Electrochemical Surface Mapping in Sodium Ion Battery Research
AUG 7, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Ion Battery Evolution and Research Objectives
Sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries, driven by the increasing demand for sustainable and cost-effective energy storage solutions. The evolution of SIB technology can be traced back to the 1980s, but significant advancements have been made in the past decade, propelling this field into the forefront of energy storage research.
The initial development of SIBs was motivated by the abundance and wide distribution of sodium resources, which offered a potential solution to the geopolitical and economic challenges associated with lithium supply. Early research focused on understanding the fundamental electrochemistry of sodium intercalation and identifying suitable electrode materials. However, progress was initially slow due to the larger ionic radius of sodium compared to lithium, which posed challenges in terms of material stability and ion diffusion kinetics.
A pivotal moment in SIB evolution came with the discovery of high-performance cathode materials, such as layered transition metal oxides and polyanionic compounds. These materials demonstrated promising sodium storage capabilities, sparking renewed interest in SIB technology. Concurrently, advancements in anode materials, including hard carbons and alloy-based materials, further improved the overall performance of SIBs.
The past five years have witnessed an acceleration in SIB research, driven by the urgent need for grid-scale energy storage and the electrification of transportation. This period has seen significant improvements in energy density, cycle life, and rate capability of SIBs, narrowing the performance gap with lithium-ion batteries. The development of novel electrolytes and the optimization of cell design have also contributed to enhancing the safety and reliability of SIB systems.
In the context of electrochemical surface mapping, the research objectives are multifaceted and ambitious. Primarily, there is a critical need to understand the complex interfacial phenomena occurring at the electrode-electrolyte interface during sodium ion insertion and extraction. This includes investigating the formation and evolution of the solid electrolyte interphase (SEI) layer, which plays a crucial role in battery performance and longevity.
Another key objective is to develop high-resolution mapping techniques that can provide real-time, in-situ insights into the spatial distribution of sodium ions within electrode materials. This information is vital for optimizing electrode architectures and improving the overall efficiency of sodium ion transport. Additionally, researchers aim to elucidate the mechanisms of structural changes and degradation in electrode materials during cycling, which is essential for developing more stable and long-lasting SIBs.
The ultimate goal of electrochemical surface mapping in SIB research is to bridge the gap between fundamental understanding and practical application. By providing detailed, nanoscale information on electrode surfaces and interfaces, these techniques can guide the rational design of next-generation SIB materials and systems, paving the way for their widespread commercialization and adoption in various energy storage applications.
The initial development of SIBs was motivated by the abundance and wide distribution of sodium resources, which offered a potential solution to the geopolitical and economic challenges associated with lithium supply. Early research focused on understanding the fundamental electrochemistry of sodium intercalation and identifying suitable electrode materials. However, progress was initially slow due to the larger ionic radius of sodium compared to lithium, which posed challenges in terms of material stability and ion diffusion kinetics.
A pivotal moment in SIB evolution came with the discovery of high-performance cathode materials, such as layered transition metal oxides and polyanionic compounds. These materials demonstrated promising sodium storage capabilities, sparking renewed interest in SIB technology. Concurrently, advancements in anode materials, including hard carbons and alloy-based materials, further improved the overall performance of SIBs.
The past five years have witnessed an acceleration in SIB research, driven by the urgent need for grid-scale energy storage and the electrification of transportation. This period has seen significant improvements in energy density, cycle life, and rate capability of SIBs, narrowing the performance gap with lithium-ion batteries. The development of novel electrolytes and the optimization of cell design have also contributed to enhancing the safety and reliability of SIB systems.
In the context of electrochemical surface mapping, the research objectives are multifaceted and ambitious. Primarily, there is a critical need to understand the complex interfacial phenomena occurring at the electrode-electrolyte interface during sodium ion insertion and extraction. This includes investigating the formation and evolution of the solid electrolyte interphase (SEI) layer, which plays a crucial role in battery performance and longevity.
Another key objective is to develop high-resolution mapping techniques that can provide real-time, in-situ insights into the spatial distribution of sodium ions within electrode materials. This information is vital for optimizing electrode architectures and improving the overall efficiency of sodium ion transport. Additionally, researchers aim to elucidate the mechanisms of structural changes and degradation in electrode materials during cycling, which is essential for developing more stable and long-lasting SIBs.
The ultimate goal of electrochemical surface mapping in SIB research is to bridge the gap between fundamental understanding and practical application. By providing detailed, nanoscale information on electrode surfaces and interfaces, these techniques can guide the rational design of next-generation SIB materials and systems, paving the way for their widespread commercialization and adoption in various energy storage applications.
Market Demand for Sodium Ion Energy Storage
The market demand for sodium-ion energy storage has been steadily growing in recent years, driven by the increasing need for sustainable and cost-effective energy storage solutions. As the world transitions towards renewable energy sources, the demand for large-scale energy storage systems has surged, creating a significant opportunity for sodium-ion battery technology.
One of the primary factors fueling this demand is the abundance and low cost of sodium resources compared to lithium. With lithium prices experiencing volatility and concerns about its long-term supply, industries are actively seeking alternative energy storage technologies. Sodium-ion batteries offer a promising solution, as sodium is the sixth most abundant element in the Earth's crust and widely available in seawater.
The electric vehicle (EV) sector represents a substantial potential market for sodium-ion batteries. As the automotive industry shifts towards electrification, there is a growing need for affordable and sustainable battery technologies. Sodium-ion batteries could potentially address the cost and resource constraints associated with lithium-ion batteries in the EV market.
Grid energy storage is another key area driving the demand for sodium-ion technology. With the increasing integration of renewable energy sources into power grids, there is a critical need for efficient and cost-effective energy storage systems to manage intermittency and ensure grid stability. Sodium-ion batteries are well-suited for stationary storage applications, offering a balance of performance and cost-effectiveness.
The consumer electronics market also presents opportunities for sodium-ion batteries, particularly in applications where cost is a primary concern. As the technology advances, sodium-ion batteries could potentially replace lithium-ion batteries in various portable devices, offering a more sustainable and affordable alternative.
Emerging markets and developing countries are showing particular interest in sodium-ion technology due to its potential for local production and reduced reliance on imported materials. This aligns with the growing trend of energy independence and sustainable development in these regions.
However, it is important to note that the market for sodium-ion energy storage is still in its early stages. While the potential is significant, the technology must overcome several challenges to achieve widespread commercial adoption. These include improving energy density, cycle life, and overall performance to compete effectively with established lithium-ion technology.
Despite these challenges, industry analysts project substantial growth in the sodium-ion battery market over the coming years. The increasing focus on sustainable energy solutions, coupled with ongoing research and development efforts, is expected to drive innovation and accelerate market adoption of sodium-ion technology across various sectors.
One of the primary factors fueling this demand is the abundance and low cost of sodium resources compared to lithium. With lithium prices experiencing volatility and concerns about its long-term supply, industries are actively seeking alternative energy storage technologies. Sodium-ion batteries offer a promising solution, as sodium is the sixth most abundant element in the Earth's crust and widely available in seawater.
The electric vehicle (EV) sector represents a substantial potential market for sodium-ion batteries. As the automotive industry shifts towards electrification, there is a growing need for affordable and sustainable battery technologies. Sodium-ion batteries could potentially address the cost and resource constraints associated with lithium-ion batteries in the EV market.
Grid energy storage is another key area driving the demand for sodium-ion technology. With the increasing integration of renewable energy sources into power grids, there is a critical need for efficient and cost-effective energy storage systems to manage intermittency and ensure grid stability. Sodium-ion batteries are well-suited for stationary storage applications, offering a balance of performance and cost-effectiveness.
The consumer electronics market also presents opportunities for sodium-ion batteries, particularly in applications where cost is a primary concern. As the technology advances, sodium-ion batteries could potentially replace lithium-ion batteries in various portable devices, offering a more sustainable and affordable alternative.
Emerging markets and developing countries are showing particular interest in sodium-ion technology due to its potential for local production and reduced reliance on imported materials. This aligns with the growing trend of energy independence and sustainable development in these regions.
However, it is important to note that the market for sodium-ion energy storage is still in its early stages. While the potential is significant, the technology must overcome several challenges to achieve widespread commercial adoption. These include improving energy density, cycle life, and overall performance to compete effectively with established lithium-ion technology.
Despite these challenges, industry analysts project substantial growth in the sodium-ion battery market over the coming years. The increasing focus on sustainable energy solutions, coupled with ongoing research and development efforts, is expected to drive innovation and accelerate market adoption of sodium-ion technology across various sectors.
Electrochemical Surface Mapping Techniques: Current Status
Electrochemical surface mapping techniques have emerged as powerful tools in sodium ion battery research, providing crucial insights into the complex interfacial processes that govern battery performance. Currently, these techniques are at the forefront of battery characterization, offering high-resolution spatial and temporal information about electrode surfaces and interfaces.
One of the most widely used techniques is Scanning Electrochemical Microscopy (SECM), which allows for the investigation of local electrochemical activity with micrometer-scale resolution. SECM has been instrumental in studying the formation and evolution of solid electrolyte interphase (SEI) layers on sodium ion battery electrodes, as well as mapping the distribution of active sites on electrode surfaces.
Another significant advancement is the development of operando electrochemical strain microscopy (ESM), which enables the visualization of ion transport and insertion processes in real-time during battery operation. This technique has provided valuable insights into the mechanical stress and strain induced by sodium ion insertion and extraction, helping researchers understand degradation mechanisms and optimize electrode materials.
X-ray photoelectron spectroscopy (XPS) combined with ion sputtering has become a standard method for depth profiling of electrode surfaces. This technique allows for the analysis of chemical composition and oxidation states of elements at different depths within the electrode, providing crucial information about the SEI layer formation and electrode-electrolyte interactions in sodium ion batteries.
Atomic force microscopy (AFM) coupled with electrochemical measurements has also gained prominence in recent years. This combination allows for simultaneous mapping of surface topography and local electrochemical properties, offering nanoscale resolution and the ability to correlate structural changes with electrochemical performance.
Raman spectroscopy, particularly in-situ and operando configurations, has become an invaluable tool for monitoring structural changes in electrode materials during cycling. This technique provides information about the local chemical environment and phase transitions, which is crucial for understanding the mechanisms of sodium ion insertion and extraction.
Recent advancements in synchrotron-based techniques, such as X-ray absorption spectroscopy (XAS) and X-ray diffraction (XRD), have enabled researchers to perform high-resolution mapping of electrode surfaces with chemical specificity. These techniques offer unprecedented insights into the spatial distribution of different chemical species and crystalline phases across the electrode surface.
The current status of electrochemical surface mapping techniques in sodium ion battery research is characterized by a trend towards multi-modal approaches, combining complementary techniques to obtain a more comprehensive understanding of electrode surfaces and interfaces. This integration of multiple characterization methods is pushing the boundaries of our understanding of sodium ion battery systems and paving the way for the development of more efficient and durable energy storage solutions.
One of the most widely used techniques is Scanning Electrochemical Microscopy (SECM), which allows for the investigation of local electrochemical activity with micrometer-scale resolution. SECM has been instrumental in studying the formation and evolution of solid electrolyte interphase (SEI) layers on sodium ion battery electrodes, as well as mapping the distribution of active sites on electrode surfaces.
Another significant advancement is the development of operando electrochemical strain microscopy (ESM), which enables the visualization of ion transport and insertion processes in real-time during battery operation. This technique has provided valuable insights into the mechanical stress and strain induced by sodium ion insertion and extraction, helping researchers understand degradation mechanisms and optimize electrode materials.
X-ray photoelectron spectroscopy (XPS) combined with ion sputtering has become a standard method for depth profiling of electrode surfaces. This technique allows for the analysis of chemical composition and oxidation states of elements at different depths within the electrode, providing crucial information about the SEI layer formation and electrode-electrolyte interactions in sodium ion batteries.
Atomic force microscopy (AFM) coupled with electrochemical measurements has also gained prominence in recent years. This combination allows for simultaneous mapping of surface topography and local electrochemical properties, offering nanoscale resolution and the ability to correlate structural changes with electrochemical performance.
Raman spectroscopy, particularly in-situ and operando configurations, has become an invaluable tool for monitoring structural changes in electrode materials during cycling. This technique provides information about the local chemical environment and phase transitions, which is crucial for understanding the mechanisms of sodium ion insertion and extraction.
Recent advancements in synchrotron-based techniques, such as X-ray absorption spectroscopy (XAS) and X-ray diffraction (XRD), have enabled researchers to perform high-resolution mapping of electrode surfaces with chemical specificity. These techniques offer unprecedented insights into the spatial distribution of different chemical species and crystalline phases across the electrode surface.
The current status of electrochemical surface mapping techniques in sodium ion battery research is characterized by a trend towards multi-modal approaches, combining complementary techniques to obtain a more comprehensive understanding of electrode surfaces and interfaces. This integration of multiple characterization methods is pushing the boundaries of our understanding of sodium ion battery systems and paving the way for the development of more efficient and durable energy storage solutions.
Existing Electrochemical Mapping Solutions for Na-ion Batteries
01 Surface modification of sodium ion battery electrodes
Various surface modification techniques are applied to sodium ion battery electrodes to enhance their performance and stability. These modifications can include coatings, treatments, or the introduction of specific materials to the electrode surface. Such modifications aim to improve the electrode-electrolyte interface, reduce side reactions, and enhance the overall battery efficiency.- Surface modification of sodium ion battery electrodes: Various surface modification techniques are applied to sodium ion battery electrodes to enhance their performance and stability. These modifications can include coatings, treatments, or the introduction of functional layers that improve the electrode-electrolyte interface, reduce side reactions, and enhance the overall battery efficiency.
- Nanostructured surfaces for sodium ion batteries: Nanostructured surfaces are developed for sodium ion battery components to increase the active surface area and improve ion transport. These nanostructures can include nanoparticles, nanotubes, or nanoporous materials that enhance the battery's capacity, rate capability, and cycling stability.
- Solid electrolyte interphase (SEI) formation and control: Research focuses on understanding and controlling the formation of the solid electrolyte interphase (SEI) on sodium ion battery electrode surfaces. Strategies are developed to optimize SEI composition and structure, which is crucial for long-term battery performance and safety.
- Surface coatings for improved sodium ion diffusion: Specialized surface coatings are designed to facilitate sodium ion diffusion at the electrode-electrolyte interface. These coatings can enhance the rate of sodium ion insertion and extraction, leading to improved battery power density and faster charging capabilities.
- Electrode surface engineering for enhanced stability: Advanced surface engineering techniques are applied to sodium ion battery electrodes to enhance their structural and chemical stability during cycling. This includes the development of protective layers, surface functionalization, and the incorporation of stabilizing additives to mitigate degradation mechanisms and extend battery life.
02 Nanostructured surfaces for sodium ion batteries
Nanostructured surfaces are developed for sodium ion battery components to increase the active surface area and improve ion transport. These nanostructures can include nanoparticles, nanotubes, or nanosheets, which are designed to enhance the electrode's capacity, rate capability, and cycling stability.Expand Specific Solutions03 Solid electrolyte interphase (SEI) formation and control
Research focuses on understanding and controlling the formation of the solid electrolyte interphase (SEI) on sodium ion battery electrode surfaces. Strategies are developed to optimize the SEI composition and structure, which plays a crucial role in battery performance, safety, and longevity.Expand Specific Solutions04 Surface functionalization for improved sodium ion storage
Surface functionalization techniques are employed to enhance the sodium ion storage capabilities of battery materials. This can involve the introduction of specific functional groups or dopants to the surface of electrode materials, improving their interaction with sodium ions and enhancing overall battery performance.Expand Specific Solutions05 Protective coatings for sodium ion battery components
Protective coatings are developed and applied to various sodium ion battery components to enhance their stability and longevity. These coatings can protect against side reactions, prevent electrode degradation, and improve the overall cycle life of the battery. Materials used for coatings may include polymers, ceramics, or composite materials.Expand Specific Solutions
Key Players in Sodium Ion Battery Development
The electrochemical surface mapping in sodium ion battery research is in an early development stage, with a growing market driven by the need for sustainable energy storage solutions. The technology's maturity is still evolving, as evidenced by the diverse range of players involved. Companies like CNGR Advanced Material, Contemporary Amperex Technology, and Broadbit Batteries are actively pursuing research and development in this field. Academic institutions such as Central South University, Fudan University, and Vanderbilt University are contributing to fundamental research. The involvement of major automotive manufacturers like BMW and Honda indicates the technology's potential for electric vehicle applications. As the field progresses, collaboration between industry and academia will likely accelerate technological advancements and commercialization efforts.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has pioneered a novel electrochemical surface mapping technique for sodium-ion batteries using a combination of atomic force microscopy (AFM) and electrochemical strain microscopy (ESM)[4]. This approach allows for nanoscale visualization of sodium ion transport and storage mechanisms within electrode materials. The company has developed custom AFM probes with sodium-sensitive coatings to enhance detection sensitivity[5]. Samsung's method incorporates in-situ X-ray photoelectron spectroscopy (XPS) to simultaneously map chemical composition changes during cycling. Their proprietary software integrates these multi-modal datasets to generate comprehensive 3D surface maps, revealing correlations between local electrochemistry, morphology, and composition[6].
Strengths: Nanoscale resolution; Multi-modal analysis capabilities; In-situ chemical composition mapping. Weaknesses: Limited to surface and near-surface phenomena; Potential tip contamination issues affecting long-term measurements.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed advanced electrochemical surface mapping techniques for sodium-ion battery research. Their approach combines high-resolution scanning electrochemical microscopy (SECM) with in-situ Raman spectroscopy to provide real-time, nanoscale insights into the electrode-electrolyte interface dynamics[1]. This method allows for precise monitoring of sodium ion insertion/extraction processes, electrolyte decomposition, and solid electrolyte interphase (SEI) formation. CATL's proprietary algorithms process the multi-dimensional data to generate 3D surface maps, revealing localized electrochemical activity and structural changes during cycling[2]. The company has also integrated machine learning models to predict long-term battery performance based on early-stage surface mapping data[3].
Strengths: High-resolution, real-time surface analysis; Integration of multiple analytical techniques; AI-powered predictive capabilities. Weaknesses: Complex and expensive equipment setup; Requires specialized expertise to interpret results.
Innovative Surface Characterization Methods for Na-ion Systems
Method for detecting electrocrystallization of lithium ion battery
PatentActiveJP2023087844A
Innovation
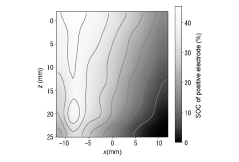
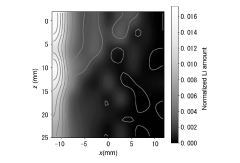
- A method involving X-ray diffraction of a positive electrode active material layer to create a SOC map, calculating the SOC difference value, and comparing it with a threshold to detect lithium electrodeposition in the negative electrode active material layer.
Method to Improve Sodium Electrochemical Interfaces of Sodium Ion-Conducting Ceramics
PatentPendingUS20230207787A1
Innovation
- Coating sodium ion-conducting ceramics with materials such as tin, bismuth, lead, antimony, germanium, silicon, or gold to form a sodium intermetallic phase, which enhances sodium ion conduction and reduces interfacial resistance, allowing for improved charge transfer and wetting at lower temperatures.
Environmental Impact of Sodium Ion Battery Technologies
The environmental impact of sodium-ion battery technologies is a crucial consideration in the development and adoption of these energy storage systems. As the demand for sustainable energy solutions grows, sodium-ion batteries have emerged as a promising alternative to lithium-ion batteries, offering potential environmental benefits.
One of the primary advantages of sodium-ion batteries is the abundance and widespread distribution of sodium resources. Unlike lithium, which is concentrated in specific geographical regions, sodium is readily available in seawater and rock salt deposits worldwide. This reduces the environmental impact associated with resource extraction and transportation, as well as the geopolitical tensions that can arise from resource scarcity.
The production process of sodium-ion batteries also presents opportunities for reduced environmental impact. The materials used in these batteries, such as sodium, iron, and manganese, are generally less toxic and more environmentally friendly compared to some components used in lithium-ion batteries. This can lead to lower emissions and reduced ecological damage during the manufacturing phase.
In terms of recycling and end-of-life management, sodium-ion batteries offer potential advantages. The materials used in these batteries are often more easily recyclable than those in lithium-ion batteries, which can contribute to a more circular economy and reduce the overall environmental footprint of the technology.
However, it is important to note that the environmental impact of sodium-ion batteries is not entirely benign. The production of battery components still requires energy and resources, and the mining of raw materials can have localized environmental effects. Additionally, the large-scale adoption of sodium-ion batteries would necessitate the development of new recycling infrastructure and processes.
The use phase of sodium-ion batteries also contributes to their environmental profile. While they may have lower energy density compared to lithium-ion batteries, their potential for longer cycle life and improved safety characteristics could lead to reduced waste generation and lower replacement rates in certain applications.
As research in electrochemical surface mapping advances, it may lead to further improvements in the environmental performance of sodium-ion batteries. Enhanced understanding of electrode-electrolyte interfaces could result in more efficient and durable batteries, potentially reducing material consumption and extending battery lifespans.
In conclusion, while sodium-ion batteries show promise for reduced environmental impact compared to current lithium-ion technologies, a comprehensive life cycle assessment is necessary to fully quantify their ecological advantages. Ongoing research and development efforts, including those in electrochemical surface mapping, will play a crucial role in optimizing the environmental performance of these emerging battery technologies.
One of the primary advantages of sodium-ion batteries is the abundance and widespread distribution of sodium resources. Unlike lithium, which is concentrated in specific geographical regions, sodium is readily available in seawater and rock salt deposits worldwide. This reduces the environmental impact associated with resource extraction and transportation, as well as the geopolitical tensions that can arise from resource scarcity.
The production process of sodium-ion batteries also presents opportunities for reduced environmental impact. The materials used in these batteries, such as sodium, iron, and manganese, are generally less toxic and more environmentally friendly compared to some components used in lithium-ion batteries. This can lead to lower emissions and reduced ecological damage during the manufacturing phase.
In terms of recycling and end-of-life management, sodium-ion batteries offer potential advantages. The materials used in these batteries are often more easily recyclable than those in lithium-ion batteries, which can contribute to a more circular economy and reduce the overall environmental footprint of the technology.
However, it is important to note that the environmental impact of sodium-ion batteries is not entirely benign. The production of battery components still requires energy and resources, and the mining of raw materials can have localized environmental effects. Additionally, the large-scale adoption of sodium-ion batteries would necessitate the development of new recycling infrastructure and processes.
The use phase of sodium-ion batteries also contributes to their environmental profile. While they may have lower energy density compared to lithium-ion batteries, their potential for longer cycle life and improved safety characteristics could lead to reduced waste generation and lower replacement rates in certain applications.
As research in electrochemical surface mapping advances, it may lead to further improvements in the environmental performance of sodium-ion batteries. Enhanced understanding of electrode-electrolyte interfaces could result in more efficient and durable batteries, potentially reducing material consumption and extending battery lifespans.
In conclusion, while sodium-ion batteries show promise for reduced environmental impact compared to current lithium-ion technologies, a comprehensive life cycle assessment is necessary to fully quantify their ecological advantages. Ongoing research and development efforts, including those in electrochemical surface mapping, will play a crucial role in optimizing the environmental performance of these emerging battery technologies.
Standardization of Surface Mapping Protocols in Battery Research
The standardization of surface mapping protocols in battery research is crucial for ensuring consistency, reproducibility, and comparability of results across different laboratories and studies. This is particularly important in the rapidly evolving field of sodium-ion battery research, where electrochemical surface mapping plays a vital role in understanding electrode-electrolyte interfaces and their impact on battery performance.
To establish standardized protocols, it is essential to define key parameters and methodologies for surface mapping techniques. These include sample preparation procedures, measurement conditions, data acquisition settings, and analysis methods. For electrochemical surface mapping in sodium-ion batteries, specific considerations must be made for the unique characteristics of sodium-based systems, such as their higher reactivity compared to lithium-ion batteries.
One critical aspect of standardization is the development of reference materials and calibration standards specifically tailored for sodium-ion battery research. These standards should account for the diverse range of electrode materials and electrolyte compositions used in sodium-ion systems. By establishing common reference points, researchers can more accurately compare results across different studies and experimental setups.
Standardized data reporting formats and metadata requirements are also essential components of surface mapping protocols. This includes specifying the minimum necessary information to be reported alongside experimental results, such as detailed descriptions of sample preparation methods, instrument specifications, and data processing techniques. Implementing standardized data formats will facilitate data sharing and meta-analyses across the research community.
Interlaboratory studies and round-robin tests can play a crucial role in validating and refining standardized protocols. These collaborative efforts help identify sources of variability and establish reproducibility limits for different surface mapping techniques applied to sodium-ion battery materials. Such studies can also highlight areas where further refinement of protocols is needed to improve consistency across different research groups.
The development and adoption of standardized protocols should be an ongoing, collaborative effort involving academic institutions, industry partners, and standardization bodies. Regular reviews and updates to these protocols will be necessary to keep pace with technological advancements in both sodium-ion battery materials and surface characterization techniques. This dynamic approach ensures that standardization efforts remain relevant and continue to support the progress of sodium-ion battery research and development.
To establish standardized protocols, it is essential to define key parameters and methodologies for surface mapping techniques. These include sample preparation procedures, measurement conditions, data acquisition settings, and analysis methods. For electrochemical surface mapping in sodium-ion batteries, specific considerations must be made for the unique characteristics of sodium-based systems, such as their higher reactivity compared to lithium-ion batteries.
One critical aspect of standardization is the development of reference materials and calibration standards specifically tailored for sodium-ion battery research. These standards should account for the diverse range of electrode materials and electrolyte compositions used in sodium-ion systems. By establishing common reference points, researchers can more accurately compare results across different studies and experimental setups.
Standardized data reporting formats and metadata requirements are also essential components of surface mapping protocols. This includes specifying the minimum necessary information to be reported alongside experimental results, such as detailed descriptions of sample preparation methods, instrument specifications, and data processing techniques. Implementing standardized data formats will facilitate data sharing and meta-analyses across the research community.
Interlaboratory studies and round-robin tests can play a crucial role in validating and refining standardized protocols. These collaborative efforts help identify sources of variability and establish reproducibility limits for different surface mapping techniques applied to sodium-ion battery materials. Such studies can also highlight areas where further refinement of protocols is needed to improve consistency across different research groups.
The development and adoption of standardized protocols should be an ongoing, collaborative effort involving academic institutions, industry partners, and standardization bodies. Regular reviews and updates to these protocols will be necessary to keep pace with technological advancements in both sodium-ion battery materials and surface characterization techniques. This dynamic approach ensures that standardization efforts remain relevant and continue to support the progress of sodium-ion battery research and development.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!