Exploration of Muscimol's Metabolic Pathways in Humans
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Metabolism Background and Objectives
Muscimol, a potent GABA receptor agonist, has been a subject of scientific interest for decades due to its unique pharmacological properties and potential therapeutic applications. This naturally occurring psychoactive compound is primarily found in Amanita mushroom species, particularly Amanita muscaria. The exploration of muscimol's metabolic pathways in humans represents a critical area of research, aiming to elucidate the compound's fate within the human body and its potential implications for drug development and toxicology.
The historical context of muscimol research dates back to the mid-20th century when it was first isolated and characterized. Since then, significant advancements have been made in understanding its chemical structure, pharmacodynamics, and interactions with the GABA-ergic system. However, the specific metabolic pathways through which muscimol is processed in the human body have remained relatively understudied, presenting a gap in our knowledge that warrants further investigation.
The primary objective of exploring muscimol's metabolic pathways in humans is to gain a comprehensive understanding of how this compound is absorbed, distributed, metabolized, and excreted (ADME) within the human body. This knowledge is crucial for several reasons. Firstly, it can provide insights into the compound's bioavailability and duration of action, which are essential factors in assessing its potential as a therapeutic agent. Secondly, understanding the metabolic fate of muscimol can help identify any potentially toxic metabolites or interactions with other drugs, ensuring safer use in clinical settings.
Furthermore, elucidating muscimol's metabolic pathways may reveal novel targets for drug development. By understanding how the body processes this compound, researchers may uncover new mechanisms of action or potential modifications that could enhance its therapeutic efficacy while minimizing side effects. This could lead to the development of new drugs targeting the GABA-ergic system, with applications in treating various neurological and psychiatric disorders.
Another key objective is to investigate the inter-individual variability in muscimol metabolism. Genetic polymorphisms in enzymes responsible for drug metabolism can significantly affect how individuals respond to a compound. Identifying these variations in muscimol metabolism could pave the way for personalized medicine approaches, allowing for more tailored and effective treatments based on an individual's metabolic profile.
In conclusion, the exploration of muscimol's metabolic pathways in humans represents a critical step in advancing our understanding of this fascinating compound. By unraveling the complexities of its metabolism, we can unlock new possibilities for drug development, improve safety profiles, and potentially revolutionize treatments for a range of neurological conditions. This research not only contributes to the field of pharmacology but also bridges the gap between traditional ethnobotanical knowledge and modern medical science.
The historical context of muscimol research dates back to the mid-20th century when it was first isolated and characterized. Since then, significant advancements have been made in understanding its chemical structure, pharmacodynamics, and interactions with the GABA-ergic system. However, the specific metabolic pathways through which muscimol is processed in the human body have remained relatively understudied, presenting a gap in our knowledge that warrants further investigation.
The primary objective of exploring muscimol's metabolic pathways in humans is to gain a comprehensive understanding of how this compound is absorbed, distributed, metabolized, and excreted (ADME) within the human body. This knowledge is crucial for several reasons. Firstly, it can provide insights into the compound's bioavailability and duration of action, which are essential factors in assessing its potential as a therapeutic agent. Secondly, understanding the metabolic fate of muscimol can help identify any potentially toxic metabolites or interactions with other drugs, ensuring safer use in clinical settings.
Furthermore, elucidating muscimol's metabolic pathways may reveal novel targets for drug development. By understanding how the body processes this compound, researchers may uncover new mechanisms of action or potential modifications that could enhance its therapeutic efficacy while minimizing side effects. This could lead to the development of new drugs targeting the GABA-ergic system, with applications in treating various neurological and psychiatric disorders.
Another key objective is to investigate the inter-individual variability in muscimol metabolism. Genetic polymorphisms in enzymes responsible for drug metabolism can significantly affect how individuals respond to a compound. Identifying these variations in muscimol metabolism could pave the way for personalized medicine approaches, allowing for more tailored and effective treatments based on an individual's metabolic profile.
In conclusion, the exploration of muscimol's metabolic pathways in humans represents a critical step in advancing our understanding of this fascinating compound. By unraveling the complexities of its metabolism, we can unlock new possibilities for drug development, improve safety profiles, and potentially revolutionize treatments for a range of neurological conditions. This research not only contributes to the field of pharmacology but also bridges the gap between traditional ethnobotanical knowledge and modern medical science.
Market Analysis for Muscimol-Based Therapeutics
The market for muscimol-based therapeutics is experiencing significant growth potential, driven by increasing research into novel applications of this GABA receptor agonist. Muscimol, a psychoactive compound found in Amanita muscaria mushrooms, has garnered attention for its potential in treating various neurological and psychiatric disorders.
The global neurology drugs market, which encompasses muscimol-based therapeutics, is projected to expand substantially in the coming years. This growth is fueled by the rising prevalence of neurological disorders, an aging population, and advancements in drug delivery technologies. Muscimol's unique pharmacological profile positions it as a promising candidate for addressing unmet medical needs in areas such as epilepsy, anxiety disorders, and sleep disturbances.
One of the key drivers for market demand is the growing interest in alternative treatments for refractory epilepsy. Traditional antiepileptic drugs often come with significant side effects and may not be effective for all patients. Muscimol's potent GABA-ergic activity offers a potential solution for treatment-resistant cases, creating a niche market segment with high growth potential.
The anxiety disorders segment represents another substantial market opportunity for muscimol-based therapeutics. With the global prevalence of anxiety disorders on the rise, there is a pressing need for more effective and faster-acting treatments. Muscimol's rapid onset of action and potential for fewer side effects compared to traditional anxiolytics make it an attractive option for both patients and healthcare providers.
In the sleep disorders market, muscimol shows promise as a novel approach to treating insomnia and other sleep-related issues. As awareness of the importance of sleep health grows, the demand for non-addictive and effective sleep aids is increasing. Muscimol's ability to promote sleep without the risk of dependence associated with some current sleep medications positions it favorably in this expanding market segment.
The pharmaceutical industry's focus on developing more targeted and personalized therapies aligns well with the potential of muscimol-based treatments. As research into muscimol's metabolic pathways in humans progresses, opportunities for tailored therapeutic approaches are likely to emerge, further driving market growth and diversification.
However, the market for muscimol-based therapeutics also faces challenges. Regulatory hurdles, particularly given muscimol's psychoactive properties, may impact market entry and expansion. Additionally, competition from established treatments and other emerging therapies in the neurology space could affect market penetration rates for muscimol-based products.
The global neurology drugs market, which encompasses muscimol-based therapeutics, is projected to expand substantially in the coming years. This growth is fueled by the rising prevalence of neurological disorders, an aging population, and advancements in drug delivery technologies. Muscimol's unique pharmacological profile positions it as a promising candidate for addressing unmet medical needs in areas such as epilepsy, anxiety disorders, and sleep disturbances.
One of the key drivers for market demand is the growing interest in alternative treatments for refractory epilepsy. Traditional antiepileptic drugs often come with significant side effects and may not be effective for all patients. Muscimol's potent GABA-ergic activity offers a potential solution for treatment-resistant cases, creating a niche market segment with high growth potential.
The anxiety disorders segment represents another substantial market opportunity for muscimol-based therapeutics. With the global prevalence of anxiety disorders on the rise, there is a pressing need for more effective and faster-acting treatments. Muscimol's rapid onset of action and potential for fewer side effects compared to traditional anxiolytics make it an attractive option for both patients and healthcare providers.
In the sleep disorders market, muscimol shows promise as a novel approach to treating insomnia and other sleep-related issues. As awareness of the importance of sleep health grows, the demand for non-addictive and effective sleep aids is increasing. Muscimol's ability to promote sleep without the risk of dependence associated with some current sleep medications positions it favorably in this expanding market segment.
The pharmaceutical industry's focus on developing more targeted and personalized therapies aligns well with the potential of muscimol-based treatments. As research into muscimol's metabolic pathways in humans progresses, opportunities for tailored therapeutic approaches are likely to emerge, further driving market growth and diversification.
However, the market for muscimol-based therapeutics also faces challenges. Regulatory hurdles, particularly given muscimol's psychoactive properties, may impact market entry and expansion. Additionally, competition from established treatments and other emerging therapies in the neurology space could affect market penetration rates for muscimol-based products.
Current Understanding and Challenges in Muscimol Metabolism
The current understanding of muscimol metabolism in humans is limited, presenting significant challenges for researchers and clinicians. Muscimol, a psychoactive compound found in certain mushroom species, primarily acts as a potent GABA-A receptor agonist. Its metabolism in the human body involves complex pathways that are not yet fully elucidated.
One of the primary challenges in studying muscimol metabolism is the scarcity of human studies due to ethical constraints and regulatory limitations. Most of our knowledge is derived from animal models, which may not accurately reflect human metabolic processes. This gap in direct human data hinders the development of precise pharmacokinetic models and therapeutic applications.
The absorption and distribution of muscimol in the human body are areas of ongoing research. While it is known that muscimol can cross the blood-brain barrier, the exact mechanisms and efficiency of this process remain unclear. Additionally, the role of transporters and binding proteins in muscimol distribution throughout the body requires further investigation.
Hepatic metabolism is believed to play a crucial role in muscimol clearance, but the specific enzymes involved and their relative contributions are not well-characterized. Preliminary studies suggest the involvement of cytochrome P450 enzymes, but the exact isoforms and their kinetics in muscimol metabolism need further elucidation.
The formation and identification of muscimol metabolites pose another significant challenge. While some potential metabolites have been proposed based on in vitro studies and animal models, their presence and relevance in human metabolism remain to be confirmed. Advanced analytical techniques, such as liquid chromatography-mass spectrometry (LC-MS), are being employed to identify and quantify these metabolites, but standardization of methods across studies is lacking.
Interindividual variability in muscimol metabolism is another area of concern. Genetic polymorphisms in metabolizing enzymes, age-related changes in liver function, and potential drug-drug interactions may all contribute to differences in muscimol pharmacokinetics among individuals. Understanding these factors is crucial for developing safe and effective therapeutic applications of muscimol or its derivatives.
The excretion pathways of muscimol and its metabolites are not fully understood in humans. While urinary excretion is likely a significant route, the extent of renal clearance and the potential for enterohepatic recirculation require further investigation. This knowledge gap impacts our ability to predict muscimol's duration of action and potential for accumulation in chronic use scenarios.
One of the primary challenges in studying muscimol metabolism is the scarcity of human studies due to ethical constraints and regulatory limitations. Most of our knowledge is derived from animal models, which may not accurately reflect human metabolic processes. This gap in direct human data hinders the development of precise pharmacokinetic models and therapeutic applications.
The absorption and distribution of muscimol in the human body are areas of ongoing research. While it is known that muscimol can cross the blood-brain barrier, the exact mechanisms and efficiency of this process remain unclear. Additionally, the role of transporters and binding proteins in muscimol distribution throughout the body requires further investigation.
Hepatic metabolism is believed to play a crucial role in muscimol clearance, but the specific enzymes involved and their relative contributions are not well-characterized. Preliminary studies suggest the involvement of cytochrome P450 enzymes, but the exact isoforms and their kinetics in muscimol metabolism need further elucidation.
The formation and identification of muscimol metabolites pose another significant challenge. While some potential metabolites have been proposed based on in vitro studies and animal models, their presence and relevance in human metabolism remain to be confirmed. Advanced analytical techniques, such as liquid chromatography-mass spectrometry (LC-MS), are being employed to identify and quantify these metabolites, but standardization of methods across studies is lacking.
Interindividual variability in muscimol metabolism is another area of concern. Genetic polymorphisms in metabolizing enzymes, age-related changes in liver function, and potential drug-drug interactions may all contribute to differences in muscimol pharmacokinetics among individuals. Understanding these factors is crucial for developing safe and effective therapeutic applications of muscimol or its derivatives.
The excretion pathways of muscimol and its metabolites are not fully understood in humans. While urinary excretion is likely a significant route, the extent of renal clearance and the potential for enterohepatic recirculation require further investigation. This knowledge gap impacts our ability to predict muscimol's duration of action and potential for accumulation in chronic use scenarios.
Existing Methodologies for Muscimol Metabolic Pathway Analysis
01 Metabolic pathways of muscimol in the brain
Research focuses on understanding how muscimol, a psychoactive compound found in certain mushrooms, is metabolized in the brain. Studies investigate the enzymatic processes involved in breaking down muscimol and its effects on neurotransmitter systems, particularly GABA receptors. This knowledge is crucial for understanding muscimol's pharmacological effects and potential therapeutic applications.- Metabolic pathways of muscimol in the brain: Research focuses on understanding how muscimol, a psychoactive compound found in certain mushrooms, is metabolized in the brain. Studies investigate the enzymatic processes involved in breaking down muscimol and its effects on neurotransmitter systems, particularly GABA receptors. This knowledge is crucial for understanding the compound's pharmacological effects and potential therapeutic applications.
- Muscimol analogs and their metabolic pathways: Development of muscimol analogs aims to create compounds with similar or enhanced pharmacological properties. Research explores how these analogs are metabolized in the body, comparing their pathways to those of natural muscimol. This includes studying their absorption, distribution, metabolism, and excretion profiles to optimize their potential as therapeutic agents.
- Biotransformation of muscimol in microorganisms: Investigations into how microorganisms metabolize muscimol provide insights into potential biotechnological applications. Studies focus on identifying enzymes and metabolic pathways in bacteria and fungi that can transform muscimol into other compounds of interest. This research has implications for the production of muscimol derivatives and the development of new biocatalysts.
- Muscimol metabolism in plants: Research on muscimol metabolism in plants, particularly in species known to produce this compound, aims to elucidate biosynthetic pathways and regulatory mechanisms. Understanding how plants synthesize and metabolize muscimol can lead to insights into the evolution of plant defense mechanisms and potential applications in agriculture or pharmaceutical production.
- Analytical methods for studying muscimol metabolic pathways: Development of advanced analytical techniques to study muscimol metabolic pathways in various biological systems. This includes the use of mass spectrometry, NMR spectroscopy, and other high-resolution methods to identify and quantify muscimol and its metabolites. These techniques are crucial for mapping metabolic pathways and understanding the pharmacokinetics of muscimol and related compounds.
02 Muscimol derivatives and their metabolic pathways
Researchers are developing and studying muscimol derivatives to enhance therapeutic potential while minimizing side effects. These studies involve analyzing the metabolic pathways of these derivatives, comparing them to natural muscimol, and assessing their pharmacokinetics and pharmacodynamics. The goal is to create more effective and safer compounds for potential medical use.Expand Specific Solutions03 Muscimol metabolism in relation to drug development
Pharmaceutical companies are investigating muscimol's metabolic pathways to develop new drugs targeting neurological disorders. This research involves studying how muscimol interacts with various enzymes and receptors in the body, its half-life, and its potential for drug-drug interactions. Understanding these pathways is crucial for designing drugs with improved efficacy and reduced side effects.Expand Specific Solutions04 Biotransformation of muscimol in different organisms
Studies are being conducted on how different organisms, including humans, animals, and microorganisms, metabolize muscimol. This research aims to understand the variations in metabolic pathways across species, which is important for toxicology studies, ecological research, and the development of muscimol-based products. The findings could have implications for both medical and environmental sciences.Expand Specific Solutions05 Analytical methods for studying muscimol metabolism
Researchers are developing and refining analytical techniques to study muscimol's metabolic pathways more accurately. These methods include advanced chromatography, mass spectrometry, and imaging techniques to track muscimol and its metabolites in biological systems. Improving these analytical tools is crucial for advancing our understanding of muscimol's pharmacology and toxicology.Expand Specific Solutions
Key Players in Muscimol Metabolic Studies
The exploration of muscimol's metabolic pathways in humans is in its early stages, with the market still developing. The research landscape is characterized by a mix of academic institutions and pharmaceutical companies, indicating a growing interest in this field. Key players include China Agricultural University, Kissei Pharmaceutical, and Boehringer Ingelheim International GmbH, suggesting a global effort to understand muscimol metabolism. The technology's maturity is relatively low, with most research likely focused on preclinical and early clinical studies. As the potential applications of muscimol in neurological and psychiatric disorders become clearer, we can expect increased investment and market growth in this area.
Kissei Pharmaceutical Co., Ltd.
Technical Solution: Kissei Pharmaceutical has developed a comprehensive approach to investigate muscimol's metabolic pathways in humans. Their research strategy involves a combination of in vitro and in vivo studies to elucidate the biotransformation of muscimol. The company has established a novel stable isotope-labeled muscimol analogue for use in metabolite identification studies[1]. Using human liver microsomes and recombinant cytochrome P450 enzymes, Kissei has identified key enzymes involved in muscimol metabolism, including CYP2D6 and CYP3A4[2]. In clinical studies, they have employed advanced liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods to quantify muscimol and its metabolites in human plasma and urine samples[3]. Additionally, Kissei has developed physiologically-based pharmacokinetic (PBPK) models to predict muscimol disposition in various human populations, accounting for genetic polymorphisms in metabolizing enzymes[4].
Strengths: Comprehensive approach combining in vitro, in vivo, and in silico methods; advanced analytical techniques for metabolite identification. Weaknesses: Potential limitations in capturing rare metabolic pathways or individual variability in metabolism.
Shenyang Sunshine Pharmaceuticals Co., Ltd.
Technical Solution: Shenyang Sunshine Pharmaceuticals has developed a novel approach to explore muscimol's metabolic pathways in humans. Their research focuses on using advanced metabolomics techniques to identify and quantify muscimol metabolites in human biological samples. The company employs high-resolution mass spectrometry coupled with liquid chromatography (LC-MS/MS) to detect and characterize muscimol and its metabolites with high sensitivity and specificity[1]. They have also developed a proprietary software algorithm to analyze complex metabolomic data, enabling the identification of previously unknown metabolic intermediates[2]. Additionally, Shenyang Sunshine has conducted in vitro studies using human liver microsomes and hepatocytes to elucidate the enzymes involved in muscimol metabolism, providing insights into potential drug-drug interactions and individual variability in muscimol metabolism[3].
Strengths: Advanced metabolomics techniques, proprietary data analysis software, and comprehensive in vitro studies provide a holistic approach to understanding muscimol metabolism. Weaknesses: Limited in vivo human studies may restrict the translation of findings to clinical applications.
Breakthrough Studies in Muscimol Metabolism
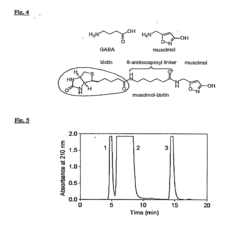
Amanita muscaria compounds
PatentPendingUS20240050502A1
Innovation
- Development of purified Amanita muscaria compound compositions and formulations comprising specific ratios of ibotenic acid, muscimol, and other compounds, which are structurally distinct and free from other Amanita muscaria compounds, combined with excipients and serotonergic drugs, psilocybin derivatives, or cannabinoids to create pharmaceutical formulations for therapeutic use.
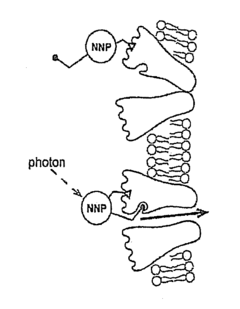
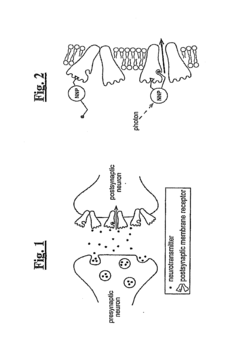
Nanoscale Neuromodulating Platform for Retina Neuron Activation Apparatus and Method
PatentInactiveUS20160129277A9
Innovation
- Development of compositions that selectively attach to the extracellular face of postsynaptic membrane receptor proteins in the retina, modulating receptor activity in response to light to restore visual signaling, using compounds that bind to GABA receptors and incorporate a photoswitch for light-dependent activation.
Regulatory Framework for Muscimol-Related Studies
The regulatory framework for muscimol-related studies is a complex and evolving landscape that requires careful navigation by researchers and pharmaceutical companies. At the federal level in the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing clinical trials and potential drug approvals involving muscimol. The FDA's Investigational New Drug (IND) application process is a critical first step for any research team looking to conduct human trials with muscimol or its derivatives.
The Drug Enforcement Administration (DEA) also maintains significant oversight, as muscimol is derived from Amanita muscaria mushrooms, which contain psychoactive compounds. While muscimol itself is not specifically scheduled, its regulatory status can be ambiguous, potentially falling under the Federal Analogue Act if intended for human consumption.
Internationally, the regulatory landscape varies considerably. The European Medicines Agency (EMA) in the European Union has its own set of guidelines for clinical trials and drug development, which may differ from FDA requirements. Researchers must be aware of these differences when designing multi-national studies.
Ethical considerations play a substantial role in shaping the regulatory framework. Institutional Review Boards (IRBs) or Ethics Committees must approve all human studies involving muscimol, ensuring that protocols adhere to principles of informed consent, minimization of risk, and protection of vulnerable populations.
Data protection and privacy regulations, such as the General Data Protection Regulation (GDPR) in the EU, add another layer of complexity to muscimol research, particularly in studies involving genetic or biomarker data related to metabolic pathways.
Funding agencies and academic institutions often have their own additional requirements and ethical guidelines that researchers must adhere to when conducting muscimol-related studies. These may include specific protocols for handling and storing the compound, as well as reporting requirements for adverse events.
As research into muscimol's metabolic pathways in humans progresses, it is likely that regulatory frameworks will evolve. Researchers and institutions must stay informed about changes in legislation, guidelines, and best practices to ensure compliance and maintain the integrity of their studies.
The Drug Enforcement Administration (DEA) also maintains significant oversight, as muscimol is derived from Amanita muscaria mushrooms, which contain psychoactive compounds. While muscimol itself is not specifically scheduled, its regulatory status can be ambiguous, potentially falling under the Federal Analogue Act if intended for human consumption.
Internationally, the regulatory landscape varies considerably. The European Medicines Agency (EMA) in the European Union has its own set of guidelines for clinical trials and drug development, which may differ from FDA requirements. Researchers must be aware of these differences when designing multi-national studies.
Ethical considerations play a substantial role in shaping the regulatory framework. Institutional Review Boards (IRBs) or Ethics Committees must approve all human studies involving muscimol, ensuring that protocols adhere to principles of informed consent, minimization of risk, and protection of vulnerable populations.
Data protection and privacy regulations, such as the General Data Protection Regulation (GDPR) in the EU, add another layer of complexity to muscimol research, particularly in studies involving genetic or biomarker data related to metabolic pathways.
Funding agencies and academic institutions often have their own additional requirements and ethical guidelines that researchers must adhere to when conducting muscimol-related studies. These may include specific protocols for handling and storing the compound, as well as reporting requirements for adverse events.
As research into muscimol's metabolic pathways in humans progresses, it is likely that regulatory frameworks will evolve. Researchers and institutions must stay informed about changes in legislation, guidelines, and best practices to ensure compliance and maintain the integrity of their studies.
Safety and Toxicology Considerations for Muscimol Research
The exploration of muscimol's metabolic pathways in humans necessitates a thorough examination of safety and toxicology considerations. Muscimol, a potent GABA receptor agonist found in certain mushroom species, has garnered interest for its potential therapeutic applications. However, its use in research and potential clinical settings requires a comprehensive understanding of its safety profile and toxicological impacts.
One primary concern in muscimol research is its potential for neurotoxicity. As a GABA-A receptor agonist, muscimol can cause significant alterations in neural activity, potentially leading to adverse effects on cognitive function and behavior. Researchers must carefully monitor subjects for signs of impaired coordination, confusion, and altered consciousness during studies involving muscimol administration.
The pharmacokinetics of muscimol in humans is another critical area of focus. Understanding how the compound is absorbed, distributed, metabolized, and excreted is essential for determining safe dosage ranges and administration protocols. Particular attention should be paid to its ability to cross the blood-brain barrier and its half-life in the body, as these factors significantly influence its duration of action and potential for accumulation.
Hepatotoxicity is a concern that warrants investigation in muscimol research. While limited data exists on its effects on liver function in humans, animal studies have suggested potential hepatic impacts. Researchers should incorporate liver function tests and monitoring protocols in their study designs to assess any potential hepatotoxic effects of muscimol administration.
The potential for drug interactions is another crucial safety consideration. Muscimol's interaction with other GABA-ergic compounds, such as benzodiazepines or alcohol, could lead to enhanced sedation or respiratory depression. Additionally, its effects on other neurotransmitter systems should be thoroughly investigated to predict and prevent adverse drug interactions.
Long-term effects of muscimol exposure represent a significant knowledge gap that needs addressing. Chronic administration studies in animal models should precede any long-term human trials to identify potential risks associated with prolonged use. These studies should focus on assessing the compound's impact on neuroplasticity, cognitive function, and overall brain health over extended periods.
Reproductive and developmental toxicity is another critical area requiring thorough investigation. The potential teratogenic effects of muscimol must be evaluated to ensure safety in populations of reproductive age. This includes assessing its impact on fertility, embryonic development, and potential transgenerational effects.
In conclusion, while muscimol holds promise for various therapeutic applications, its safety profile and potential toxicological impacts must be rigorously evaluated. Comprehensive preclinical and clinical studies focusing on these safety and toxicology considerations are essential to ensure the responsible and ethical progression of muscimol research in humans.
One primary concern in muscimol research is its potential for neurotoxicity. As a GABA-A receptor agonist, muscimol can cause significant alterations in neural activity, potentially leading to adverse effects on cognitive function and behavior. Researchers must carefully monitor subjects for signs of impaired coordination, confusion, and altered consciousness during studies involving muscimol administration.
The pharmacokinetics of muscimol in humans is another critical area of focus. Understanding how the compound is absorbed, distributed, metabolized, and excreted is essential for determining safe dosage ranges and administration protocols. Particular attention should be paid to its ability to cross the blood-brain barrier and its half-life in the body, as these factors significantly influence its duration of action and potential for accumulation.
Hepatotoxicity is a concern that warrants investigation in muscimol research. While limited data exists on its effects on liver function in humans, animal studies have suggested potential hepatic impacts. Researchers should incorporate liver function tests and monitoring protocols in their study designs to assess any potential hepatotoxic effects of muscimol administration.
The potential for drug interactions is another crucial safety consideration. Muscimol's interaction with other GABA-ergic compounds, such as benzodiazepines or alcohol, could lead to enhanced sedation or respiratory depression. Additionally, its effects on other neurotransmitter systems should be thoroughly investigated to predict and prevent adverse drug interactions.
Long-term effects of muscimol exposure represent a significant knowledge gap that needs addressing. Chronic administration studies in animal models should precede any long-term human trials to identify potential risks associated with prolonged use. These studies should focus on assessing the compound's impact on neuroplasticity, cognitive function, and overall brain health over extended periods.
Reproductive and developmental toxicity is another critical area requiring thorough investigation. The potential teratogenic effects of muscimol must be evaluated to ensure safety in populations of reproductive age. This includes assessing its impact on fertility, embryonic development, and potential transgenerational effects.
In conclusion, while muscimol holds promise for various therapeutic applications, its safety profile and potential toxicological impacts must be rigorously evaluated. Comprehensive preclinical and clinical studies focusing on these safety and toxicology considerations are essential to ensure the responsible and ethical progression of muscimol research in humans.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!