Exploring Biocompatibility of Self-Powered Sensors in Medicine
OCT 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biocompatible Self-Powered Sensors: Background and Objectives
Self-powered sensors represent a revolutionary advancement in medical technology, emerging from the convergence of energy harvesting techniques and biomedical sensing capabilities. These devices have evolved significantly over the past decade, transitioning from rudimentary prototypes to sophisticated systems capable of continuous health monitoring without external power sources. The historical trajectory began with basic piezoelectric and thermoelectric generators in the early 2000s, followed by the integration of triboelectric nanogenerators (TENGs) around 2012, which marked a pivotal turning point in self-powered sensing technology.
The evolution of biocompatible materials has paralleled this development, moving from conventional medical-grade polymers to advanced nanocomposites and biodegradable substrates. This progression has been driven by the increasing demand for minimally invasive, long-term implantable devices that can operate autonomously within the human body without causing adverse reactions or requiring frequent replacement.
Current technological trends indicate a shift toward multifunctional self-powered sensors that can simultaneously harvest energy from multiple biological sources (such as blood flow, muscle movement, and temperature gradients) while monitoring various physiological parameters. The miniaturization of these devices continues to advance, with recent breakthroughs in nanofabrication enabling sensors at the microscale that can navigate through the bloodstream or be embedded in tissue with minimal disruption.
The primary technical objectives for biocompatible self-powered sensors encompass several critical dimensions. First, achieving complete biocompatibility with zero immunological response or tissue damage during long-term implantation. Second, developing sufficient energy harvesting efficiency to power not only the sensing components but also data processing and wireless transmission capabilities. Third, ensuring reliable operation under the variable and often harsh conditions of the human body, including fluctuating temperatures, pH levels, and mechanical stresses.
Additionally, these sensors must demonstrate exceptional sensitivity and specificity in detecting biomarkers at physiologically relevant concentrations, often in the nanomolar to picomolar range. The ultimate goal is to create fully integrated, autonomous sensing systems that can provide real-time, continuous monitoring of health parameters without external intervention, potentially revolutionizing disease management, drug delivery, and personalized medicine.
The convergence of nanotechnology, materials science, and biomedical engineering is expected to accelerate innovation in this field, with particular emphasis on biodegradable sensors that can perform their function and then safely dissolve, eliminating the need for surgical removal and reducing the risk of long-term complications.
The evolution of biocompatible materials has paralleled this development, moving from conventional medical-grade polymers to advanced nanocomposites and biodegradable substrates. This progression has been driven by the increasing demand for minimally invasive, long-term implantable devices that can operate autonomously within the human body without causing adverse reactions or requiring frequent replacement.
Current technological trends indicate a shift toward multifunctional self-powered sensors that can simultaneously harvest energy from multiple biological sources (such as blood flow, muscle movement, and temperature gradients) while monitoring various physiological parameters. The miniaturization of these devices continues to advance, with recent breakthroughs in nanofabrication enabling sensors at the microscale that can navigate through the bloodstream or be embedded in tissue with minimal disruption.
The primary technical objectives for biocompatible self-powered sensors encompass several critical dimensions. First, achieving complete biocompatibility with zero immunological response or tissue damage during long-term implantation. Second, developing sufficient energy harvesting efficiency to power not only the sensing components but also data processing and wireless transmission capabilities. Third, ensuring reliable operation under the variable and often harsh conditions of the human body, including fluctuating temperatures, pH levels, and mechanical stresses.
Additionally, these sensors must demonstrate exceptional sensitivity and specificity in detecting biomarkers at physiologically relevant concentrations, often in the nanomolar to picomolar range. The ultimate goal is to create fully integrated, autonomous sensing systems that can provide real-time, continuous monitoring of health parameters without external intervention, potentially revolutionizing disease management, drug delivery, and personalized medicine.
The convergence of nanotechnology, materials science, and biomedical engineering is expected to accelerate innovation in this field, with particular emphasis on biodegradable sensors that can perform their function and then safely dissolve, eliminating the need for surgical removal and reducing the risk of long-term complications.
Market Analysis for Medical Self-Powered Sensing Technologies
The global market for self-powered sensing technologies in medical applications is experiencing robust growth, driven by increasing demand for continuous health monitoring solutions and advancements in energy harvesting technologies. Current market valuations indicate that the medical wearable device sector, which includes self-powered sensors, reached approximately 30 billion USD in 2022 and is projected to grow at a compound annual growth rate of 14-16% through 2028.
Patient monitoring represents the largest application segment, accounting for nearly 40% of the market share. This dominance stems from the rising prevalence of chronic diseases requiring continuous monitoring and the growing elderly population worldwide. Implantable medical devices incorporating self-powered sensors constitute a rapidly expanding segment, with particular growth in cardiac monitoring and glucose sensing applications.
Geographically, North America leads the market with approximately 45% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is demonstrating the fastest growth rate, primarily due to increasing healthcare expenditure, improving healthcare infrastructure, and rising awareness about preventive healthcare in countries like China, Japan, and India.
Key market drivers include the growing emphasis on preventive healthcare, increasing adoption of remote patient monitoring systems, and technological advancements in miniaturization and biocompatibility of sensors. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of remote monitoring capabilities and reducing hospital visits for routine check-ups.
Consumer demand trends indicate strong preference for non-invasive, comfortable, and long-lasting monitoring solutions. There is particular interest in devices that can operate without frequent battery replacements or recharging, making self-powered sensors especially attractive. Market research shows that patients are increasingly willing to pay premium prices for devices offering extended operational lifetimes and reduced maintenance requirements.
Reimbursement policies are evolving to accommodate these new technologies, with several insurance providers now covering continuous monitoring devices for specific conditions. This trend is expected to continue as evidence accumulates regarding the cost-effectiveness of preventive monitoring versus reactive treatment approaches.
Market challenges include concerns about data security and privacy, regulatory hurdles for novel biocompatible materials, and the need for clinical validation of long-term biocompatibility. Despite these challenges, the market outlook remains highly positive, with significant opportunities for companies that can successfully address biocompatibility concerns while maintaining reliable power generation capabilities.
Patient monitoring represents the largest application segment, accounting for nearly 40% of the market share. This dominance stems from the rising prevalence of chronic diseases requiring continuous monitoring and the growing elderly population worldwide. Implantable medical devices incorporating self-powered sensors constitute a rapidly expanding segment, with particular growth in cardiac monitoring and glucose sensing applications.
Geographically, North America leads the market with approximately 45% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is demonstrating the fastest growth rate, primarily due to increasing healthcare expenditure, improving healthcare infrastructure, and rising awareness about preventive healthcare in countries like China, Japan, and India.
Key market drivers include the growing emphasis on preventive healthcare, increasing adoption of remote patient monitoring systems, and technological advancements in miniaturization and biocompatibility of sensors. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of remote monitoring capabilities and reducing hospital visits for routine check-ups.
Consumer demand trends indicate strong preference for non-invasive, comfortable, and long-lasting monitoring solutions. There is particular interest in devices that can operate without frequent battery replacements or recharging, making self-powered sensors especially attractive. Market research shows that patients are increasingly willing to pay premium prices for devices offering extended operational lifetimes and reduced maintenance requirements.
Reimbursement policies are evolving to accommodate these new technologies, with several insurance providers now covering continuous monitoring devices for specific conditions. This trend is expected to continue as evidence accumulates regarding the cost-effectiveness of preventive monitoring versus reactive treatment approaches.
Market challenges include concerns about data security and privacy, regulatory hurdles for novel biocompatible materials, and the need for clinical validation of long-term biocompatibility. Despite these challenges, the market outlook remains highly positive, with significant opportunities for companies that can successfully address biocompatibility concerns while maintaining reliable power generation capabilities.
Current Biocompatibility Challenges in Implantable Sensor Technology
Despite significant advancements in implantable sensor technology, biocompatibility remains a critical challenge that limits widespread clinical adoption. Current implantable sensors face several biological integration issues, including foreign body response, which triggers inflammation, fibrosis, and eventual sensor encapsulation. This immune reaction not only compromises sensor functionality but also reduces device longevity, with most implantable sensors experiencing significant performance degradation within weeks to months after implantation.
Material toxicity presents another substantial challenge, as conventional electronic components often contain heavy metals and other potentially harmful substances. Even trace leaching of these materials can cause localized tissue damage or systemic toxicity. Self-powered sensors, while solving energy constraints, introduce additional biocompatibility concerns through their energy harvesting mechanisms, which may create mechanical stress, temperature fluctuations, or electrical field interactions with surrounding tissues.
Surface modification technologies have shown promise but remain insufficient for long-term implantation. Current coating approaches using hydrogels, anti-fouling polymers, and drug-eluting materials provide temporary mitigation but fail to address the fundamental incompatibility between rigid electronic systems and soft biological tissues. This mechanical mismatch leads to micromotion at the tissue-sensor interface, causing chronic irritation and accelerated foreign body response.
Sterilization compatibility represents another significant hurdle, as many innovative biomaterials and sensitive electronic components cannot withstand standard sterilization protocols. This creates a paradoxical situation where improving sensor biocompatibility often reduces compatibility with established sterilization methods, complicating regulatory approval pathways.
The challenge of biodegradation control also remains largely unsolved. Conventional implantable sensors are designed to resist biodegradation, necessitating surgical removal after their functional lifespan. Conversely, emerging biodegradable sensors face difficulties in precisely controlling their degradation timeline to match clinical needs while ensuring degradation byproducts remain non-toxic.
Regulatory frameworks have struggled to keep pace with technological innovation in this field. Current standards for biocompatibility testing were largely developed for passive implants and may not adequately address the unique challenges of active, self-powered sensing systems. This regulatory uncertainty creates additional barriers to clinical translation and commercialization of novel implantable sensor technologies.
Cross-disciplinary collaboration between materials scientists, biomedical engineers, and clinicians remains insufficient, resulting in sensors that excel in technical performance but fail to address practical biocompatibility requirements for real-world clinical applications. Bridging this gap requires integrated development approaches that consider biocompatibility as a primary design parameter rather than a secondary consideration.
Material toxicity presents another substantial challenge, as conventional electronic components often contain heavy metals and other potentially harmful substances. Even trace leaching of these materials can cause localized tissue damage or systemic toxicity. Self-powered sensors, while solving energy constraints, introduce additional biocompatibility concerns through their energy harvesting mechanisms, which may create mechanical stress, temperature fluctuations, or electrical field interactions with surrounding tissues.
Surface modification technologies have shown promise but remain insufficient for long-term implantation. Current coating approaches using hydrogels, anti-fouling polymers, and drug-eluting materials provide temporary mitigation but fail to address the fundamental incompatibility between rigid electronic systems and soft biological tissues. This mechanical mismatch leads to micromotion at the tissue-sensor interface, causing chronic irritation and accelerated foreign body response.
Sterilization compatibility represents another significant hurdle, as many innovative biomaterials and sensitive electronic components cannot withstand standard sterilization protocols. This creates a paradoxical situation where improving sensor biocompatibility often reduces compatibility with established sterilization methods, complicating regulatory approval pathways.
The challenge of biodegradation control also remains largely unsolved. Conventional implantable sensors are designed to resist biodegradation, necessitating surgical removal after their functional lifespan. Conversely, emerging biodegradable sensors face difficulties in precisely controlling their degradation timeline to match clinical needs while ensuring degradation byproducts remain non-toxic.
Regulatory frameworks have struggled to keep pace with technological innovation in this field. Current standards for biocompatibility testing were largely developed for passive implants and may not adequately address the unique challenges of active, self-powered sensing systems. This regulatory uncertainty creates additional barriers to clinical translation and commercialization of novel implantable sensor technologies.
Cross-disciplinary collaboration between materials scientists, biomedical engineers, and clinicians remains insufficient, resulting in sensors that excel in technical performance but fail to address practical biocompatibility requirements for real-world clinical applications. Bridging this gap requires integrated development approaches that consider biocompatibility as a primary design parameter rather than a secondary consideration.
Current Biocompatible Materials and Energy Harvesting Solutions
01 Biocompatible materials for self-powered sensors
Various biocompatible materials can be used in the fabrication of self-powered sensors to ensure compatibility with biological systems. These materials include biodegradable polymers, natural fibers, and biocompatible metals that minimize immune responses when implanted in the body. The selection of these materials is crucial for long-term monitoring applications in healthcare and can significantly improve sensor performance while reducing adverse reactions in biological environments.- Biocompatible materials for self-powered sensors: Various biocompatible materials can be used in the fabrication of self-powered sensors to ensure compatibility with biological systems. These materials include biodegradable polymers, natural fibers, and biocompatible metals that minimize immune responses when implanted in the body. The selection of these materials is crucial for long-term monitoring applications, particularly for implantable devices that need to operate within the body without causing inflammation or rejection.
- Energy harvesting mechanisms for biocompatible sensors: Self-powered sensors utilize various energy harvesting mechanisms to operate autonomously within biological environments. These mechanisms include piezoelectric generators that convert mechanical motion into electrical energy, triboelectric nanogenerators that harvest energy from friction, and biofuel cells that generate power from bodily fluids. These energy harvesting technologies eliminate the need for batteries, making the sensors more suitable for long-term implantation and reducing the risk of toxic battery leakage.
- Encapsulation techniques for biocompatibility enhancement: Encapsulation techniques play a vital role in enhancing the biocompatibility of self-powered sensors. These techniques involve coating the sensors with biocompatible materials such as hydrogels, parylene, or silicone to create a protective barrier between the device and surrounding tissues. Advanced encapsulation methods not only improve biocompatibility but also maintain sensor functionality by allowing selective permeability to target analytes while protecting electronic components from bodily fluids.
- Implantable self-powered biosensors: Implantable self-powered biosensors are designed specifically for in vivo applications, featuring miniaturized designs and biocompatible interfaces. These sensors can monitor various physiological parameters such as glucose levels, pH, oxygen concentration, and specific biomarkers. The integration of biocompatible materials with efficient energy harvesting mechanisms allows these sensors to function autonomously within the body for extended periods, providing continuous health monitoring without external power sources.
- Flexible and stretchable biocompatible sensors: Flexible and stretchable biocompatible sensors are designed to conform to the natural contours and movements of biological tissues. These sensors incorporate elastic substrates, serpentine conductive traces, and stretchable electrodes that can withstand mechanical deformation while maintaining functionality. The flexibility and stretchability of these sensors reduce mechanical mismatch with soft tissues, minimizing irritation and foreign body responses, which is crucial for wearable health monitoring applications and epidermal electronic systems.
02 Energy harvesting mechanisms for biocompatible sensors
Self-powered sensors utilize various energy harvesting mechanisms to operate without external power sources, enhancing their biocompatibility and longevity. These mechanisms include piezoelectric generators that convert mechanical motion into electrical energy, triboelectric nanogenerators that harvest energy from friction, and biofuel cells that generate power from biological fluids. These technologies enable continuous operation of sensors in biological environments without the need for battery replacement.Expand Specific Solutions03 Implantable self-powered biosensors
Implantable self-powered biosensors are designed to monitor physiological parameters within the body while maintaining biocompatibility. These sensors incorporate biocompatible coatings, anti-fouling surfaces, and tissue-friendly interfaces to minimize foreign body responses. Advanced designs include flexible and stretchable components that conform to tissue surfaces, reducing mechanical irritation and improving long-term stability for continuous health monitoring applications.Expand Specific Solutions04 Wearable biocompatible self-powered sensors
Wearable self-powered sensors designed with biocompatible interfaces can monitor health parameters non-invasively. These sensors utilize skin-friendly adhesives, breathable substrates, and hypoallergenic materials to prevent skin irritation during prolonged wear. The integration of flexible electronics and soft materials allows these sensors to conform to body contours, enhancing comfort while efficiently harvesting energy from body heat, motion, or ambient light to power sensing functions.Expand Specific Solutions05 Biocompatible encapsulation techniques
Encapsulation techniques play a crucial role in enhancing the biocompatibility of self-powered sensors. Methods such as parylene coating, silicone encapsulation, and hydrogel embedding protect electronic components from bodily fluids while preventing leaching of potentially harmful substances. These techniques extend sensor lifetime in biological environments and maintain stable performance by creating effective barriers against biofouling and degradation, which is essential for reliable long-term monitoring applications.Expand Specific Solutions
Leading Innovators in Medical Self-Powered Sensor Development
The self-powered sensor market in medicine is currently in an early growth phase, characterized by increasing research activity and emerging commercial applications. The global biocompatible sensor market is projected to expand significantly, driven by growing demand for minimally invasive medical monitoring solutions. Leading players include established medical technology companies like Medtronic, Johnson & Johnson Vision Care, and Abbott Diabetes Care, which leverage their clinical expertise and distribution networks. Academic institutions such as California Institute of Technology, Zhejiang University, and University of Washington are advancing fundamental research in biocompatible materials and energy harvesting techniques. Specialized sensor companies like Opteev Technologies and ArgusEye are developing innovative solutions, while semiconductor manufacturers including TSMC provide critical component technologies. The field remains technically challenging, with biocompatibility and long-term reliability representing key hurdles to widespread clinical adoption.
Medtronic, Inc.
Technical Solution: Medtronic has developed advanced self-powered biosensors utilizing triboelectric nanogenerators (TENG) technology for implantable medical devices. Their approach integrates biocompatible polymers like PDMS (polydimethylsiloxane) and PTFE (polytetrafluoroethylene) with nanostructured surfaces to enhance energy harvesting efficiency while maintaining excellent biocompatibility. These materials undergo rigorous ISO 10993 biocompatibility testing to ensure safety for long-term implantation. Medtronic's self-powered sensors can harvest energy from natural body movements, cardiac contractions, and blood flow to power various monitoring functions including glucose sensing, cardiac monitoring, and neural activity detection. Their proprietary encapsulation technology uses ultra-thin biocompatible coatings that prevent immune responses while allowing sufficient mechanical deformation for energy generation. Recent clinical trials have demonstrated stable performance of these sensors for over 18 months in vivo with minimal foreign body response.
Strengths: Extensive clinical validation experience, established regulatory pathway expertise, and comprehensive biocompatibility testing infrastructure. Weaknesses: Higher production costs compared to non-implantable alternatives, and potential challenges with long-term stability of biocompatible materials in dynamic physiological environments.
California Institute of Technology
Technical Solution: Caltech has developed groundbreaking biodegradable self-powered sensors using piezoelectric materials derived from natural polymers like cellulose, chitin, and silk fibroin. Their approach focuses on transient electronics that can perform critical monitoring functions before harmlessly dissolving in the body, eliminating the need for surgical removal. The technology employs specialized surface modification techniques using zwitterionic polymers that create an ultra-hydrophilic interface, significantly reducing protein adsorption and subsequent inflammatory responses. Caltech's sensors utilize magnesium-based biodegradable electrodes and interconnects encapsulated in poly(lactic-co-glycolic acid) (PLGA) with controlled degradation profiles tailored to specific clinical applications. Their innovative "mechanically adaptive" materials change mechanical properties upon implantation, transitioning from rigid during insertion to soft and compliant in physiological environments, minimizing mechanical mismatch with surrounding tissues. Recent publications demonstrate successful in vivo testing of these sensors for post-operative monitoring of surgical sites with complete biodegradation within 3-6 months and minimal inflammatory response.
Strengths: Cutting-edge biodegradable materials science expertise, elimination of long-term biocompatibility concerns through transient electronics approach, and strong academic research capabilities. Weaknesses: Limited commercialization experience, challenges in scaling production, and potential variability in biodegradation rates under different physiological conditions.
Key Patents and Research in Biocompatible Self-Powered Sensing
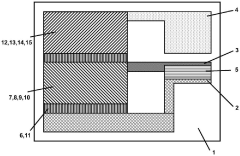
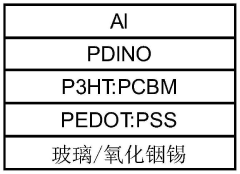
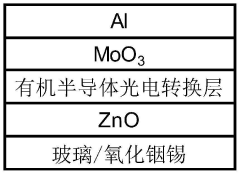
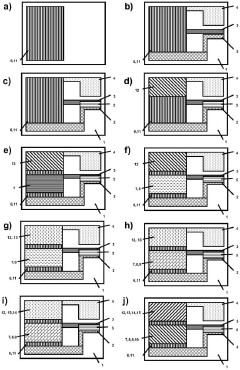
A self-powered electrochemical sensor and solar cell and processing method thereof
PatentActiveCN114927614B
Innovation
- 设计一种自供电的电化学传感器,采用太阳能电池作为电源,通过溶液法和真空热蒸镀法制备,包括漏极和栅极太阳能电池,结合半导体沟道层和选择性检测膜,实现对泪液组分浓度的高灵敏度检测。
Self-power sensor
PatentPendingUS20220047183A1
Innovation
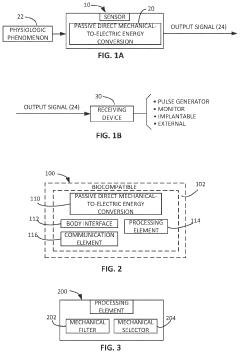
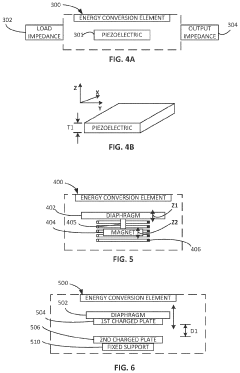
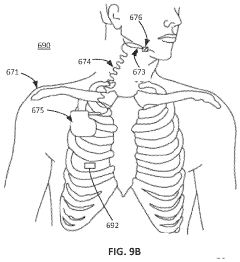
- Development of zero-external-input power sensors that utilize mechanical-to-electrical energy conversion elements, such as piezoelectric or electromagnetic components, to generate power from physiological movements, eliminating the need for external power sources and enabling real-time data capture without storage or additional power transfer.
Regulatory Framework for Implantable Self-Powered Medical Devices
The regulatory landscape for implantable self-powered medical devices represents a complex framework that spans multiple jurisdictions and oversight bodies. In the United States, the Food and Drug Administration (FDA) classifies these devices primarily under Class III, requiring the most stringent Premarket Approval (PMA) process due to their implantable nature and novel power generation mechanisms. The FDA's guidance specifically addresses biocompatibility concerns through ISO 10993 standards, which manufacturers must satisfy through comprehensive testing protocols.
The European Union approaches regulation through the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive in 2021, introducing more rigorous requirements for clinical evaluation and post-market surveillance. Self-powered implantable devices face particular scrutiny under Annex IX, which mandates extensive documentation of energy sources and potential electromagnetic interference risks.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the "Sakigake" designation system that can expedite approval for innovative medical technologies, including self-powered sensors, while maintaining strict safety standards. This pathway has become increasingly relevant for biocompatible energy harvesting technologies that demonstrate significant medical advantages.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) have produced guidance documents specifically addressing software as a medical device (SaMD) and cybersecurity concerns, which are particularly relevant for connected self-powered sensors that transmit patient data.
Regulatory challenges specific to self-powered devices include demonstrating long-term stability of power generation mechanisms, validating performance under varying physiological conditions, and ensuring that energy harvesting components do not introduce additional biocompatibility risks. The FDA's recent "Safer Technologies Program" (STeP) offers potential accelerated pathways for devices that demonstrate substantial safety improvements over existing technologies.
Emerging regulatory considerations include the development of specific standards for bioenergy harvesting mechanisms, such as piezoelectric materials in contact with tissue, thermoelectric generators utilizing body temperature differentials, and triboelectric nanogenerators converting biomechanical energy. These technologies currently face regulatory evaluation on a case-by-case basis without standardized testing protocols.
The European Union approaches regulation through the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive in 2021, introducing more rigorous requirements for clinical evaluation and post-market surveillance. Self-powered implantable devices face particular scrutiny under Annex IX, which mandates extensive documentation of energy sources and potential electromagnetic interference risks.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the "Sakigake" designation system that can expedite approval for innovative medical technologies, including self-powered sensors, while maintaining strict safety standards. This pathway has become increasingly relevant for biocompatible energy harvesting technologies that demonstrate significant medical advantages.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) have produced guidance documents specifically addressing software as a medical device (SaMD) and cybersecurity concerns, which are particularly relevant for connected self-powered sensors that transmit patient data.
Regulatory challenges specific to self-powered devices include demonstrating long-term stability of power generation mechanisms, validating performance under varying physiological conditions, and ensuring that energy harvesting components do not introduce additional biocompatibility risks. The FDA's recent "Safer Technologies Program" (STeP) offers potential accelerated pathways for devices that demonstrate substantial safety improvements over existing technologies.
Emerging regulatory considerations include the development of specific standards for bioenergy harvesting mechanisms, such as piezoelectric materials in contact with tissue, thermoelectric generators utilizing body temperature differentials, and triboelectric nanogenerators converting biomechanical energy. These technologies currently face regulatory evaluation on a case-by-case basis without standardized testing protocols.
Long-Term Tissue Integration and Biocompatibility Assessment Methods
The assessment of long-term tissue integration and biocompatibility for self-powered sensors represents a critical challenge in medical device development. Current methodologies typically involve a multi-phase approach beginning with in vitro cytotoxicity testing using standardized ISO 10993 protocols, where sensor materials are exposed to cell cultures to evaluate potential toxic effects on cellular viability, proliferation, and morphology.
Advanced biocompatibility assessment has evolved to include more sophisticated in vitro models such as 3D organoids and tissue-on-chip platforms, which better simulate the complex cellular interactions within human tissues. These models provide more physiologically relevant data on how self-powered sensors might interact with specific tissue microenvironments over extended periods.
In vivo evaluation remains the gold standard for long-term tissue integration assessment, typically involving implantation studies in animal models over periods ranging from several weeks to multiple years. These studies monitor local tissue responses including inflammation, fibrosis, and foreign body reactions through histopathological analysis and immunohistochemistry techniques that identify specific cellular markers of biocompatibility issues.
Recent advances in non-invasive imaging technologies have significantly enhanced biocompatibility monitoring capabilities. Techniques such as magnetic resonance imaging (MRI), micro-computed tomography (micro-CT), and intravital microscopy now allow researchers to observe tissue-sensor interactions in real-time without sacrificing experimental subjects, providing longitudinal data on integration processes.
Molecular and genetic biomarkers have emerged as valuable tools for assessing subtle tissue responses to implanted sensors. Techniques such as transcriptomics, proteomics, and metabolomics can detect early signs of adverse reactions before they manifest as visible tissue damage, enabling more proactive intervention in sensor design.
The development of standardized degradation testing protocols represents another important advancement, particularly for biodegradable self-powered sensors. These protocols simulate physiological conditions to predict how sensor materials will break down over time and whether degradation products might cause delayed biocompatibility issues.
Functional integration assessment has become increasingly important, focusing on whether the sensor maintains its operational capabilities while achieving tissue compatibility. This involves evaluating signal stability, power generation consistency, and data transmission reliability in the context of tissue integration, recognizing that mechanical and electrical performance can be compromised by biological responses.
Advanced biocompatibility assessment has evolved to include more sophisticated in vitro models such as 3D organoids and tissue-on-chip platforms, which better simulate the complex cellular interactions within human tissues. These models provide more physiologically relevant data on how self-powered sensors might interact with specific tissue microenvironments over extended periods.
In vivo evaluation remains the gold standard for long-term tissue integration assessment, typically involving implantation studies in animal models over periods ranging from several weeks to multiple years. These studies monitor local tissue responses including inflammation, fibrosis, and foreign body reactions through histopathological analysis and immunohistochemistry techniques that identify specific cellular markers of biocompatibility issues.
Recent advances in non-invasive imaging technologies have significantly enhanced biocompatibility monitoring capabilities. Techniques such as magnetic resonance imaging (MRI), micro-computed tomography (micro-CT), and intravital microscopy now allow researchers to observe tissue-sensor interactions in real-time without sacrificing experimental subjects, providing longitudinal data on integration processes.
Molecular and genetic biomarkers have emerged as valuable tools for assessing subtle tissue responses to implanted sensors. Techniques such as transcriptomics, proteomics, and metabolomics can detect early signs of adverse reactions before they manifest as visible tissue damage, enabling more proactive intervention in sensor design.
The development of standardized degradation testing protocols represents another important advancement, particularly for biodegradable self-powered sensors. These protocols simulate physiological conditions to predict how sensor materials will break down over time and whether degradation products might cause delayed biocompatibility issues.
Functional integration assessment has become increasingly important, focusing on whether the sensor maintains its operational capabilities while achieving tissue compatibility. This involves evaluating signal stability, power generation consistency, and data transmission reliability in the context of tissue integration, recognizing that mechanical and electrical performance can be compromised by biological responses.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!