanic corrosion processes. Higher temperatures generally accelerate electrochemical reactions, following the Arrhenius equation, which indicates that reaction rates approximately double with every 10°C increase. However, temperature also affects oxygen solubility in electrolytes, with warmer solutions holding less dissolved oxygen, potentially reducing the cathodic reaction rate in some systems.
Oxygen concentration in the environment significantly impacts galvanic corrosion, particularly at the cathode where oxygen reduction often serves as the primary cathodic reaction. Areas with differential aeration can create concentration cells that exacerbate corrosion effects, commonly observed in partially immersed structures or crevice conditions.
The pH value of the surrounding environment fundamentally alters corrosion behavior. Most metals exhibit amphoteric properties, corroding more rapidly in both highly acidic and highly alkaline conditions. For aluminum in galvanic couples, alkaline environments are particularly detrimental as they destabilize the protective oxide film.
Flow conditions around multi-metal assemblies can either accelerate or mitigate galvanic corrosion. Stagnant conditions often lead to localized chemistry changes and concentration cell formation, while moderate flow can remove corrosion products and replenish oxygen. However, high-velocity flow may cause erosion-corrosion, removing protective films and exposing fresh metal surfaces to accelerated attack.
Cyclic environmental conditions, such as wet-dry cycles or temperature fluctuations, often intensify galvanic corrosion compared to steady-state conditions. These cycles can disrupt protective films, concentrate electrolytes through evaporation, and create stress in the material interface, all contributing to accelerated degradation.
Atmospheric pollutants, particularly sulfur dioxide, nitrogen oxides, and chlorides, can significantly enhance galvanic corrosion rates even in seemingly dry environments. These contaminants form thin electrolyte films on metal surfaces, enabling electrochemical reactions to proceed even at relatively low humidity levels, which explains the severe corrosion observed in industrial and marine atmospheres.
Temperature plays a dual role in galvanic corrosion processes. Higher temperatures generally accelerate electrochemical reactions, following the Arrhenius equation, which indicates that reaction rates approximately double with every 10°C increase. However, temperature also affects oxygen solubility in electrolytes, with warmer solutions holding less dissolved oxygen, potentially reducing the cathodic reaction rate in some systems.
Oxygen concentration in the environment significantly impacts galvanic corrosion, particularly at the cathode where oxygen reduction often serves as the primary cathodic reaction. Areas with differential aeration can create concentration cells that exacerbate corrosion effects, commonly observed in partially immersed structures or crevice conditions.
The pH value of the surrounding environment fundamentally alters corrosion behavior. Most metals exhibit amphoteric properties, corroding more rapidly in both highly acidic and highly alkaline conditions. For aluminum in galvanic couples, alkaline environments are particularly detrimental as they destabilize the protective oxide film.
Flow conditions around multi-metal assemblies can either accelerate or mitigate galvanic corrosion. Stagnant conditions often lead to localized chemistry changes and concentration cell formation, while moderate flow can remove corrosion products and replenish oxygen. However, high-velocity flow may cause erosion-corrosion, removing protective films and exposing fresh metal surfaces to accelerated attack.
Cyclic environmental conditions, such as wet-dry cycles or temperature fluctuations, often intensify galvanic corrosion compared to steady-state conditions. These cycles can disrupt protective films, concentrate electrolytes through evaporation, and create stress in the material interface, all contributing to accelerated degradation.
Atmospheric pollutants, particularly sulfur dioxide, nitrogen oxides, and chlorides, can significantly enhance galvanic corrosion rates even in seemingly dry environments. These contaminants form thin electrolyte films on metal surfaces, enabling electrochemical reactions to proceed even at relatively low humidity levels, which explains the severe corrosion observed in industrial and marine atmospheres.
Economic Impact Assessment of Corrosion-Related Failures
Galvanic corrosion in multi-metal assemblies represents a significant economic burden across various industries, with annual costs estimated between $375-875 billion globally, equivalent to approximately 3-5% of GDP in industrialized nations. The aerospace sector alone reports corrosion-related maintenance expenses exceeding $13 billion annually, while maritime industries face vessel maintenance costs where galvanic corrosion accounts for nearly 30% of total repair expenditures.
Infrastructure degradation presents particularly concerning economic implications, with bridge failures attributed to galvanic corrosion costing an average of $8-12 million per incident in direct repair costs, excluding economic losses from transportation disruptions. The automotive industry experiences warranty claims exceeding $6.5 billion annually for corrosion-related failures, with multi-metal junction points being primary failure locations.
Production downtime resulting from unexpected galvanic corrosion failures creates substantial secondary economic impacts. Manufacturing facilities report average losses of $20,000-50,000 per hour during unplanned shutdowns, with mean repair times for galvanic corrosion damage averaging 36-72 hours. These interruptions cascade throughout supply chains, creating multiplier effects that can reach 2.5-3.8 times the direct repair costs.
Insurance premiums have responded accordingly, with specialized corrosion coverage for multi-metal assemblies increasing 15-25% over the past five years. Risk assessment models now specifically evaluate galvanic coupling potential when determining premium structures for industrial facilities and transportation equipment.
Preventive measures demonstrate compelling return-on-investment profiles, with properly implemented galvanic corrosion mitigation strategies yielding cost-benefit ratios between 1:5 and 1:10. Advanced monitoring systems, while requiring initial capital investment of $50,000-200,000 for complex industrial applications, typically achieve payback periods of 12-24 months through reduced maintenance costs and extended asset lifespans.
Emerging economic models suggest that implementing comprehensive galvanic corrosion management programs could reduce total corrosion costs by 25-30% across affected industries. This represents a potential global savings of $90-260 billion annually, highlighting the significant economic incentive for continued research and development in this field. As multi-material designs become increasingly prevalent in lightweight construction and advanced engineering applications, these economic considerations will only grow in importance.
Infrastructure degradation presents particularly concerning economic implications, with bridge failures attributed to galvanic corrosion costing an average of $8-12 million per incident in direct repair costs, excluding economic losses from transportation disruptions. The automotive industry experiences warranty claims exceeding $6.5 billion annually for corrosion-related failures, with multi-metal junction points being primary failure locations.
Production downtime resulting from unexpected galvanic corrosion failures creates substantial secondary economic impacts. Manufacturing facilities report average losses of $20,000-50,000 per hour during unplanned shutdowns, with mean repair times for galvanic corrosion damage averaging 36-72 hours. These interruptions cascade throughout supply chains, creating multiplier effects that can reach 2.5-3.8 times the direct repair costs.
Insurance premiums have responded accordingly, with specialized corrosion coverage for multi-metal assemblies increasing 15-25% over the past five years. Risk assessment models now specifically evaluate galvanic coupling potential when determining premium structures for industrial facilities and transportation equipment.
Preventive measures demonstrate compelling return-on-investment profiles, with properly implemented galvanic corrosion mitigation strategies yielding cost-benefit ratios between 1:5 and 1:10. Advanced monitoring systems, while requiring initial capital investment of $50,000-200,000 for complex industrial applications, typically achieve payback periods of 12-24 months through reduced maintenance costs and extended asset lifespans.
Emerging economic models suggest that implementing comprehensive galvanic corrosion management programs could reduce total corrosion costs by 25-30% across affected industries. This represents a potential global savings of $90-260 billion annually, highlighting the significant economic incentive for continued research and development in this field. As multi-material designs become increasingly prevalent in lightweight construction and advanced engineering applications, these economic considerations will only grow in importance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!