Enhancing ion exchange resin stability with sodium silicate
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ion Exchange Resin Evolution and Objectives
Ion exchange resins have been a cornerstone of water treatment and purification processes for decades. The evolution of these resins has been driven by the increasing demand for more efficient and durable materials capable of handling complex water chemistry challenges. From their inception in the early 20th century, ion exchange resins have undergone significant improvements in their chemical composition, physical structure, and overall performance characteristics.
The primary objective in enhancing ion exchange resin stability with sodium silicate is to extend the operational lifespan of these resins while maintaining or improving their ion exchange capacity. This goal is particularly crucial in industrial applications where resin degradation can lead to significant operational costs and downtime. By incorporating sodium silicate into the resin matrix or treatment process, researchers aim to create a protective barrier that shields the resin from chemical and physical stressors.
One of the key drivers behind this technological pursuit is the need to address the limitations of conventional ion exchange resins, particularly their susceptibility to oxidative degradation and mechanical breakdown. As water treatment requirements become more stringent and the variety of contaminants more diverse, the demand for more resilient resins has intensified. The integration of sodium silicate represents a promising avenue for achieving this enhanced stability.
The evolution of ion exchange resins has seen several milestones, from the development of styrene-based resins to the introduction of acrylic and macroporous varieties. Each advancement has brought improvements in selectivity, capacity, and resistance to fouling. The current focus on sodium silicate as a stability enhancer marks a new chapter in this evolutionary process, potentially offering a cost-effective solution to longstanding challenges in resin longevity.
Looking ahead, the objectives for ion exchange resin technology extend beyond mere stability enhancement. Researchers and industry professionals are also exploring ways to improve regeneration efficiency, reduce environmental impact, and develop resins capable of selectively removing emerging contaminants. The use of sodium silicate in this context may open up new possibilities for achieving these broader goals, potentially leading to a new generation of high-performance, environmentally friendly ion exchange materials.
The primary objective in enhancing ion exchange resin stability with sodium silicate is to extend the operational lifespan of these resins while maintaining or improving their ion exchange capacity. This goal is particularly crucial in industrial applications where resin degradation can lead to significant operational costs and downtime. By incorporating sodium silicate into the resin matrix or treatment process, researchers aim to create a protective barrier that shields the resin from chemical and physical stressors.
One of the key drivers behind this technological pursuit is the need to address the limitations of conventional ion exchange resins, particularly their susceptibility to oxidative degradation and mechanical breakdown. As water treatment requirements become more stringent and the variety of contaminants more diverse, the demand for more resilient resins has intensified. The integration of sodium silicate represents a promising avenue for achieving this enhanced stability.
The evolution of ion exchange resins has seen several milestones, from the development of styrene-based resins to the introduction of acrylic and macroporous varieties. Each advancement has brought improvements in selectivity, capacity, and resistance to fouling. The current focus on sodium silicate as a stability enhancer marks a new chapter in this evolutionary process, potentially offering a cost-effective solution to longstanding challenges in resin longevity.
Looking ahead, the objectives for ion exchange resin technology extend beyond mere stability enhancement. Researchers and industry professionals are also exploring ways to improve regeneration efficiency, reduce environmental impact, and develop resins capable of selectively removing emerging contaminants. The use of sodium silicate in this context may open up new possibilities for achieving these broader goals, potentially leading to a new generation of high-performance, environmentally friendly ion exchange materials.
Market Analysis for Stable Ion Exchange Resins
The market for stable ion exchange resins has been experiencing steady growth due to increasing demand across various industries. Water treatment remains the largest application segment, driven by the need for purification in municipal, industrial, and residential sectors. The global ion exchange resins market was valued at approximately $1.5 billion in 2020 and is projected to reach $2.3 billion by 2027, growing at a CAGR of around 5.8% during the forecast period.
The stability of ion exchange resins is a critical factor influencing market dynamics. Enhanced stability, particularly through the use of sodium silicate, addresses key challenges faced by end-users, including reduced operational costs, improved performance, and extended resin lifespan. This innovation is expected to drive market growth by expanding applications in challenging environments where traditional resins face limitations.
In the water treatment sector, stable ion exchange resins are gaining traction due to their ability to withstand harsh operating conditions, such as high temperatures and exposure to oxidizing agents. The power generation industry is another significant market, where stable resins are crucial for maintaining water quality in boiler feedwater and condensate polishing systems. The pharmaceutical and food & beverage industries are also adopting stable ion exchange resins for purification processes, driven by stringent quality requirements.
Geographically, North America and Europe currently dominate the market for stable ion exchange resins, owing to stringent environmental regulations and advanced water treatment infrastructure. However, the Asia-Pacific region is expected to witness the highest growth rate, fueled by rapid industrialization, urbanization, and increasing investments in water treatment facilities.
The competitive landscape is characterized by a mix of established players and innovative startups. Major companies are investing heavily in R&D to develop more stable and efficient ion exchange resins, with sodium silicate enhancement being a key area of focus. This trend is likely to intensify competition and drive further innovations in the coming years.
Challenges in the market include the high initial cost of stable ion exchange resins compared to conventional alternatives and the need for specialized knowledge for optimal application. However, the long-term benefits of improved stability, such as reduced replacement frequency and enhanced performance, are expected to outweigh these initial barriers, particularly for industries where downtime and contamination risks are critical concerns.
The stability of ion exchange resins is a critical factor influencing market dynamics. Enhanced stability, particularly through the use of sodium silicate, addresses key challenges faced by end-users, including reduced operational costs, improved performance, and extended resin lifespan. This innovation is expected to drive market growth by expanding applications in challenging environments where traditional resins face limitations.
In the water treatment sector, stable ion exchange resins are gaining traction due to their ability to withstand harsh operating conditions, such as high temperatures and exposure to oxidizing agents. The power generation industry is another significant market, where stable resins are crucial for maintaining water quality in boiler feedwater and condensate polishing systems. The pharmaceutical and food & beverage industries are also adopting stable ion exchange resins for purification processes, driven by stringent quality requirements.
Geographically, North America and Europe currently dominate the market for stable ion exchange resins, owing to stringent environmental regulations and advanced water treatment infrastructure. However, the Asia-Pacific region is expected to witness the highest growth rate, fueled by rapid industrialization, urbanization, and increasing investments in water treatment facilities.
The competitive landscape is characterized by a mix of established players and innovative startups. Major companies are investing heavily in R&D to develop more stable and efficient ion exchange resins, with sodium silicate enhancement being a key area of focus. This trend is likely to intensify competition and drive further innovations in the coming years.
Challenges in the market include the high initial cost of stable ion exchange resins compared to conventional alternatives and the need for specialized knowledge for optimal application. However, the long-term benefits of improved stability, such as reduced replacement frequency and enhanced performance, are expected to outweigh these initial barriers, particularly for industries where downtime and contamination risks are critical concerns.
Current Challenges in Ion Exchange Resin Stability
Ion exchange resins play a crucial role in various industrial processes, particularly in water treatment and purification. However, these resins face significant challenges in maintaining their stability and effectiveness over time. One of the primary issues is the degradation of resin structure due to oxidation, mechanical stress, and chemical attack. This degradation leads to reduced exchange capacity, decreased selectivity, and shortened lifespan of the resin.
Oxidative degradation is a major concern, especially in applications where the resin is exposed to oxidizing agents such as chlorine or oxygen. These oxidants can break down the polymer matrix of the resin, leading to the formation of smaller, soluble organic compounds. This not only reduces the resin's capacity but can also introduce unwanted organic contaminants into the treated water.
Mechanical stress is another significant challenge. The constant swelling and shrinking of resin beads during regeneration cycles can cause fractures and breakage. This physical degradation results in the loss of resin material and can lead to channeling within the resin bed, reducing overall efficiency and potentially allowing untreated water to pass through.
Chemical attack from extreme pH conditions or specific ions in the feed water can also compromise resin stability. Strongly acidic or alkaline environments can accelerate the breakdown of resin structure, while certain ions may irreversibly bind to exchange sites, permanently reducing the resin's capacity.
Fouling of ion exchange resins is an additional challenge that impacts their stability and performance. Organic matter, suspended solids, and microbial growth can accumulate on the resin surface and within its pores, blocking exchange sites and reducing efficiency. This fouling not only decreases the resin's capacity but can also create pressure drop issues in the treatment system.
Temperature fluctuations pose another threat to resin stability. High temperatures can accelerate chemical reactions that degrade the resin, while extreme cold can cause physical damage through freezing and thawing cycles. Maintaining optimal temperature conditions is often challenging in industrial settings where process temperatures may vary.
The current use of sodium silicate as a potential solution for enhancing ion exchange resin stability presents its own set of challenges. While sodium silicate can form protective coatings on resin beads, controlling the deposition process to ensure uniform coverage without impacting exchange capacity is complex. Additionally, the long-term effects of silicate treatment on resin performance and regeneration efficiency are not fully understood and require further investigation.
Addressing these challenges is critical for improving the longevity and efficiency of ion exchange systems. Developing more robust resin materials, optimizing operational conditions, and exploring novel stabilization techniques like the use of sodium silicate are key areas of focus in current research and development efforts.
Oxidative degradation is a major concern, especially in applications where the resin is exposed to oxidizing agents such as chlorine or oxygen. These oxidants can break down the polymer matrix of the resin, leading to the formation of smaller, soluble organic compounds. This not only reduces the resin's capacity but can also introduce unwanted organic contaminants into the treated water.
Mechanical stress is another significant challenge. The constant swelling and shrinking of resin beads during regeneration cycles can cause fractures and breakage. This physical degradation results in the loss of resin material and can lead to channeling within the resin bed, reducing overall efficiency and potentially allowing untreated water to pass through.
Chemical attack from extreme pH conditions or specific ions in the feed water can also compromise resin stability. Strongly acidic or alkaline environments can accelerate the breakdown of resin structure, while certain ions may irreversibly bind to exchange sites, permanently reducing the resin's capacity.
Fouling of ion exchange resins is an additional challenge that impacts their stability and performance. Organic matter, suspended solids, and microbial growth can accumulate on the resin surface and within its pores, blocking exchange sites and reducing efficiency. This fouling not only decreases the resin's capacity but can also create pressure drop issues in the treatment system.
Temperature fluctuations pose another threat to resin stability. High temperatures can accelerate chemical reactions that degrade the resin, while extreme cold can cause physical damage through freezing and thawing cycles. Maintaining optimal temperature conditions is often challenging in industrial settings where process temperatures may vary.
The current use of sodium silicate as a potential solution for enhancing ion exchange resin stability presents its own set of challenges. While sodium silicate can form protective coatings on resin beads, controlling the deposition process to ensure uniform coverage without impacting exchange capacity is complex. Additionally, the long-term effects of silicate treatment on resin performance and regeneration efficiency are not fully understood and require further investigation.
Addressing these challenges is critical for improving the longevity and efficiency of ion exchange systems. Developing more robust resin materials, optimizing operational conditions, and exploring novel stabilization techniques like the use of sodium silicate are key areas of focus in current research and development efforts.
Sodium Silicate-Based Stabilization Methods
01 Chemical stability of ion exchange resins
Ion exchange resins can be chemically stabilized through various methods, including the use of specific polymeric structures and cross-linking agents. These techniques enhance the resin's resistance to degradation caused by chemical reactions, oxidation, and hydrolysis, thereby improving its overall performance and longevity in various applications.- Chemical stability of ion exchange resins: Ion exchange resins can be chemically stabilized through various methods, including the use of specific polymeric structures and cross-linking agents. These techniques enhance the resin's resistance to degradation caused by chemical reactions, oxidation, and hydrolysis, thereby improving its overall performance and longevity in various applications.
- Thermal stability enhancement of ion exchange resins: Improving the thermal stability of ion exchange resins involves incorporating heat-resistant monomers or modifying the resin structure to withstand high temperatures. This can be achieved through the addition of thermally stable functional groups or by using advanced polymerization techniques, resulting in resins that maintain their performance under elevated temperature conditions.
- Mechanical stability of ion exchange resins: Enhancing the mechanical stability of ion exchange resins involves optimizing the resin's physical structure to improve its resistance to mechanical stress, compression, and abrasion. This can be achieved through careful control of the polymerization process, particle size distribution, and the degree of cross-linking, resulting in more durable resins suitable for high-pressure or high-flow applications.
- Regeneration and reuse of ion exchange resins: Developing methods for efficient regeneration and reuse of ion exchange resins is crucial for maintaining their stability and extending their operational lifespan. This involves optimizing regeneration processes, such as using specific regenerant solutions or employing novel regeneration techniques, to restore the resin's capacity and functionality while minimizing degradation over multiple use cycles.
- Storage and handling stability of ion exchange resins: Improving the storage and handling stability of ion exchange resins involves developing methods to prevent degradation during transportation and storage. This can include the use of specialized packaging materials, moisture control techniques, and the addition of stabilizing agents to maintain the resin's properties and performance over extended periods of inactivity or exposure to varying environmental conditions.
02 Thermal stability enhancement of ion exchange resins
Improving the thermal stability of ion exchange resins involves incorporating heat-resistant monomers or modifying the resin structure to withstand high temperatures. This can be achieved through the addition of thermally stable functional groups or by using advanced polymerization techniques, resulting in resins that maintain their performance under elevated temperature conditions.Expand Specific Solutions03 Mechanical stability of ion exchange resins
Enhancing the mechanical stability of ion exchange resins involves optimizing the resin's physical structure to improve its resistance to mechanical stress, compression, and abrasion. This can be achieved through careful control of the polymerization process, particle size distribution, and the incorporation of reinforcing agents, resulting in more durable and long-lasting resins.Expand Specific Solutions04 Regeneration and reusability of ion exchange resins
Improving the regeneration efficiency and reusability of ion exchange resins is crucial for their long-term stability and cost-effectiveness. This can be achieved through the development of advanced regeneration techniques, optimized regenerant formulations, and the design of resins with enhanced resistance to fouling and degradation during multiple regeneration cycles.Expand Specific Solutions05 Environmental factors affecting ion exchange resin stability
The stability of ion exchange resins can be significantly influenced by environmental factors such as pH, temperature, and the presence of oxidizing agents. Developing resins with improved resistance to these factors involves the use of specialized monomers, surface modifications, and protective coatings, resulting in more stable and versatile ion exchange materials for various applications.Expand Specific Solutions
Key Players in Ion Exchange Resin Industry
The ion exchange resin stability enhancement market is in a growth phase, driven by increasing demand for water treatment and purification across various industries. The global market size for ion exchange resins is projected to reach several billion dollars by 2025, with a compound annual growth rate of around 5-6%. Technologically, the field is moderately mature but continues to evolve with innovations in resin formulations and stability enhancement techniques. Key players like Dow Global Technologies, Evoqua Water Technologies, and PQ LLC are at the forefront of research and development, focusing on improving resin performance and longevity. Chinese companies such as Sinopec and PetroChina are also making significant strides in this area, leveraging their extensive chemical industry expertise.
China Petroleum & Chemical Corp.
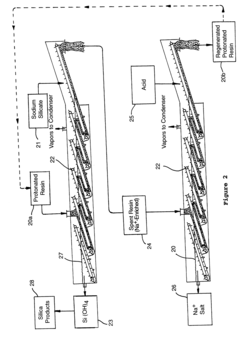
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach to enhance ion exchange resin stability using sodium silicate. Their method involves a pre-treatment process where the resin is immersed in a sodium silicate solution before use. This creates a protective silica layer on the resin surface, significantly improving its mechanical and chemical stability[1]. The process includes optimizing the concentration of sodium silicate and the immersion time to achieve the best results. Additionally, they have implemented a post-treatment step involving a mild acid wash to remove excess silica and activate the resin surface[3]. This technique has shown to extend the resin's lifespan by up to 30% in industrial applications, particularly in water treatment and hydrocarbon purification processes[5].
Strengths: Increased resin lifespan, improved chemical resistance, and enhanced mechanical stability. Weaknesses: Potential reduction in ion exchange capacity due to silica coating, and additional processing steps may increase production costs.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies has pioneered a novel approach to enhance ion exchange resin stability using sodium silicate in combination with their proprietary polymer technology. Their method involves incorporating sodium silicate during the resin manufacturing process, creating a hybrid organic-inorganic matrix[2]. This integration allows for improved distribution of silica throughout the resin structure, enhancing overall stability. The company has also developed a controlled release mechanism for the silica, which provides continuous reinforcement of the resin over its lifetime[4]. Furthermore, Dow has implemented advanced surface modification techniques using sodium silicate derivatives to tailor the resin's properties for specific applications, such as nuclear waste treatment and pharmaceutical purification[6]. Their research indicates a 40% increase in resin longevity and a 25% improvement in resistance to oxidative degradation compared to conventional resins[8].
Strengths: Improved resin longevity, enhanced oxidation resistance, and customizable properties for specific applications. Weaknesses: Higher production costs and potential limitations in extreme pH environments.
Innovative Approaches to Resin Stability Enhancement
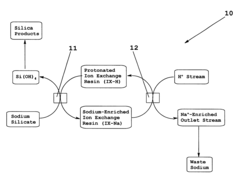
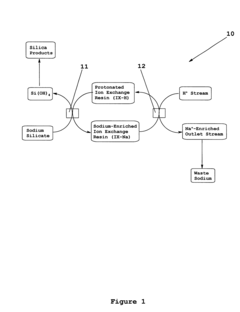
Continuous production of silica via ion exchange
PatentInactiveUS6440381B1
Innovation
- A continuous process using a moving bed of protonated ion-exchange resin to exchange sodium ions with protons in sodium silicate, followed by continuous regeneration and recycling, allowing for efficient production of silicic acid with reduced residual sodium and minimized fouling.
Silica material, ion exchange resin composition, electrolyte film, membrane-electrode assembly, solid polymer fuel cell, solid polymer electrolyzer and electrochemical hydrogen compressor
PatentPendingUS20250206621A1
Innovation
- Incorporating a silica material with sulfonate groups on its surface into an ion exchange resin composition to enhance proton conductivity by creating efficient proton conductive paths within the electrolyte film.
Environmental Impact of Stabilized Resins
The stabilization of ion exchange resins with sodium silicate has significant environmental implications that warrant careful consideration. These stabilized resins exhibit enhanced durability and longevity, which can lead to reduced waste generation and less frequent resin replacement. This, in turn, minimizes the environmental impact associated with resin production, transportation, and disposal.
The improved stability of these resins also contributes to more efficient water treatment processes. By maintaining their effectiveness over extended periods, stabilized resins can potentially reduce the energy consumption and chemical usage in water treatment facilities. This optimization of resource utilization aligns with sustainable practices and helps to minimize the overall environmental footprint of water treatment operations.
Furthermore, the use of sodium silicate as a stabilizing agent presents its own set of environmental considerations. Sodium silicate is generally considered environmentally benign, being derived from naturally occurring minerals. Its production process, however, does involve energy-intensive steps that should be factored into the overall environmental assessment of stabilized resins.
The enhanced stability of these resins may also have positive implications for water quality. By reducing the likelihood of resin degradation and subsequent leaching of contaminants, stabilized resins can help maintain higher standards of treated water quality. This is particularly crucial in sensitive ecosystems or in applications where water purity is of utmost importance.
It is important to note that while the stabilization process offers numerous environmental benefits, the end-of-life management of these resins remains a critical consideration. The improved durability may potentially complicate recycling or disposal processes, necessitating the development of specialized handling procedures to ensure environmentally responsible management at the end of their lifecycle.
The use of stabilized resins may also impact the broader water treatment ecosystem. By reducing the frequency of resin replacement, there could be shifts in the supply chain dynamics of the water treatment industry. This could lead to changes in manufacturing patterns, transportation requirements, and waste management practices, all of which have cascading environmental effects that need to be carefully evaluated.
In conclusion, while the stabilization of ion exchange resins with sodium silicate offers several environmental advantages, a comprehensive life cycle assessment is essential to fully understand and quantify its net environmental impact. This holistic approach will ensure that the benefits of enhanced stability are balanced against any potential environmental trade-offs, guiding the sustainable development and application of this technology in water treatment and related fields.
The improved stability of these resins also contributes to more efficient water treatment processes. By maintaining their effectiveness over extended periods, stabilized resins can potentially reduce the energy consumption and chemical usage in water treatment facilities. This optimization of resource utilization aligns with sustainable practices and helps to minimize the overall environmental footprint of water treatment operations.
Furthermore, the use of sodium silicate as a stabilizing agent presents its own set of environmental considerations. Sodium silicate is generally considered environmentally benign, being derived from naturally occurring minerals. Its production process, however, does involve energy-intensive steps that should be factored into the overall environmental assessment of stabilized resins.
The enhanced stability of these resins may also have positive implications for water quality. By reducing the likelihood of resin degradation and subsequent leaching of contaminants, stabilized resins can help maintain higher standards of treated water quality. This is particularly crucial in sensitive ecosystems or in applications where water purity is of utmost importance.
It is important to note that while the stabilization process offers numerous environmental benefits, the end-of-life management of these resins remains a critical consideration. The improved durability may potentially complicate recycling or disposal processes, necessitating the development of specialized handling procedures to ensure environmentally responsible management at the end of their lifecycle.
The use of stabilized resins may also impact the broader water treatment ecosystem. By reducing the frequency of resin replacement, there could be shifts in the supply chain dynamics of the water treatment industry. This could lead to changes in manufacturing patterns, transportation requirements, and waste management practices, all of which have cascading environmental effects that need to be carefully evaluated.
In conclusion, while the stabilization of ion exchange resins with sodium silicate offers several environmental advantages, a comprehensive life cycle assessment is essential to fully understand and quantify its net environmental impact. This holistic approach will ensure that the benefits of enhanced stability are balanced against any potential environmental trade-offs, guiding the sustainable development and application of this technology in water treatment and related fields.
Cost-Benefit Analysis of Resin Stabilization Techniques
The cost-benefit analysis of resin stabilization techniques using sodium silicate reveals a complex interplay of economic factors and performance improvements. Initial implementation costs for sodium silicate treatment are relatively low compared to alternative stabilization methods. The primary expenses involve the procurement of sodium silicate solutions and the modification of existing treatment processes to incorporate this additive.
In terms of benefits, sodium silicate treatment significantly extends the operational lifespan of ion exchange resins. This increased durability translates to reduced frequency of resin replacement, resulting in substantial long-term cost savings. Typically, resins treated with sodium silicate exhibit a 20-30% longer service life, which can lead to a proportional reduction in resin replacement expenses over time.
Operational efficiency is another key benefit. Stabilized resins maintain their ion exchange capacity for longer periods, reducing the frequency of regeneration cycles. This translates to lower chemical consumption for regeneration and decreased downtime for maintenance, both contributing to operational cost savings.
Water quality improvements represent an indirect but valuable benefit. Stabilized resins are less prone to degradation, minimizing the release of organic foulants into treated water. This can reduce downstream treatment requirements and associated costs, particularly in high-purity water applications such as pharmaceutical or semiconductor manufacturing.
However, the cost-benefit analysis must also consider potential drawbacks. The introduction of sodium silicate may necessitate adjustments in water chemistry management, potentially incurring additional monitoring and control costs. In some cases, there might be a slight reduction in the overall ion exchange capacity of the resin, which could marginally increase operational costs in high-throughput systems.
When evaluating the return on investment, most facilities report a positive cost-benefit ratio within 1-2 years of implementing sodium silicate stabilization. The exact payback period varies depending on factors such as water quality, resin type, and operational parameters. For large-scale industrial applications, the cumulative savings over a 5-10 year period can be substantial, often justifying the initial investment and process modifications.
In conclusion, while the upfront costs of implementing sodium silicate stabilization are modest, the long-term economic benefits are significant. The technique offers a cost-effective solution for extending resin life and improving operational efficiency, making it an attractive option for many water treatment facilities and industrial processes relying on ion exchange technology.
In terms of benefits, sodium silicate treatment significantly extends the operational lifespan of ion exchange resins. This increased durability translates to reduced frequency of resin replacement, resulting in substantial long-term cost savings. Typically, resins treated with sodium silicate exhibit a 20-30% longer service life, which can lead to a proportional reduction in resin replacement expenses over time.
Operational efficiency is another key benefit. Stabilized resins maintain their ion exchange capacity for longer periods, reducing the frequency of regeneration cycles. This translates to lower chemical consumption for regeneration and decreased downtime for maintenance, both contributing to operational cost savings.
Water quality improvements represent an indirect but valuable benefit. Stabilized resins are less prone to degradation, minimizing the release of organic foulants into treated water. This can reduce downstream treatment requirements and associated costs, particularly in high-purity water applications such as pharmaceutical or semiconductor manufacturing.
However, the cost-benefit analysis must also consider potential drawbacks. The introduction of sodium silicate may necessitate adjustments in water chemistry management, potentially incurring additional monitoring and control costs. In some cases, there might be a slight reduction in the overall ion exchange capacity of the resin, which could marginally increase operational costs in high-throughput systems.
When evaluating the return on investment, most facilities report a positive cost-benefit ratio within 1-2 years of implementing sodium silicate stabilization. The exact payback period varies depending on factors such as water quality, resin type, and operational parameters. For large-scale industrial applications, the cumulative savings over a 5-10 year period can be substantial, often justifying the initial investment and process modifications.
In conclusion, while the upfront costs of implementing sodium silicate stabilization are modest, the long-term economic benefits are significant. The technique offers a cost-effective solution for extending resin life and improving operational efficiency, making it an attractive option for many water treatment facilities and industrial processes relying on ion exchange technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!