How Salt Chemistry Influences the Development of Sodium Ion Batteries
AUG 7, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Salt Chemistry Background and Objectives
Salt chemistry plays a pivotal role in the development of sodium-ion batteries, a promising alternative to lithium-ion batteries. The evolution of salt chemistry in this field has been driven by the need for more efficient, cost-effective, and sustainable energy storage solutions. Historically, the focus on sodium-ion batteries emerged as a response to concerns about the long-term availability and cost of lithium resources.
The primary objective in salt chemistry for sodium-ion batteries is to develop electrolytes that facilitate efficient sodium ion transport while maintaining stability and safety. This involves exploring various sodium salts and their interactions with solvents and electrode materials. The aim is to achieve high ionic conductivity, wide electrochemical stability windows, and compatibility with both anode and cathode materials.
One of the key challenges in sodium-ion battery development has been finding suitable salt compositions that can withstand the larger size of sodium ions compared to lithium ions. This size difference affects the intercalation processes and overall battery performance. Consequently, researchers have been investigating a wide range of sodium salts, including NaPF6, NaClO4, and NaTFSI, among others, to optimize their properties for battery applications.
The evolution of salt chemistry in this field has seen significant progress over the past decade. Initial efforts focused on adapting lithium-ion battery electrolytes for sodium-ion systems. However, it quickly became apparent that sodium-specific solutions were necessary. This realization led to the exploration of novel salt compositions and electrolyte formulations tailored to the unique characteristics of sodium-ion chemistry.
Current research objectives in salt chemistry for sodium-ion batteries include developing electrolytes with enhanced thermal and electrochemical stability, improving the solid electrolyte interphase (SEI) formation, and mitigating unwanted side reactions. Additionally, there is a growing emphasis on environmentally friendly and sustainable electrolyte compositions, aligning with global efforts to reduce the environmental impact of energy storage technologies.
The future trajectory of salt chemistry in sodium-ion batteries is expected to focus on multi-functional electrolytes that not only facilitate ion transport but also contribute to overall battery performance and longevity. This may involve the development of additives and co-solvents that work synergistically with sodium salts to enhance various aspects of battery operation.
The primary objective in salt chemistry for sodium-ion batteries is to develop electrolytes that facilitate efficient sodium ion transport while maintaining stability and safety. This involves exploring various sodium salts and their interactions with solvents and electrode materials. The aim is to achieve high ionic conductivity, wide electrochemical stability windows, and compatibility with both anode and cathode materials.
One of the key challenges in sodium-ion battery development has been finding suitable salt compositions that can withstand the larger size of sodium ions compared to lithium ions. This size difference affects the intercalation processes and overall battery performance. Consequently, researchers have been investigating a wide range of sodium salts, including NaPF6, NaClO4, and NaTFSI, among others, to optimize their properties for battery applications.
The evolution of salt chemistry in this field has seen significant progress over the past decade. Initial efforts focused on adapting lithium-ion battery electrolytes for sodium-ion systems. However, it quickly became apparent that sodium-specific solutions were necessary. This realization led to the exploration of novel salt compositions and electrolyte formulations tailored to the unique characteristics of sodium-ion chemistry.
Current research objectives in salt chemistry for sodium-ion batteries include developing electrolytes with enhanced thermal and electrochemical stability, improving the solid electrolyte interphase (SEI) formation, and mitigating unwanted side reactions. Additionally, there is a growing emphasis on environmentally friendly and sustainable electrolyte compositions, aligning with global efforts to reduce the environmental impact of energy storage technologies.
The future trajectory of salt chemistry in sodium-ion batteries is expected to focus on multi-functional electrolytes that not only facilitate ion transport but also contribute to overall battery performance and longevity. This may involve the development of additives and co-solvents that work synergistically with sodium salts to enhance various aspects of battery operation.
Market Analysis for Sodium Ion Batteries
The sodium-ion battery market is experiencing rapid growth and attracting significant attention from both industry players and investors. This emerging technology is positioned as a potential alternative to lithium-ion batteries, particularly in large-scale energy storage applications and electric vehicles. The market demand for sodium-ion batteries is driven by several factors, including the abundance and low cost of sodium resources, the increasing need for sustainable energy storage solutions, and the growing concerns over the long-term supply of lithium.
Current market projections indicate a compound annual growth rate (CAGR) of over 20% for the sodium-ion battery market in the coming years. This growth is primarily fueled by the rising demand for renewable energy integration and grid stabilization. The Asia-Pacific region, particularly China, is expected to dominate the market due to its strong manufacturing capabilities and government support for clean energy technologies.
The electric vehicle sector represents a significant potential market for sodium-ion batteries. While lithium-ion batteries currently dominate this space, sodium-ion technology offers advantages in terms of cost and resource availability. As the technology matures and performance improves, it is anticipated that sodium-ion batteries could capture a notable share of the electric vehicle battery market, especially in regions where cost is a primary consideration.
Grid-scale energy storage is another key market segment for sodium-ion batteries. The increasing integration of renewable energy sources into power grids necessitates efficient and cost-effective energy storage solutions. Sodium-ion batteries are well-suited for this application due to their scalability, safety, and potentially lower lifecycle costs compared to some existing technologies.
Consumer electronics represent a smaller but growing market for sodium-ion batteries. While the energy density of sodium-ion cells is currently lower than that of lithium-ion, improvements in salt chemistry and electrode materials are narrowing this gap. As performance continues to improve, sodium-ion batteries may find applications in portable devices, particularly in markets where cost is a critical factor.
The market landscape for sodium-ion batteries is characterized by a mix of established battery manufacturers diversifying their portfolios and startups focused exclusively on sodium-ion technology. Major players are investing in research and development to overcome technical challenges and improve the performance of sodium-ion cells. Collaborations between industry and academia are also driving innovation in this field.
However, the market faces challenges, including the need for further technological advancements to improve energy density and cycle life. Additionally, the established infrastructure and economies of scale for lithium-ion batteries present a significant barrier to entry for sodium-ion technology. Despite these challenges, the long-term market outlook for sodium-ion batteries remains positive, driven by the increasing demand for sustainable and cost-effective energy storage solutions across various sectors.
Current market projections indicate a compound annual growth rate (CAGR) of over 20% for the sodium-ion battery market in the coming years. This growth is primarily fueled by the rising demand for renewable energy integration and grid stabilization. The Asia-Pacific region, particularly China, is expected to dominate the market due to its strong manufacturing capabilities and government support for clean energy technologies.
The electric vehicle sector represents a significant potential market for sodium-ion batteries. While lithium-ion batteries currently dominate this space, sodium-ion technology offers advantages in terms of cost and resource availability. As the technology matures and performance improves, it is anticipated that sodium-ion batteries could capture a notable share of the electric vehicle battery market, especially in regions where cost is a primary consideration.
Grid-scale energy storage is another key market segment for sodium-ion batteries. The increasing integration of renewable energy sources into power grids necessitates efficient and cost-effective energy storage solutions. Sodium-ion batteries are well-suited for this application due to their scalability, safety, and potentially lower lifecycle costs compared to some existing technologies.
Consumer electronics represent a smaller but growing market for sodium-ion batteries. While the energy density of sodium-ion cells is currently lower than that of lithium-ion, improvements in salt chemistry and electrode materials are narrowing this gap. As performance continues to improve, sodium-ion batteries may find applications in portable devices, particularly in markets where cost is a critical factor.
The market landscape for sodium-ion batteries is characterized by a mix of established battery manufacturers diversifying their portfolios and startups focused exclusively on sodium-ion technology. Major players are investing in research and development to overcome technical challenges and improve the performance of sodium-ion cells. Collaborations between industry and academia are also driving innovation in this field.
However, the market faces challenges, including the need for further technological advancements to improve energy density and cycle life. Additionally, the established infrastructure and economies of scale for lithium-ion batteries present a significant barrier to entry for sodium-ion technology. Despite these challenges, the long-term market outlook for sodium-ion batteries remains positive, driven by the increasing demand for sustainable and cost-effective energy storage solutions across various sectors.
Current Challenges in Salt Chemistry for NIBs
The development of sodium-ion batteries (NIBs) faces several critical challenges related to salt chemistry. One of the primary issues is the limited choice of suitable electrolyte salts. Unlike lithium-ion batteries, which have a well-established range of electrolyte options, NIBs struggle with finding salts that can provide optimal performance and stability.
A significant challenge lies in the reactivity of sodium with conventional electrolyte solvents. This reactivity often leads to the formation of a thick and resistive solid electrolyte interphase (SEI) layer on the anode surface. The nature and composition of this SEI layer are crucial for battery performance, but controlling its formation and properties remains a complex task in NIB systems.
Another major hurdle is the larger ionic radius of sodium compared to lithium. This size difference affects the intercalation kinetics and structural stability of electrode materials. Consequently, finding electrolyte salts that can facilitate efficient sodium ion transport while maintaining electrode integrity is a persistent challenge.
The corrosion of current collectors, particularly aluminum, poses another significant problem in NIB salt chemistry. Many sodium salts are more corrosive than their lithium counterparts, leading to degradation of battery components and reduced long-term stability. Developing salt formulations that mitigate this corrosion while maintaining high ionic conductivity is a key research focus.
Furthermore, the solubility and dissociation of sodium salts in organic solvents present additional complications. Achieving high salt concentrations without precipitation or phase separation is crucial for maintaining battery performance across a wide temperature range. This challenge is particularly acute in NIBs due to the different solvation behavior of sodium ions compared to lithium ions.
The thermal stability of electrolyte salts in NIBs is another area of concern. Many potential sodium salts exhibit lower thermal decomposition temperatures compared to their lithium analogues, limiting the operational temperature range of NIBs. This constraint impacts the safety and applicability of NIBs in various environments and applications.
Lastly, the environmental impact and cost of electrolyte salts for NIBs are important considerations. While sodium is more abundant than lithium, developing sustainable and cost-effective salt chemistries that can compete with established lithium-ion technologies remains a significant challenge in the commercialization of NIBs.
A significant challenge lies in the reactivity of sodium with conventional electrolyte solvents. This reactivity often leads to the formation of a thick and resistive solid electrolyte interphase (SEI) layer on the anode surface. The nature and composition of this SEI layer are crucial for battery performance, but controlling its formation and properties remains a complex task in NIB systems.
Another major hurdle is the larger ionic radius of sodium compared to lithium. This size difference affects the intercalation kinetics and structural stability of electrode materials. Consequently, finding electrolyte salts that can facilitate efficient sodium ion transport while maintaining electrode integrity is a persistent challenge.
The corrosion of current collectors, particularly aluminum, poses another significant problem in NIB salt chemistry. Many sodium salts are more corrosive than their lithium counterparts, leading to degradation of battery components and reduced long-term stability. Developing salt formulations that mitigate this corrosion while maintaining high ionic conductivity is a key research focus.
Furthermore, the solubility and dissociation of sodium salts in organic solvents present additional complications. Achieving high salt concentrations without precipitation or phase separation is crucial for maintaining battery performance across a wide temperature range. This challenge is particularly acute in NIBs due to the different solvation behavior of sodium ions compared to lithium ions.
The thermal stability of electrolyte salts in NIBs is another area of concern. Many potential sodium salts exhibit lower thermal decomposition temperatures compared to their lithium analogues, limiting the operational temperature range of NIBs. This constraint impacts the safety and applicability of NIBs in various environments and applications.
Lastly, the environmental impact and cost of electrolyte salts for NIBs are important considerations. While sodium is more abundant than lithium, developing sustainable and cost-effective salt chemistries that can compete with established lithium-ion technologies remains a significant challenge in the commercialization of NIBs.
Current Salt Solutions for NIBs
01 Novel electrolyte compositions for sodium-ion batteries
Development of new electrolyte formulations specifically designed for sodium-ion batteries. These compositions aim to improve the overall performance, stability, and safety of the batteries. The focus is on optimizing the salt chemistry to enhance ionic conductivity and electrochemical stability.- Novel electrolyte compositions for sodium-ion batteries: Development of new electrolyte formulations specifically designed for sodium-ion batteries. These compositions aim to improve the performance, stability, and safety of the batteries by optimizing the salt chemistry and solvent combinations.
- Sodium-based salt alternatives for battery electrolytes: Exploration of various sodium-based salts as alternatives to traditional lithium-based electrolytes. These salts are investigated for their potential to enhance sodium-ion transport, improve cycling stability, and reduce costs in sodium-ion battery systems.
- Additives for enhancing sodium-ion battery performance: Incorporation of specific additives into the electrolyte to enhance various aspects of sodium-ion battery performance. These additives may improve the formation of stable solid electrolyte interphase (SEI) layers, increase conductivity, or mitigate unwanted side reactions.
- Solid-state electrolytes for sodium-ion batteries: Development of solid-state electrolytes tailored for sodium-ion batteries. These materials aim to improve safety, increase energy density, and enable the use of sodium metal anodes while addressing challenges related to interfacial stability and ionic conductivity.
- Electrode-electrolyte interface optimization: Strategies for optimizing the interface between electrodes and electrolytes in sodium-ion batteries. This includes surface modifications, protective coatings, and tailored electrolyte compositions to enhance compatibility, reduce side reactions, and improve overall battery performance.
02 Sodium-based salt compounds for battery electrolytes
Research into various sodium-based salt compounds suitable for use in battery electrolytes. These compounds are investigated for their potential to improve the energy density, cycle life, and rate capability of sodium-ion batteries. The studies explore different anion structures and their impact on battery performance.Expand Specific Solutions03 Solid electrolyte interfaces (SEI) in sodium-ion batteries
Investigation of the formation and properties of solid electrolyte interfaces in sodium-ion batteries. The research focuses on understanding how different salt chemistries affect SEI formation, stability, and overall battery performance. Strategies for optimizing SEI layers through electrolyte engineering are explored.Expand Specific Solutions04 Additives for sodium-ion battery electrolytes
Development and evaluation of various additives for sodium-ion battery electrolytes. These additives are designed to enhance the performance, stability, and safety of the batteries. The research explores how different additives interact with the salt chemistry to improve battery characteristics such as capacity retention and cycling stability.Expand Specific Solutions05 High-concentration electrolytes for sodium-ion batteries
Investigation of high-concentration electrolytes, also known as 'solvent-in-salt' electrolytes, for sodium-ion batteries. This approach involves using a higher salt-to-solvent ratio to achieve improved electrochemical stability, reduced electrolyte decomposition, and enhanced battery performance. The research focuses on optimizing salt concentrations and compositions for specific battery applications.Expand Specific Solutions
Key Players in NIB Salt Chemistry Research
The development of sodium-ion batteries is currently in an early growth stage, with increasing market potential as the technology matures. The global market size for sodium-ion batteries is projected to expand significantly in the coming years, driven by the demand for sustainable energy storage solutions. Companies like Faradion, CATL, and Shenzhen Zhenhua New Material are at the forefront of this technology, with varying levels of technical maturity. While some firms have achieved commercial-scale production, others are still in the research and development phase. The salt chemistry influencing sodium-ion batteries is a critical area of focus, with companies like Sumitomo Electric Industries and Arkema France contributing to advancements in electrolyte formulations and electrode materials.
Faradion Ltd.
Technical Solution: Faradion has pioneered a non-aqueous sodium-ion technology that delivers leading energy density. Their patented technology utilizes sodium and iron-based cathode materials combined with hard carbon anodes[4]. The company has developed a proprietary electrolyte formulation that enhances the stability and performance of their sodium-ion cells. Faradion's batteries have demonstrated excellent cycle life, with over 1000 cycles achieved while maintaining 80% capacity retention[5]. Their technology also shows promise in fast-charging capabilities, reaching 80% charge in less than 30 minutes[6].
Strengths: High energy density for sodium-ion technology, excellent cycle life, fast charging capabilities. Weaknesses: Limited large-scale production experience, potential challenges in scaling up manufacturing processes.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed a first-generation sodium-ion battery with an energy density of up to 160Wh/kg[1]. Their approach focuses on optimizing the cathode material using Prussian white with a higher specific capacity and redesigning the bulk structure of the anode material. They have also developed a hard carbon material with a unique porous structure that enables efficient storage and fast movement of sodium ions[2]. CATL's sodium-ion batteries utilize an electrolyte with high purity and stability, which is crucial for the overall performance and longevity of the battery[3].
Strengths: High energy density for sodium-ion technology, innovative cathode and anode materials, potential for fast charging. Weaknesses: Still lower energy density compared to lithium-ion batteries, technology relatively new and unproven in large-scale applications.
Innovative Salt Chemistries for NIBs
Electrolyte and sodium-ion battery
PatentWO2025098091A1
Innovation
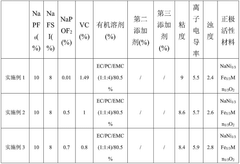
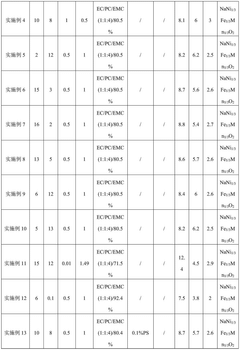
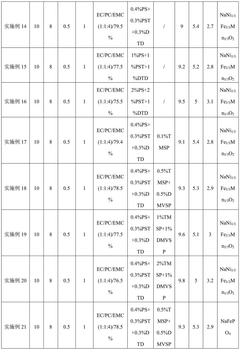
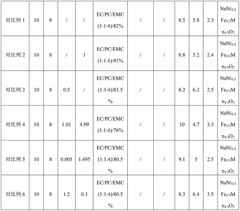
- An electrolyte solution is developed, which includes a sodium salt main salt, a sodium salt auxiliary salt, an organic solvent and a first additive. The sodium salt auxiliary salt is sodium difluorophosphate, and the first additive is vinyl carbonate. By limiting its mass percentage content, a SEI film with high stability and a specific content of sodium ions and phosphorus elements is formed on the negative electrode surface of the battery, thereby improving the mobility of sodium ions and reducing radicals at active sites.
Environmental Impact of NIB Salt Production
The production of salts for sodium-ion batteries (NIBs) has significant environmental implications that warrant careful consideration. The extraction and processing of raw materials for these salts, primarily sodium and other elements like phosphorus or sulfur, can lead to habitat disruption and ecosystem damage. Mining activities often result in land degradation, water pollution, and increased greenhouse gas emissions.
The refining processes for NIB salts typically involve energy-intensive operations, contributing to carbon dioxide emissions and climate change concerns. The use of chemical solvents and reagents in salt production can also lead to the generation of hazardous waste, which requires proper management and disposal to prevent environmental contamination.
Water consumption is another critical environmental factor in NIB salt production. The extraction and purification processes often require substantial amounts of water, potentially straining local water resources, especially in water-scarce regions. This can lead to conflicts with other water users and impact aquatic ecosystems.
However, it's important to note that the environmental impact of NIB salt production should be evaluated in comparison to the production of materials for other battery technologies, such as lithium-ion batteries. In some cases, NIB salts may have a lower environmental footprint due to the greater abundance and accessibility of sodium compared to lithium.
Efforts are being made to mitigate the environmental impact of NIB salt production. These include developing more efficient extraction and refining processes, implementing closed-loop systems to reduce waste and water consumption, and exploring alternative, more environmentally friendly synthesis routes for battery-grade salts.
The recycling of NIB salts at the end of battery life is another crucial aspect of reducing environmental impact. Effective recycling processes can recover valuable materials, reducing the need for primary raw material extraction and minimizing waste. However, the development of efficient and economically viable recycling technologies for NIB salts is still an ongoing challenge.
As the demand for NIBs grows, it is essential to continue research into more sustainable salt production methods and to implement stringent environmental regulations and best practices across the industry. This will help ensure that the environmental benefits of NIBs in terms of renewable energy storage are not overshadowed by the environmental costs of their production.
The refining processes for NIB salts typically involve energy-intensive operations, contributing to carbon dioxide emissions and climate change concerns. The use of chemical solvents and reagents in salt production can also lead to the generation of hazardous waste, which requires proper management and disposal to prevent environmental contamination.
Water consumption is another critical environmental factor in NIB salt production. The extraction and purification processes often require substantial amounts of water, potentially straining local water resources, especially in water-scarce regions. This can lead to conflicts with other water users and impact aquatic ecosystems.
However, it's important to note that the environmental impact of NIB salt production should be evaluated in comparison to the production of materials for other battery technologies, such as lithium-ion batteries. In some cases, NIB salts may have a lower environmental footprint due to the greater abundance and accessibility of sodium compared to lithium.
Efforts are being made to mitigate the environmental impact of NIB salt production. These include developing more efficient extraction and refining processes, implementing closed-loop systems to reduce waste and water consumption, and exploring alternative, more environmentally friendly synthesis routes for battery-grade salts.
The recycling of NIB salts at the end of battery life is another crucial aspect of reducing environmental impact. Effective recycling processes can recover valuable materials, reducing the need for primary raw material extraction and minimizing waste. However, the development of efficient and economically viable recycling technologies for NIB salts is still an ongoing challenge.
As the demand for NIBs grows, it is essential to continue research into more sustainable salt production methods and to implement stringent environmental regulations and best practices across the industry. This will help ensure that the environmental benefits of NIBs in terms of renewable energy storage are not overshadowed by the environmental costs of their production.
Safety Considerations in NIB Salt Chemistry
Safety considerations play a crucial role in the development and implementation of sodium-ion battery (NIB) technology, particularly in relation to salt chemistry. The choice of salts and their chemical properties significantly impact the safety profile of NIBs, necessitating careful evaluation and mitigation strategies.
One of the primary safety concerns in NIB salt chemistry is the potential for thermal runaway. The reactivity of sodium with organic electrolytes can lead to exothermic reactions, potentially causing battery overheating or even fires. To address this, researchers are exploring salt additives that can form stable solid electrolyte interphase (SEI) layers, reducing the risk of uncontrolled reactions between the electrode and electrolyte.
Electrolyte decomposition is another critical safety issue influenced by salt chemistry. Certain salts may undergo degradation at high voltages or temperatures, producing flammable or toxic gases. This risk has led to the investigation of more stable salt formulations and the development of non-flammable electrolytes, such as ionic liquids or solid-state electrolytes, which can enhance the overall safety of NIBs.
The hygroscopic nature of many sodium salts poses additional safety challenges. Moisture absorption can lead to the formation of corrosive compounds, potentially compromising the structural integrity of the battery and increasing the risk of short circuits. Consequently, stringent moisture control during manufacturing and the use of moisture-resistant packaging materials are essential safety measures in NIB production.
Salt concentration also plays a role in safety considerations. High salt concentrations can improve ionic conductivity but may increase the risk of salt precipitation at low temperatures, potentially leading to battery failure or internal short circuits. Balancing salt concentration for optimal performance while maintaining safety across a wide temperature range is a key challenge in NIB development.
Furthermore, the environmental and health impacts of NIB salts must be carefully evaluated. While sodium-based chemistries are generally considered less toxic than their lithium counterparts, the large-scale production and disposal of NIBs necessitate a thorough assessment of potential environmental risks associated with salt leakage or improper disposal.
To address these safety concerns, researchers are exploring advanced characterization techniques to better understand the behavior of salts in NIB systems. In-situ monitoring methods, such as differential scanning calorimetry and electrochemical impedance spectroscopy, are being employed to study salt-related safety issues in real-time, enabling the development of more robust safety protocols.
As NIB technology advances, the integration of smart battery management systems (BMS) that can monitor and respond to salt-related safety issues is becoming increasingly important. These systems can detect anomalies in salt behavior, such as unexpected temperature increases or voltage fluctuations, and take preventive actions to maintain safe operating conditions.
One of the primary safety concerns in NIB salt chemistry is the potential for thermal runaway. The reactivity of sodium with organic electrolytes can lead to exothermic reactions, potentially causing battery overheating or even fires. To address this, researchers are exploring salt additives that can form stable solid electrolyte interphase (SEI) layers, reducing the risk of uncontrolled reactions between the electrode and electrolyte.
Electrolyte decomposition is another critical safety issue influenced by salt chemistry. Certain salts may undergo degradation at high voltages or temperatures, producing flammable or toxic gases. This risk has led to the investigation of more stable salt formulations and the development of non-flammable electrolytes, such as ionic liquids or solid-state electrolytes, which can enhance the overall safety of NIBs.
The hygroscopic nature of many sodium salts poses additional safety challenges. Moisture absorption can lead to the formation of corrosive compounds, potentially compromising the structural integrity of the battery and increasing the risk of short circuits. Consequently, stringent moisture control during manufacturing and the use of moisture-resistant packaging materials are essential safety measures in NIB production.
Salt concentration also plays a role in safety considerations. High salt concentrations can improve ionic conductivity but may increase the risk of salt precipitation at low temperatures, potentially leading to battery failure or internal short circuits. Balancing salt concentration for optimal performance while maintaining safety across a wide temperature range is a key challenge in NIB development.
Furthermore, the environmental and health impacts of NIB salts must be carefully evaluated. While sodium-based chemistries are generally considered less toxic than their lithium counterparts, the large-scale production and disposal of NIBs necessitate a thorough assessment of potential environmental risks associated with salt leakage or improper disposal.
To address these safety concerns, researchers are exploring advanced characterization techniques to better understand the behavior of salts in NIB systems. In-situ monitoring methods, such as differential scanning calorimetry and electrochemical impedance spectroscopy, are being employed to study salt-related safety issues in real-time, enabling the development of more robust safety protocols.
As NIB technology advances, the integration of smart battery management systems (BMS) that can monitor and respond to salt-related safety issues is becoming increasingly important. These systems can detect anomalies in salt behavior, such as unexpected temperature increases or voltage fluctuations, and take preventive actions to maintain safe operating conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!