High-Temperature Oxidation Resistance of Corrosion-Resistant Alloys
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
High-Temperature Alloy Development History and Objectives
The development of high-temperature oxidation-resistant alloys traces back to the early 20th century, with significant advancements occurring during World War II when the need for jet engine materials catalyzed research. The 1940s saw the introduction of the first nickel-based superalloys, which represented a breakthrough in combining strength and oxidation resistance at elevated temperatures. By the 1950s, researchers had begun to understand the critical role of chromium and aluminum in forming protective oxide scales.
The 1960s and 1970s marked a period of rapid innovation with the development of directionally solidified and single-crystal superalloys, dramatically improving high-temperature performance. Concurrently, the addition of reactive elements such as yttrium and hafnium was discovered to enhance oxide scale adhesion, a phenomenon that became known as the "reactive element effect."
The 1980s and 1990s witnessed the emergence of thermal barrier coating systems and the refinement of alloy compositions through computational methods. This era also saw increased focus on understanding oxidation mechanisms at the microstructural level, leading to more targeted alloy designs.
In the early 2000s, research shifted toward developing alloys for ultra-high temperature applications exceeding 1100°C, with emphasis on refractory metal additions and intermetallic compounds. The past decade has seen growing interest in alloys that can withstand extreme environments combining high temperature, corrosive species, and mechanical stresses.
Current technological objectives center on developing alloys with oxidation resistance above 1200°C while maintaining mechanical properties and manufacturability. Researchers aim to extend component lifetimes in aggressive environments by improving oxide scale stability and self-healing capabilities. There is particular focus on reducing or eliminating strategic elements like cobalt and rhenium due to supply chain vulnerabilities.
Another key objective is the development of predictive models that can accurately forecast oxidation behavior over component lifetimes, reducing the need for extensive testing. The industry is also pursuing alloys compatible with advanced manufacturing techniques such as additive manufacturing, which presents unique challenges for microstructural control and oxidation performance.
Looking forward, the field is trending toward multi-principal element alloys (MPEAs) or high-entropy alloys as a promising direction for achieving unprecedented combinations of oxidation resistance and mechanical properties. The ultimate goal remains creating materials that can push the efficiency boundaries of energy generation, aerospace, and industrial systems through higher operating temperatures.
The 1960s and 1970s marked a period of rapid innovation with the development of directionally solidified and single-crystal superalloys, dramatically improving high-temperature performance. Concurrently, the addition of reactive elements such as yttrium and hafnium was discovered to enhance oxide scale adhesion, a phenomenon that became known as the "reactive element effect."
The 1980s and 1990s witnessed the emergence of thermal barrier coating systems and the refinement of alloy compositions through computational methods. This era also saw increased focus on understanding oxidation mechanisms at the microstructural level, leading to more targeted alloy designs.
In the early 2000s, research shifted toward developing alloys for ultra-high temperature applications exceeding 1100°C, with emphasis on refractory metal additions and intermetallic compounds. The past decade has seen growing interest in alloys that can withstand extreme environments combining high temperature, corrosive species, and mechanical stresses.
Current technological objectives center on developing alloys with oxidation resistance above 1200°C while maintaining mechanical properties and manufacturability. Researchers aim to extend component lifetimes in aggressive environments by improving oxide scale stability and self-healing capabilities. There is particular focus on reducing or eliminating strategic elements like cobalt and rhenium due to supply chain vulnerabilities.
Another key objective is the development of predictive models that can accurately forecast oxidation behavior over component lifetimes, reducing the need for extensive testing. The industry is also pursuing alloys compatible with advanced manufacturing techniques such as additive manufacturing, which presents unique challenges for microstructural control and oxidation performance.
Looking forward, the field is trending toward multi-principal element alloys (MPEAs) or high-entropy alloys as a promising direction for achieving unprecedented combinations of oxidation resistance and mechanical properties. The ultimate goal remains creating materials that can push the efficiency boundaries of energy generation, aerospace, and industrial systems through higher operating temperatures.
Market Analysis for Heat-Resistant Materials
The global market for heat-resistant materials, particularly corrosion-resistant alloys with high-temperature oxidation resistance, has been experiencing robust growth driven by increasing demands across multiple industrial sectors. The market size was valued at approximately $7.2 billion in 2022 and is projected to reach $10.5 billion by 2028, representing a compound annual growth rate (CAGR) of 6.4%.
Aerospace and defense industries remain the largest consumers of these specialized alloys, accounting for nearly 35% of the total market share. The continuous pursuit of higher operating temperatures in aircraft engines to improve fuel efficiency and performance has significantly boosted demand for advanced heat-resistant materials capable of withstanding temperatures exceeding 1000°C while maintaining structural integrity.
Power generation follows closely as the second-largest application segment, particularly with the growing installation of advanced gas turbines and next-generation nuclear reactors. The transition toward cleaner energy sources has paradoxically increased the need for materials that can withstand extreme conditions, as higher operating temperatures improve energy conversion efficiency.
Regionally, North America and Europe currently dominate the market with a combined share of approximately 58%, primarily due to their established aerospace, defense, and power generation industries. However, the Asia-Pacific region is emerging as the fastest-growing market with an estimated CAGR of 8.2%, driven by rapid industrialization in China and India, alongside significant investments in energy infrastructure.
The automotive sector represents an expanding application area, particularly with the growing interest in high-efficiency engines and exhaust systems. The market penetration in this sector is expected to grow by 25% over the next five years as emission standards become increasingly stringent worldwide.
A notable trend is the increasing demand for nickel-based superalloys, which currently hold approximately 42% of the heat-resistant alloys market. These materials offer exceptional resistance to oxidation at temperatures up to 1200°C, making them indispensable for critical high-temperature applications.
The market is also witnessing growing interest in ceramic matrix composites (CMCs) as potential alternatives to traditional metallic alloys in extreme temperature environments. Though currently representing only about 8% of the market, CMCs are projected to grow at a CAGR of 12.3%, significantly outpacing the overall market growth rate.
Customer requirements are evolving toward materials that offer longer service life and reduced maintenance intervals, with lifecycle cost increasingly becoming a key decision factor rather than initial acquisition cost alone. This shift is driving manufacturers to focus on developing alloys with enhanced durability and predictable degradation patterns.
Aerospace and defense industries remain the largest consumers of these specialized alloys, accounting for nearly 35% of the total market share. The continuous pursuit of higher operating temperatures in aircraft engines to improve fuel efficiency and performance has significantly boosted demand for advanced heat-resistant materials capable of withstanding temperatures exceeding 1000°C while maintaining structural integrity.
Power generation follows closely as the second-largest application segment, particularly with the growing installation of advanced gas turbines and next-generation nuclear reactors. The transition toward cleaner energy sources has paradoxically increased the need for materials that can withstand extreme conditions, as higher operating temperatures improve energy conversion efficiency.
Regionally, North America and Europe currently dominate the market with a combined share of approximately 58%, primarily due to their established aerospace, defense, and power generation industries. However, the Asia-Pacific region is emerging as the fastest-growing market with an estimated CAGR of 8.2%, driven by rapid industrialization in China and India, alongside significant investments in energy infrastructure.
The automotive sector represents an expanding application area, particularly with the growing interest in high-efficiency engines and exhaust systems. The market penetration in this sector is expected to grow by 25% over the next five years as emission standards become increasingly stringent worldwide.
A notable trend is the increasing demand for nickel-based superalloys, which currently hold approximately 42% of the heat-resistant alloys market. These materials offer exceptional resistance to oxidation at temperatures up to 1200°C, making them indispensable for critical high-temperature applications.
The market is also witnessing growing interest in ceramic matrix composites (CMCs) as potential alternatives to traditional metallic alloys in extreme temperature environments. Though currently representing only about 8% of the market, CMCs are projected to grow at a CAGR of 12.3%, significantly outpacing the overall market growth rate.
Customer requirements are evolving toward materials that offer longer service life and reduced maintenance intervals, with lifecycle cost increasingly becoming a key decision factor rather than initial acquisition cost alone. This shift is driving manufacturers to focus on developing alloys with enhanced durability and predictable degradation patterns.
Current Challenges in Oxidation Resistance Technology
Despite significant advancements in high-temperature alloy development, several critical challenges persist in oxidation resistance technology for corrosion-resistant alloys. The formation of protective oxide scales, primarily chromia (Cr2O3) and alumina (Al2O3), remains fundamental to oxidation resistance, yet these protective layers face stability issues at ultra-high temperatures exceeding 1000°C. The volatilization of chromia as CrO3 in environments containing water vapor presents a particularly vexing problem, leading to accelerated material degradation in steam-containing atmospheres relevant to power generation applications.
Cyclic oxidation resistance represents another significant challenge, as thermal cycling induces mechanical stresses at the metal-oxide interface, resulting in spallation and subsequent accelerated oxidation. This issue is exacerbated in components experiencing frequent temperature fluctuations, such as gas turbine blades and industrial furnace components, where the coefficient of thermal expansion mismatch between the substrate and oxide layer becomes critical.
The presence of contaminants in service environments, particularly sulfur, vanadium, and alkali metals, introduces complex degradation mechanisms through hot corrosion. These contaminants can form low-melting eutectics that dissolve protective oxide scales, creating a fluxing mechanism that continuously exposes fresh metal surfaces to oxidizing environments. This phenomenon is particularly problematic in marine environments, waste incineration systems, and certain petrochemical processes.
Interdiffusion between coating systems and substrate alloys presents another technical hurdle. While overlay coatings and diffusion coatings provide enhanced oxidation resistance, the migration of elements between coating and substrate during high-temperature service can lead to the formation of detrimental phases, reduced mechanical properties, and eventually coating failure. The depletion of scale-forming elements like chromium and aluminum from the substrate further complicates this issue.
Manufacturing challenges also persist, particularly in achieving uniform protective coatings on complex geometries. Advanced coating technologies such as electron beam physical vapor deposition (EB-PVD) and high-velocity oxygen fuel (HVOF) spraying require precise control parameters, and their application to intricate components with internal cooling passages remains problematic. Additionally, the cost-effectiveness of these advanced coating technologies presents economic barriers to widespread industrial implementation.
The development of predictive models for oxidation behavior represents a significant scientific challenge. Current models struggle to accurately predict the complex interplay between oxidation kinetics, mechanical stresses, and microstructural evolution during long-term service. This limitation hampers the ability to forecast component lifetimes accurately and optimize maintenance schedules in critical high-temperature applications.
Cyclic oxidation resistance represents another significant challenge, as thermal cycling induces mechanical stresses at the metal-oxide interface, resulting in spallation and subsequent accelerated oxidation. This issue is exacerbated in components experiencing frequent temperature fluctuations, such as gas turbine blades and industrial furnace components, where the coefficient of thermal expansion mismatch between the substrate and oxide layer becomes critical.
The presence of contaminants in service environments, particularly sulfur, vanadium, and alkali metals, introduces complex degradation mechanisms through hot corrosion. These contaminants can form low-melting eutectics that dissolve protective oxide scales, creating a fluxing mechanism that continuously exposes fresh metal surfaces to oxidizing environments. This phenomenon is particularly problematic in marine environments, waste incineration systems, and certain petrochemical processes.
Interdiffusion between coating systems and substrate alloys presents another technical hurdle. While overlay coatings and diffusion coatings provide enhanced oxidation resistance, the migration of elements between coating and substrate during high-temperature service can lead to the formation of detrimental phases, reduced mechanical properties, and eventually coating failure. The depletion of scale-forming elements like chromium and aluminum from the substrate further complicates this issue.
Manufacturing challenges also persist, particularly in achieving uniform protective coatings on complex geometries. Advanced coating technologies such as electron beam physical vapor deposition (EB-PVD) and high-velocity oxygen fuel (HVOF) spraying require precise control parameters, and their application to intricate components with internal cooling passages remains problematic. Additionally, the cost-effectiveness of these advanced coating technologies presents economic barriers to widespread industrial implementation.
The development of predictive models for oxidation behavior represents a significant scientific challenge. Current models struggle to accurately predict the complex interplay between oxidation kinetics, mechanical stresses, and microstructural evolution during long-term service. This limitation hampers the ability to forecast component lifetimes accurately and optimize maintenance schedules in critical high-temperature applications.
Current Oxidation Protection Strategies
01 Nickel-based superalloys for high temperature oxidation resistance
Nickel-based superalloys are widely used for applications requiring high temperature oxidation resistance. These alloys typically contain chromium, aluminum, and other elements that form protective oxide scales. The addition of elements such as yttrium, hafnium, or rare earth metals can further enhance the adhesion of the protective oxide layer, improving long-term oxidation resistance in extreme environments like gas turbines and aerospace applications.- Chromium-containing alloys for oxidation resistance: Chromium is a key element in developing corrosion-resistant alloys with enhanced oxidation resistance. These alloys form protective chromium oxide (Cr2O3) layers when exposed to high temperatures, creating a barrier against further oxidation. The chromium content typically ranges from 10-30% depending on the application environment, with higher chromium content generally providing better oxidation resistance. These alloys may also contain other elements to enhance the stability and adherence of the protective oxide layer.
- Nickel-based superalloys with improved oxidation resistance: Nickel-based superalloys are widely used in high-temperature applications requiring excellent oxidation resistance. These alloys typically contain chromium, aluminum, and reactive elements that form protective oxide scales. The addition of elements such as yttrium, hafnium, and rare earth metals improves the adhesion of the protective oxide layer. These superalloys can maintain their mechanical properties and oxidation resistance at temperatures exceeding 1000°C, making them suitable for turbine components and other high-temperature applications.
- Aluminum-containing alloys for high-temperature oxidation resistance: Aluminum-containing alloys form protective alumina (Al2O3) scales that provide exceptional oxidation resistance at elevated temperatures. These alloys typically contain sufficient aluminum (usually 4-6%) to form and maintain a continuous alumina layer. The alumina scale grows more slowly than chromia scales at high temperatures, providing longer-term protection. Some of these alloys also incorporate reactive elements like yttrium or zirconium to improve the adherence and stability of the alumina scale, particularly during thermal cycling conditions.
- Silicon-containing alloys for specialized oxidation environments: Silicon additions to alloys can significantly improve oxidation resistance in certain environments by forming protective silica (SiO2) layers. These alloys are particularly effective in environments containing water vapor or acidic components where other protective oxides might fail. Silicon-containing alloys typically incorporate 1-4% silicon along with chromium and other elements to create multi-layered protective scales. These materials find applications in petrochemical processing, waste incineration systems, and other aggressive environments where both oxidation and corrosion resistance are required.
- Surface treatments and coatings for enhanced oxidation resistance: Various surface treatments and coatings can be applied to alloys to enhance their oxidation resistance. These include aluminizing, chromizing, and the application of thermal barrier coatings. Diffusion coatings enrich the surface with elements like aluminum or chromium, promoting the formation of protective oxide scales. Overlay coatings such as MCrAlY (where M is nickel, cobalt, or a combination) provide an additional protective layer. These surface modifications can significantly extend the service life of components in high-temperature oxidizing environments without changing the bulk properties of the base alloy.
02 Chromium-containing alloys for corrosion protection
Chromium is a key element in corrosion-resistant alloys due to its ability to form a passive chromium oxide layer. Alloys with chromium content typically between 12-30% demonstrate excellent resistance to oxidizing environments. The protective chromium oxide film self-heals when damaged, providing continuous protection against corrosive media. These alloys may be further enhanced with molybdenum, nitrogen, or other elements to improve specific corrosion resistance properties.Expand Specific Solutions03 Aluminum-containing alloys for high-temperature applications
Aluminum additions to alloys promote the formation of aluminum oxide (Al₂O₃) scales that provide exceptional protection against high-temperature oxidation. These alloys typically contain sufficient aluminum to form a continuous, adherent alumina layer while maintaining mechanical properties. The alumina scale grows slowly and is highly stable at elevated temperatures, making these alloys suitable for combustion environments, heat exchangers, and other high-temperature applications where oxidation resistance is critical.Expand Specific Solutions04 Silicon additions for improved oxidation resistance
Silicon is an effective alloying element for enhancing oxidation resistance in various alloy systems. When added in controlled amounts, silicon promotes the formation of silica (SiO₂) or complex silicate layers that act as diffusion barriers against oxygen penetration. Silicon-containing alloys show improved performance in cyclic oxidation conditions and in environments containing water vapor. These alloys find applications in power generation, petrochemical processing, and other industries requiring resistance to aggressive oxidizing environments.Expand Specific Solutions05 Surface treatments and coatings for enhanced oxidation resistance
Various surface treatments and coatings can significantly improve the oxidation resistance of alloys. These include aluminizing, chromizing, silicon diffusion treatments, and the application of thermal barrier coatings. Advanced coating systems may incorporate multiple layers with different compositions to provide optimal protection. Some treatments modify the surface composition to promote the formation of more protective oxide scales, while others provide a physical barrier against oxidizing species, extending component life in harsh environments.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The high-temperature oxidation resistance of corrosion-resistant alloys market is currently in a growth phase, with increasing demand driven by energy, aerospace, and industrial applications. The global market size is estimated to exceed $5 billion, expanding at 6-8% CAGR. Leading players demonstrate varying levels of technological maturity: Mitsubishi Heavy Industries, Sumitomo Metal, and Nippon Yakin Kogyo possess advanced proprietary technologies; Institute of Metal Research CAS and Central South University contribute significant research innovations; while companies like Thyssenkrupp VDM, Oerlikon Surface Solutions, and Proterial are developing specialized commercial applications. Chinese companies including Xi'an Thermal Power Research Institute and Chongqing Instrument Materials Research Institute are rapidly advancing their capabilities, narrowing the technology gap with established Japanese and European manufacturers.
Mitsubishi Heavy Industries, Ltd.
Technical Solution: Mitsubishi Heavy Industries has developed advanced high-temperature oxidation resistant alloys featuring proprietary Ni-Cr-Fe compositions with controlled additions of Al, Si, and rare earth elements. Their technology employs a dual-layer protection mechanism where an initial chromia (Cr2O3) scale forms rapidly, followed by a more stable alumina (Al2O3) layer that provides long-term protection. MHI's alloys incorporate precise microstructural control through specialized heat treatments that optimize grain boundary characteristics, reducing diffusion pathways for oxygen penetration. The company has implemented a surface modification technique involving pre-oxidation treatments under controlled atmospheres to establish protective oxide layers before service. Their research has demonstrated that these alloys maintain structural integrity at temperatures exceeding 1000°C for extended periods, with oxidation rates significantly lower than conventional heat-resistant steels.
Strengths: Superior long-term stability at extreme temperatures with oxidation resistance maintained for over 10,000 hours at 1000°C. Their dual-layer protection mechanism provides redundancy against scale spallation. Weaknesses: Higher manufacturing costs compared to conventional alloys due to complex processing requirements and the addition of expensive rare earth elements. Limited weldability can complicate repairs and fabrication.
Institute of Metal Research Chinese Academy of Sciences
Technical Solution: The Institute of Metal Research (IMR) has pioneered innovative high-temperature oxidation resistant alloys through their multi-element alloying strategy. Their approach focuses on developing Ni-Cr-Al based superalloys with precisely controlled additions of reactive elements (Y, Hf, Zr) that significantly enhance oxide scale adhesion. IMR has developed a unique microstructural engineering technique that creates nano-dispersed oxide particles within the alloy matrix, serving as nucleation sites for protective oxide formation and anchoring points for the oxide scale. Their research has demonstrated that controlling the distribution of these particles dramatically improves scale adhesion during thermal cycling. Additionally, IMR has implemented advanced surface modification technologies including pack cementation and slurry coating methods to create aluminum-enriched surface layers that form stable α-Al2O3 scales. Their alloys have demonstrated exceptional resistance to cyclic oxidation with minimal mass change even after hundreds of thermal cycles between room temperature and 1100°C.
Strengths: Exceptional resistance to oxide spallation during thermal cycling due to their nano-dispersed oxide particle technology. Their alloys demonstrate superior performance in fluctuating temperature environments typical in power generation applications. Weaknesses: The complex manufacturing process requires precise control of multiple elements and processing parameters, leading to potential batch-to-batch variability. The high aluminum content in surface-modified variants can reduce ductility and complicate forming operations.
Key Patents in High-Temperature Alloy Protection
High temperature corrosion resistant alloy, thermal barrier coating material, and gas turbine using high temperature corrosion resistant alloy
PatentInactiveUS6756131B2
Innovation
- A high-temperature corrosion-resistant alloy with a composition of 0.1-12% Co, 10-30% Cr, 4-15% Al, 0.1-5% Y, and 0.5-10% Re, with the rest being Ni, is used to form a metal bonding layer that enhances ductility and oxidation resistance, allowing for a stable thermal barrier coating that prevents ceramic layer separation and oxidation of the base material.
High-temperature corrosion-resistant alloy with high toughness, and structure used under high-temperature corrosive environment
PatentActiveJP2009221523A
Innovation
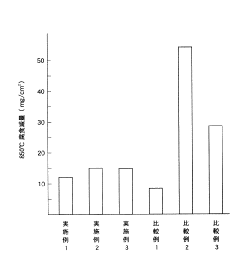
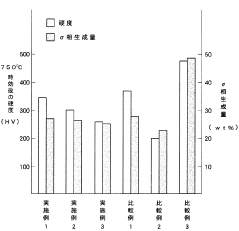
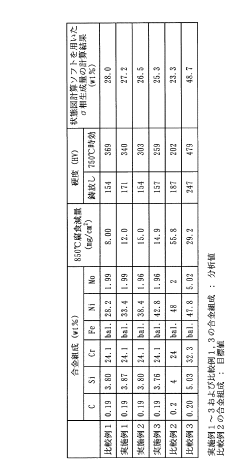
- A high-toughness high-temperature corrosion-resistant alloy with specific compositions of Ni (33-43 wt%), Cr (23-29 wt%), Mo (1.0-3.0 wt%), Si (3.0-4.0 wt%), and C (0.15-0.25 wt%), balanced with Fe and unavoidable impurities, is developed to minimize molten salt corrosion and prevent σ phase precipitation, maintaining toughness and corrosion resistance.
Environmental Impact of Alloy Manufacturing
The manufacturing processes of corrosion-resistant alloys with high-temperature oxidation resistance involve significant environmental considerations that extend throughout their lifecycle. Primary production of these specialized alloys typically requires extensive mining operations for extracting nickel, chromium, molybdenum, and other critical elements, leading to habitat disruption, soil degradation, and potential water contamination in mining regions. The subsequent refining and processing stages are notably energy-intensive, with some specialty alloy production consuming 10-15 times more energy per unit weight than conventional steel manufacturing.
Emissions from alloy manufacturing facilities present another environmental challenge. The production of high-temperature resistant alloys releases substantial quantities of greenhouse gases, primarily CO2, with estimates suggesting that specialty alloy production generates 4-7 times higher carbon emissions compared to standard steel production per ton of material. Additionally, the release of particulate matter containing heavy metals and volatile organic compounds poses localized air quality concerns in manufacturing zones.
Water usage represents a significant environmental footprint in alloy manufacturing, with cooling processes and chemical treatments requiring 20-40 cubic meters of water per ton of finished alloy. Wastewater from these operations often contains dissolved metals and processing chemicals that require extensive treatment before discharge to prevent aquatic ecosystem damage.
Recent advancements in manufacturing technologies have begun addressing these environmental challenges. Closed-loop water systems have reduced freshwater consumption by up to 60% in modern facilities. Electric arc furnace technologies powered by renewable energy sources have demonstrated potential to decrease carbon emissions by 30-50% compared to traditional methods. Additionally, improved filtration systems have reduced particulate emissions by up to 95% in state-of-the-art production facilities.
The recycling potential of these alloys represents a significant environmental advantage. With recovery rates exceeding 80% for nickel and chromium from end-of-life components, the circular economy approach substantially reduces the need for primary resource extraction. Life cycle assessments indicate that using recycled content in alloy production can reduce energy consumption by 60-75% and decrease carbon emissions by similar proportions.
Regulatory frameworks worldwide are increasingly focusing on the environmental impact of specialty alloy manufacturing, with stricter emissions standards and extended producer responsibility requirements emerging in major manufacturing regions. These regulations are driving innovation in cleaner production technologies and more sustainable supply chain practices throughout the industry.
Emissions from alloy manufacturing facilities present another environmental challenge. The production of high-temperature resistant alloys releases substantial quantities of greenhouse gases, primarily CO2, with estimates suggesting that specialty alloy production generates 4-7 times higher carbon emissions compared to standard steel production per ton of material. Additionally, the release of particulate matter containing heavy metals and volatile organic compounds poses localized air quality concerns in manufacturing zones.
Water usage represents a significant environmental footprint in alloy manufacturing, with cooling processes and chemical treatments requiring 20-40 cubic meters of water per ton of finished alloy. Wastewater from these operations often contains dissolved metals and processing chemicals that require extensive treatment before discharge to prevent aquatic ecosystem damage.
Recent advancements in manufacturing technologies have begun addressing these environmental challenges. Closed-loop water systems have reduced freshwater consumption by up to 60% in modern facilities. Electric arc furnace technologies powered by renewable energy sources have demonstrated potential to decrease carbon emissions by 30-50% compared to traditional methods. Additionally, improved filtration systems have reduced particulate emissions by up to 95% in state-of-the-art production facilities.
The recycling potential of these alloys represents a significant environmental advantage. With recovery rates exceeding 80% for nickel and chromium from end-of-life components, the circular economy approach substantially reduces the need for primary resource extraction. Life cycle assessments indicate that using recycled content in alloy production can reduce energy consumption by 60-75% and decrease carbon emissions by similar proportions.
Regulatory frameworks worldwide are increasingly focusing on the environmental impact of specialty alloy manufacturing, with stricter emissions standards and extended producer responsibility requirements emerging in major manufacturing regions. These regulations are driving innovation in cleaner production technologies and more sustainable supply chain practices throughout the industry.
Standardization and Testing Protocols
The standardization of testing protocols for high-temperature oxidation resistance of corrosion-resistant alloys is critical for ensuring reliable performance evaluation and comparison across different materials and applications. Currently, several international standards govern these testing procedures, including ASTM G54, ISO 21608, and NACE TM0169, each providing specific guidelines for different aspects of oxidation testing.
These standardized protocols typically define key parameters such as temperature ranges (commonly 600-1200°C), exposure durations (from short-term 100-hour tests to long-term 10,000+ hour evaluations), atmosphere compositions (including air, steam, CO2, and mixed gases), and pressure conditions that simulate various industrial environments. The standardization ensures that test results from different laboratories and research institutions can be meaningfully compared.
Weight change measurement remains the most widely adopted quantitative method, with standards specifying precise procedures for sample preparation, weighing intervals, and data reporting. Complementary analytical techniques have also been standardized, including cross-sectional microscopy protocols for oxide scale characterization, X-ray diffraction procedures for phase identification, and electron microscopy methods for microstructural analysis.
Cyclic oxidation testing has received particular attention in recent standardization efforts, with protocols now addressing cooling rates, thermal cycling frequencies, and sample handling between cycles. These parameters significantly influence spallation behavior and overall oxidation resistance, making standardization essential for meaningful material comparisons.
Industry-specific testing protocols have emerged for specialized applications, such as ASTM D6557 for petrochemical environments and specialized procedures for nuclear, aerospace, and power generation sectors. These tailored standards address unique conditions such as radiation exposure, high-velocity gas flows, or specific contaminants that may accelerate degradation.
Round-robin testing programs conducted by organizations like NIST, NPL, and industrial consortia have been instrumental in validating and refining these standards. These collaborative efforts have identified critical variables affecting test reproducibility and established statistical frameworks for data interpretation and uncertainty quantification.
The digital transformation of testing protocols represents the newest frontier in standardization, with emerging guidelines for automated data collection, machine learning approaches for predictive modeling, and standardized formats for sharing oxidation test data across research communities. These developments aim to accelerate material development through more efficient knowledge transfer and data utilization.
These standardized protocols typically define key parameters such as temperature ranges (commonly 600-1200°C), exposure durations (from short-term 100-hour tests to long-term 10,000+ hour evaluations), atmosphere compositions (including air, steam, CO2, and mixed gases), and pressure conditions that simulate various industrial environments. The standardization ensures that test results from different laboratories and research institutions can be meaningfully compared.
Weight change measurement remains the most widely adopted quantitative method, with standards specifying precise procedures for sample preparation, weighing intervals, and data reporting. Complementary analytical techniques have also been standardized, including cross-sectional microscopy protocols for oxide scale characterization, X-ray diffraction procedures for phase identification, and electron microscopy methods for microstructural analysis.
Cyclic oxidation testing has received particular attention in recent standardization efforts, with protocols now addressing cooling rates, thermal cycling frequencies, and sample handling between cycles. These parameters significantly influence spallation behavior and overall oxidation resistance, making standardization essential for meaningful material comparisons.
Industry-specific testing protocols have emerged for specialized applications, such as ASTM D6557 for petrochemical environments and specialized procedures for nuclear, aerospace, and power generation sectors. These tailored standards address unique conditions such as radiation exposure, high-velocity gas flows, or specific contaminants that may accelerate degradation.
Round-robin testing programs conducted by organizations like NIST, NPL, and industrial consortia have been instrumental in validating and refining these standards. These collaborative efforts have identified critical variables affecting test reproducibility and established statistical frameworks for data interpretation and uncertainty quantification.
The digital transformation of testing protocols represents the newest frontier in standardization, with emerging guidelines for automated data collection, machine learning approaches for predictive modeling, and standardized formats for sharing oxidation test data across research communities. These developments aim to accelerate material development through more efficient knowledge transfer and data utilization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!