Exploring Carbonyl Group Applications in Pharmaceuticals
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonyl Group Overview
The carbonyl group, characterized by a carbon atom double-bonded to an oxygen atom (C=O), is a fundamental structural feature in organic chemistry and plays a crucial role in pharmaceutical compounds. This functional group is ubiquitous in nature and synthetic molecules, contributing significantly to the chemical and biological properties of various substances.
In the context of pharmaceuticals, carbonyl groups are essential for drug efficacy and functionality. They participate in hydrogen bonding, which is vital for drug-target interactions and influences drug solubility and bioavailability. The presence of carbonyl groups can affect a drug's ability to cross biological membranes, impacting its distribution within the body.
Carbonyl compounds in pharmaceuticals encompass a wide range of functional groups, including aldehydes, ketones, carboxylic acids, esters, amides, and their derivatives. Each of these subgroups exhibits unique chemical properties and reactivity, allowing for diverse applications in drug design and synthesis. For instance, aldehydes and ketones are often used as reactive intermediates in drug synthesis, while carboxylic acids and their derivatives are common in many drug molecules due to their ability to form salts and improve water solubility.
The versatility of carbonyl groups extends to their role in prodrug design, where they can be strategically incorporated to enhance drug delivery or activation. In some cases, the carbonyl group serves as a site for enzymatic cleavage, releasing the active drug compound at the target site. This approach can improve drug efficacy and reduce side effects by enabling more targeted delivery.
Furthermore, carbonyl groups are integral to many biologically active natural products that serve as templates for drug development. These include steroids, prostaglandins, and various alkaloids, where the carbonyl functionality often contributes to the molecule's biological activity. Understanding the behavior and reactivity of carbonyl groups in these natural compounds has led to the development of numerous synthetic analogs with improved pharmacological properties.
In recent years, the exploration of carbonyl chemistry has expanded into new territories within pharmaceutical research. Advanced synthetic methodologies, such as organocatalysis and transition metal-catalyzed reactions, have provided novel ways to manipulate carbonyl groups, enabling the creation of complex drug molecules with precise stereochemistry and functionality. These advancements have opened up new possibilities for drug discovery and optimization, particularly in the development of small molecule therapeutics.
In the context of pharmaceuticals, carbonyl groups are essential for drug efficacy and functionality. They participate in hydrogen bonding, which is vital for drug-target interactions and influences drug solubility and bioavailability. The presence of carbonyl groups can affect a drug's ability to cross biological membranes, impacting its distribution within the body.
Carbonyl compounds in pharmaceuticals encompass a wide range of functional groups, including aldehydes, ketones, carboxylic acids, esters, amides, and their derivatives. Each of these subgroups exhibits unique chemical properties and reactivity, allowing for diverse applications in drug design and synthesis. For instance, aldehydes and ketones are often used as reactive intermediates in drug synthesis, while carboxylic acids and their derivatives are common in many drug molecules due to their ability to form salts and improve water solubility.
The versatility of carbonyl groups extends to their role in prodrug design, where they can be strategically incorporated to enhance drug delivery or activation. In some cases, the carbonyl group serves as a site for enzymatic cleavage, releasing the active drug compound at the target site. This approach can improve drug efficacy and reduce side effects by enabling more targeted delivery.
Furthermore, carbonyl groups are integral to many biologically active natural products that serve as templates for drug development. These include steroids, prostaglandins, and various alkaloids, where the carbonyl functionality often contributes to the molecule's biological activity. Understanding the behavior and reactivity of carbonyl groups in these natural compounds has led to the development of numerous synthetic analogs with improved pharmacological properties.
In recent years, the exploration of carbonyl chemistry has expanded into new territories within pharmaceutical research. Advanced synthetic methodologies, such as organocatalysis and transition metal-catalyzed reactions, have provided novel ways to manipulate carbonyl groups, enabling the creation of complex drug molecules with precise stereochemistry and functionality. These advancements have opened up new possibilities for drug discovery and optimization, particularly in the development of small molecule therapeutics.
Pharmaceutical Market Trends
The pharmaceutical market has been experiencing significant growth and transformation in recent years, driven by various factors including technological advancements, changing demographics, and evolving healthcare needs. The global pharmaceutical market size was valued at $1.27 trillion in 2020 and is projected to reach $1.70 trillion by 2025, growing at a CAGR of 6.0% during the forecast period.
One of the key trends shaping the pharmaceutical industry is the increasing focus on personalized medicine and targeted therapies. This shift towards precision medicine is driven by advancements in genomics, proteomics, and other "-omics" technologies, enabling the development of drugs tailored to specific patient populations. As a result, there is a growing demand for biomarkers and companion diagnostics to identify patients who are most likely to benefit from particular treatments.
Another significant trend is the rise of biologics and biosimilars. Biologic drugs, including monoclonal antibodies, recombinant proteins, and cell and gene therapies, are becoming increasingly important in treating complex diseases such as cancer and autoimmune disorders. The global biologics market is expected to grow at a CAGR of 9.5% from 2021 to 2026, reaching $567.9 billion by the end of the forecast period.
The pharmaceutical industry is also witnessing a shift towards digital health solutions and telemedicine. The COVID-19 pandemic has accelerated the adoption of digital technologies in healthcare, leading to increased investment in areas such as remote patient monitoring, digital therapeutics, and artificial intelligence-driven drug discovery platforms. This trend is expected to continue, with the global digital health market projected to reach $509.2 billion by 2025, growing at a CAGR of 27.7% from 2020 to 2025.
In terms of therapeutic areas, oncology remains the largest and fastest-growing segment in the pharmaceutical market. The global oncology drugs market is expected to reach $394.2 billion by 2027, growing at a CAGR of 11.6% from 2020 to 2027. Other key therapeutic areas experiencing significant growth include neurology, immunology, and rare diseases.
Geographically, emerging markets are playing an increasingly important role in the pharmaceutical industry's growth. Countries such as China, India, and Brazil are experiencing rapid economic development, expanding healthcare access, and growing middle-class populations, driving demand for pharmaceutical products. The Asia-Pacific region is expected to be the fastest-growing pharmaceutical market, with a projected CAGR of 8.4% from 2020 to 2025.
One of the key trends shaping the pharmaceutical industry is the increasing focus on personalized medicine and targeted therapies. This shift towards precision medicine is driven by advancements in genomics, proteomics, and other "-omics" technologies, enabling the development of drugs tailored to specific patient populations. As a result, there is a growing demand for biomarkers and companion diagnostics to identify patients who are most likely to benefit from particular treatments.
Another significant trend is the rise of biologics and biosimilars. Biologic drugs, including monoclonal antibodies, recombinant proteins, and cell and gene therapies, are becoming increasingly important in treating complex diseases such as cancer and autoimmune disorders. The global biologics market is expected to grow at a CAGR of 9.5% from 2021 to 2026, reaching $567.9 billion by the end of the forecast period.
The pharmaceutical industry is also witnessing a shift towards digital health solutions and telemedicine. The COVID-19 pandemic has accelerated the adoption of digital technologies in healthcare, leading to increased investment in areas such as remote patient monitoring, digital therapeutics, and artificial intelligence-driven drug discovery platforms. This trend is expected to continue, with the global digital health market projected to reach $509.2 billion by 2025, growing at a CAGR of 27.7% from 2020 to 2025.
In terms of therapeutic areas, oncology remains the largest and fastest-growing segment in the pharmaceutical market. The global oncology drugs market is expected to reach $394.2 billion by 2027, growing at a CAGR of 11.6% from 2020 to 2027. Other key therapeutic areas experiencing significant growth include neurology, immunology, and rare diseases.
Geographically, emerging markets are playing an increasingly important role in the pharmaceutical industry's growth. Countries such as China, India, and Brazil are experiencing rapid economic development, expanding healthcare access, and growing middle-class populations, driving demand for pharmaceutical products. The Asia-Pacific region is expected to be the fastest-growing pharmaceutical market, with a projected CAGR of 8.4% from 2020 to 2025.
Carbonyl Challenges
The carbonyl group, a fundamental functional group in organic chemistry, presents several challenges in pharmaceutical applications. One of the primary difficulties lies in its reactivity, which can lead to unwanted side reactions during drug synthesis or storage. This reactivity stems from the polarized nature of the carbon-oxygen double bond, making it susceptible to nucleophilic attack and reduction reactions.
Another significant challenge is the potential for carbonyl-containing drugs to form unwanted adducts with proteins or other biomolecules in the body. This can result in altered drug efficacy, increased toxicity, or unexpected immune responses. The formation of these adducts, known as haptenization, can be particularly problematic in the development of certain classes of drugs, such as antibiotics and anti-inflammatory agents.
The stability of carbonyl-containing compounds is also a concern in pharmaceutical formulations. Many carbonyl groups are prone to oxidation or hydrolysis, which can lead to degradation of the active pharmaceutical ingredient (API) over time. This instability can significantly impact the shelf life of medications and necessitate special storage conditions or packaging requirements.
In drug design, the presence of carbonyl groups can affect the solubility and bioavailability of compounds. While carbonyl groups can enhance water solubility in some cases, they may also contribute to poor membrane permeability, limiting the drug's ability to reach its intended target. Balancing these properties is crucial for developing orally bioavailable drugs.
The carbonyl group's impact on a drug's metabolism and pharmacokinetics presents another set of challenges. Carbonyl-containing drugs may undergo rapid metabolism in the liver, leading to short half-lives and reduced efficacy. Additionally, some carbonyl metabolites can be toxic or contribute to adverse drug reactions, necessitating careful consideration during drug development.
Synthetic challenges also arise when incorporating carbonyl groups into complex drug molecules. Controlling the stereochemistry and regioselectivity of carbonyl-forming reactions can be difficult, especially in the presence of other functional groups. This complexity often requires the development of novel synthetic methodologies or the use of protecting group strategies, which can increase the cost and complexity of drug manufacturing processes.
Another significant challenge is the potential for carbonyl-containing drugs to form unwanted adducts with proteins or other biomolecules in the body. This can result in altered drug efficacy, increased toxicity, or unexpected immune responses. The formation of these adducts, known as haptenization, can be particularly problematic in the development of certain classes of drugs, such as antibiotics and anti-inflammatory agents.
The stability of carbonyl-containing compounds is also a concern in pharmaceutical formulations. Many carbonyl groups are prone to oxidation or hydrolysis, which can lead to degradation of the active pharmaceutical ingredient (API) over time. This instability can significantly impact the shelf life of medications and necessitate special storage conditions or packaging requirements.
In drug design, the presence of carbonyl groups can affect the solubility and bioavailability of compounds. While carbonyl groups can enhance water solubility in some cases, they may also contribute to poor membrane permeability, limiting the drug's ability to reach its intended target. Balancing these properties is crucial for developing orally bioavailable drugs.
The carbonyl group's impact on a drug's metabolism and pharmacokinetics presents another set of challenges. Carbonyl-containing drugs may undergo rapid metabolism in the liver, leading to short half-lives and reduced efficacy. Additionally, some carbonyl metabolites can be toxic or contribute to adverse drug reactions, necessitating careful consideration during drug development.
Synthetic challenges also arise when incorporating carbonyl groups into complex drug molecules. Controlling the stereochemistry and regioselectivity of carbonyl-forming reactions can be difficult, especially in the presence of other functional groups. This complexity often requires the development of novel synthetic methodologies or the use of protecting group strategies, which can increase the cost and complexity of drug manufacturing processes.
Current Carbonyl Solutions
01 Synthesis of carbonyl compounds
Various methods for synthesizing carbonyl compounds are described, including oxidation reactions, rearrangements, and addition reactions. These processes are essential in organic chemistry for producing aldehydes, ketones, and carboxylic acids, which are important intermediates in many industrial applications.- Synthesis of carbonyl compounds: Various methods for synthesizing carbonyl compounds are described, including oxidation reactions, rearrangements, and catalytic processes. These techniques are crucial in organic chemistry for producing aldehydes and ketones, which are important intermediates in many industrial and pharmaceutical applications.
- Carbonyl group protection and modification: Strategies for protecting and modifying carbonyl groups are outlined, including the use of protecting groups, reduction reactions, and nucleophilic additions. These methods are essential in multi-step organic syntheses to control reactivity and achieve desired transformations of carbonyl-containing compounds.
- Carbonyl compounds in polymer chemistry: The role of carbonyl groups in polymer chemistry is explored, including their use in polymerization reactions, polymer modifications, and the development of functional materials. Carbonyl-containing monomers and polymers are utilized in various applications such as adhesives, coatings, and biomaterials.
- Carbonyl compounds in pharmaceutical applications: The importance of carbonyl-containing compounds in pharmaceutical research and development is discussed. This includes the synthesis of drug precursors, the design of bioactive molecules, and the use of carbonyl chemistry in drug delivery systems and prodrug approaches.
- Analysis and characterization of carbonyl groups: Techniques for analyzing and characterizing carbonyl groups in various compounds are presented. These methods include spectroscopic techniques such as infrared spectroscopy and NMR, as well as chemical derivatization approaches for identifying and quantifying carbonyl functionalities in complex mixtures.
02 Carbonyl group protection and modification
Techniques for protecting and modifying carbonyl groups are discussed, including the use of protecting groups, reduction reactions, and nucleophilic addition. These methods are crucial in multi-step organic syntheses to control reactivity and achieve desired transformations.Expand Specific Solutions03 Carbonyl compounds in polymer chemistry
The role of carbonyl groups in polymer chemistry is explored, including their use in polymerization reactions, polymer modifications, and the development of functional materials. Carbonyl-containing monomers and polymers find applications in various industries, such as adhesives, coatings, and biomaterials.Expand Specific Solutions04 Analytical methods for carbonyl compounds
Various analytical techniques for detecting, quantifying, and characterizing carbonyl compounds are presented. These methods include spectroscopic techniques, chromatography, and derivatization approaches, which are essential for quality control, environmental monitoring, and research purposes.Expand Specific Solutions05 Carbonyl compounds in pharmaceutical applications
The importance of carbonyl compounds in pharmaceutical research and development is discussed, including their role as active pharmaceutical ingredients, intermediates, and building blocks for drug synthesis. Methods for optimizing carbonyl-containing drug candidates and improving their properties are also explored.Expand Specific Solutions
Key Pharma Players
The exploration of carbonyl group applications in pharmaceuticals is currently in a mature stage of development, with a significant market size and established technological foundations. Key players in this field include major pharmaceutical companies like Novartis AG, GlaxoSmithKline, and Pfizer Inc., as well as research-focused organizations such as FibroGen, Inc. and Zhejiang University. The competitive landscape is characterized by ongoing innovation and research collaborations between industry and academia. Companies are investing heavily in R&D to develop novel drug candidates and improve existing formulations, leveraging the versatile chemistry of carbonyl groups to enhance drug efficacy and bioavailability.
Novartis AG
Technical Solution: Novartis AG has been at the forefront of exploring carbonyl group applications in pharmaceuticals. They have developed a novel approach using carbonyl-containing compounds as key intermediates in the synthesis of potential drug candidates. Their research focuses on the reactivity of carbonyl groups to create diverse molecular scaffolds. Novartis has successfully applied this strategy in the development of several drug candidates, including a promising anti-inflammatory agent that utilizes a carbonyl-based warhead to target specific enzymes[1]. Additionally, they have explored the use of carbonyl groups in prodrug design, enhancing the bioavailability and efficacy of existing drugs[3].
Strengths: Strong research capabilities, diverse drug pipeline, and expertise in medicinal chemistry. Weaknesses: High R&D costs and potential regulatory challenges in bringing novel compounds to market.
Glaxo Group Ltd.
Technical Solution: Glaxo Group Ltd. has made significant strides in leveraging carbonyl group chemistry for pharmaceutical applications. Their approach involves utilizing carbonyl groups as reactive centers for creating complex drug molecules. They have developed a platform technology that exploits the versatility of carbonyl chemistry in drug design, particularly in the field of respiratory medicines. Glaxo's researchers have successfully employed carbonyl-based reactions to synthesize novel bronchodilators with improved efficacy and duration of action[2]. Furthermore, they have explored the use of carbonyl groups in creating targeted drug delivery systems, enhancing the specificity of their compounds[4].
Strengths: Extensive experience in respiratory medicine, strong patent portfolio, and global research network. Weaknesses: Potential for patent expirations and increasing competition in key therapeutic areas.
Carbonyl Innovations
Compound
PatentInactiveUS20100120789A1
Innovation
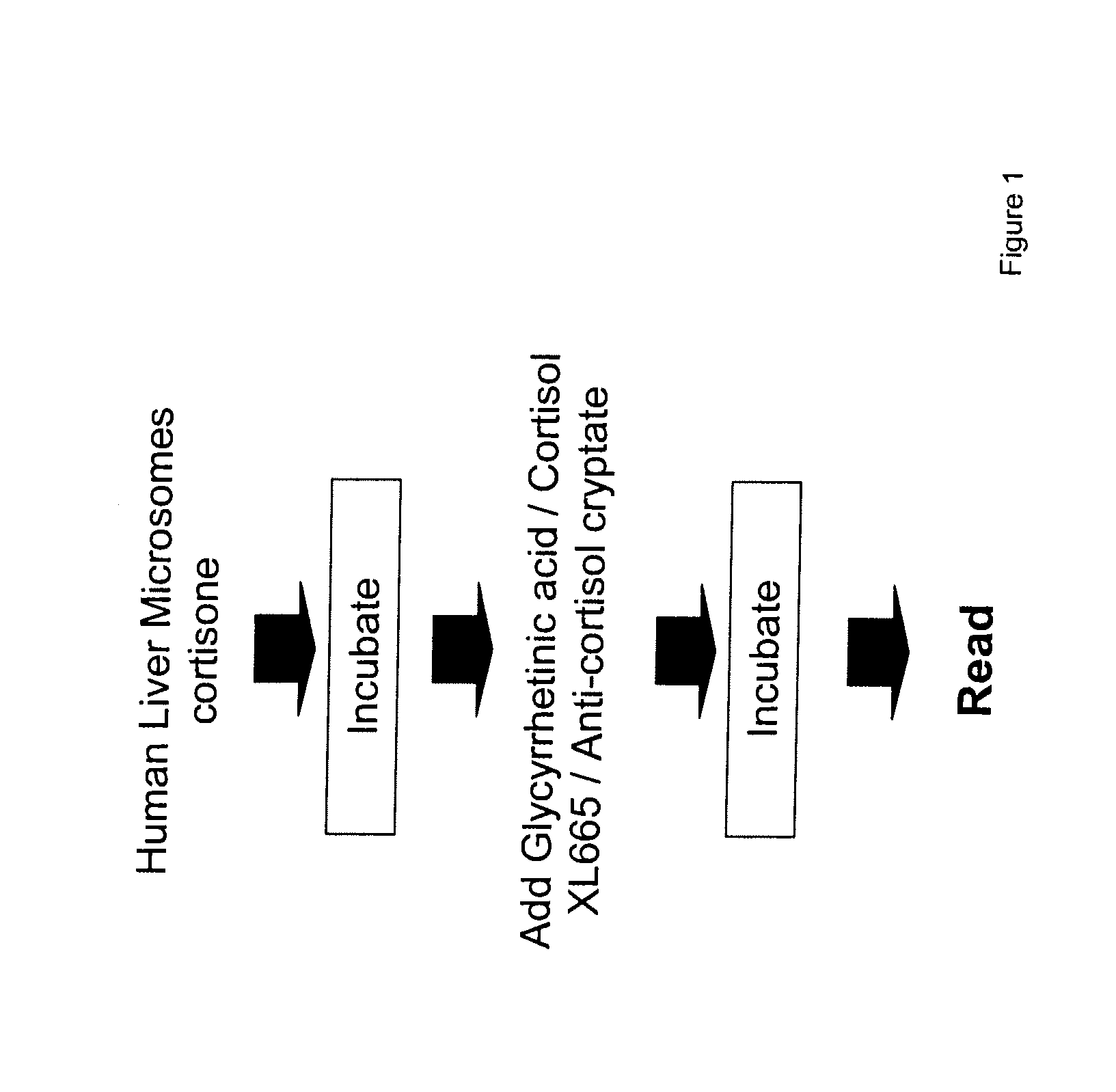
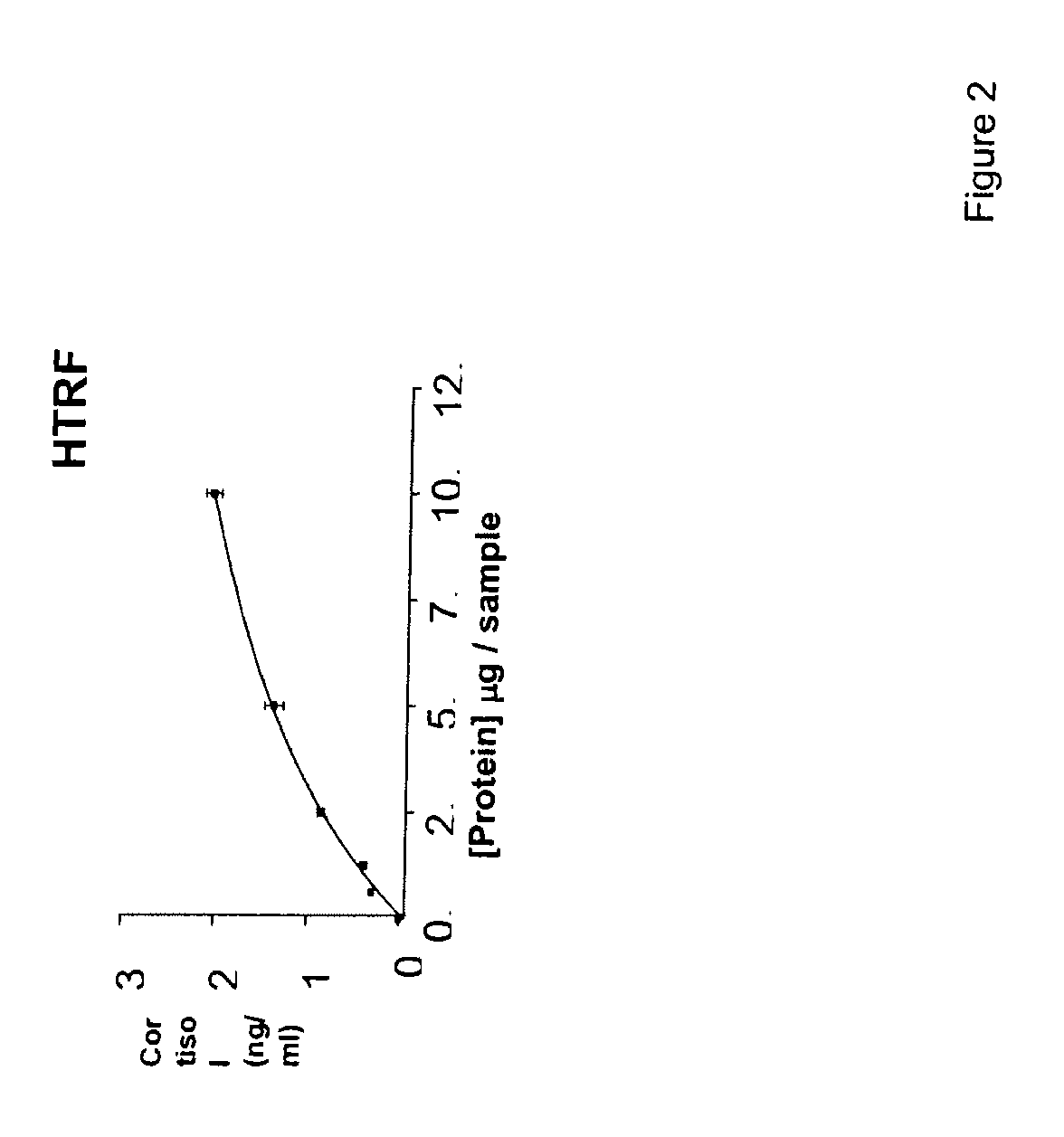
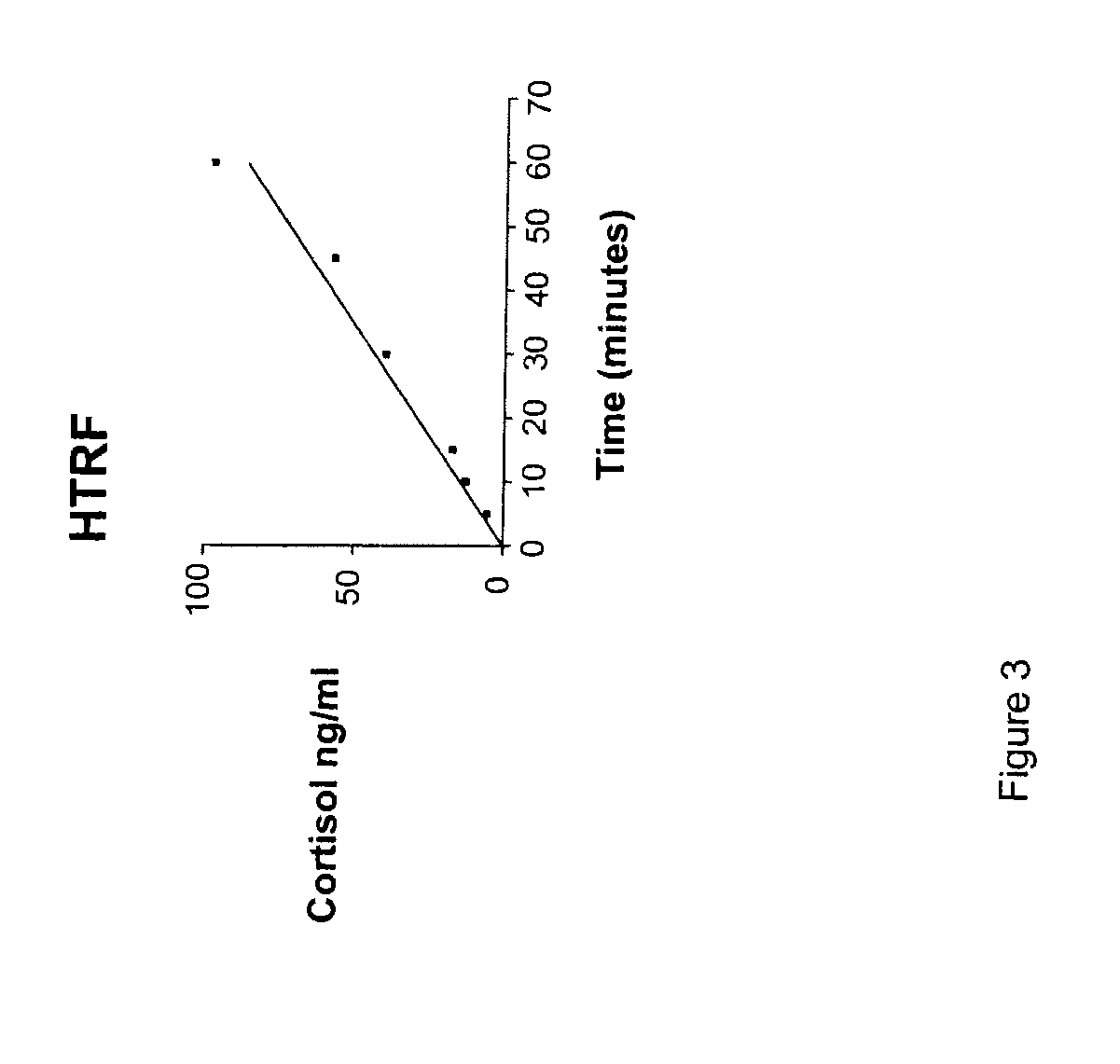
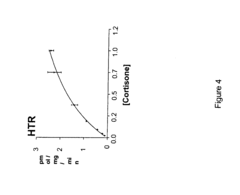
- Development of compounds with Formula I, specifically designed to inhibit 11.beta.-HSD, which can modulate the interconversion between cortisone and cortisol, offering therapeutic benefits for regulating carbohydrate, protein, and lipid metabolism, as well as influencing cognitive function and immune responses.
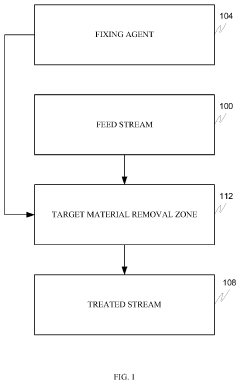
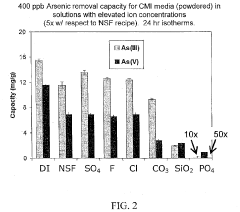
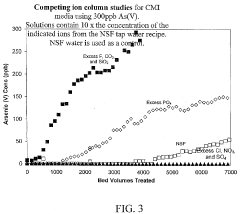
Remediation of physiologically active compounds from waste water
PatentInactiveUS20200140290A1
Innovation
- A process involving rare earth metals as soluble or insoluble fixing agents to form insoluble target material-containing agents, which precipitate out and can be easily removed from the water stream, thereby stabilizing and eliminating these materials.
Regulatory Compliance
Regulatory compliance is a critical aspect of pharmaceutical development, particularly when exploring carbonyl group applications. The carbonyl functional group, characterized by its C=O double bond, is prevalent in many drug molecules and plays a crucial role in their efficacy and safety profiles. As such, regulatory bodies worldwide have established stringent guidelines to ensure the proper use and control of carbonyl-containing compounds in pharmaceuticals.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of carbonyl-containing drugs through various guidance documents and regulatory frameworks. The FDA's Good Manufacturing Practice (GMP) guidelines specifically address the synthesis, purification, and quality control of carbonyl-containing compounds. These regulations emphasize the importance of process validation, impurity profiling, and stability testing to ensure the safety and efficacy of carbonyl-based pharmaceuticals.
The European Medicines Agency (EMA) has also implemented comprehensive regulations for carbonyl-containing drugs. The EMA's guidelines on impurities in drug substances and products specifically address carbonyl-related impurities, such as aldehydes and ketones, which may form during synthesis or storage. Manufacturers must demonstrate thorough control and characterization of these impurities to obtain regulatory approval.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has established similar regulatory requirements for carbonyl-containing drugs. The PMDA's guidelines on impurity profiling and stability testing align with international standards, ensuring consistency in the global pharmaceutical market.
Regulatory compliance for carbonyl group applications extends beyond the final drug product. Environmental regulations, such as those set by the Environmental Protection Agency (EPA) in the United States, govern the handling and disposal of carbonyl-containing compounds used in pharmaceutical manufacturing. These regulations aim to minimize environmental impact and protect public health.
As the pharmaceutical industry continues to explore novel applications of carbonyl groups, regulatory bodies are adapting their guidelines to address emerging challenges. For instance, the development of carbonyl-based prodrugs and targeted drug delivery systems has prompted regulatory agencies to update their guidance on bioequivalence studies and pharmacokinetic assessments.
Compliance with these regulations requires pharmaceutical companies to implement robust quality management systems and maintain detailed documentation throughout the drug development process. This includes comprehensive analytical methods for detecting and quantifying carbonyl-containing compounds, as well as validated stability-indicating assays to monitor potential degradation products.
In conclusion, regulatory compliance in the exploration of carbonyl group applications in pharmaceuticals is a complex and evolving field. As research continues to uncover new potential uses for carbonyl-containing compounds, regulatory bodies will likely refine and expand their guidelines to ensure the safety and efficacy of these innovative pharmaceutical products.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of carbonyl-containing drugs through various guidance documents and regulatory frameworks. The FDA's Good Manufacturing Practice (GMP) guidelines specifically address the synthesis, purification, and quality control of carbonyl-containing compounds. These regulations emphasize the importance of process validation, impurity profiling, and stability testing to ensure the safety and efficacy of carbonyl-based pharmaceuticals.
The European Medicines Agency (EMA) has also implemented comprehensive regulations for carbonyl-containing drugs. The EMA's guidelines on impurities in drug substances and products specifically address carbonyl-related impurities, such as aldehydes and ketones, which may form during synthesis or storage. Manufacturers must demonstrate thorough control and characterization of these impurities to obtain regulatory approval.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has established similar regulatory requirements for carbonyl-containing drugs. The PMDA's guidelines on impurity profiling and stability testing align with international standards, ensuring consistency in the global pharmaceutical market.
Regulatory compliance for carbonyl group applications extends beyond the final drug product. Environmental regulations, such as those set by the Environmental Protection Agency (EPA) in the United States, govern the handling and disposal of carbonyl-containing compounds used in pharmaceutical manufacturing. These regulations aim to minimize environmental impact and protect public health.
As the pharmaceutical industry continues to explore novel applications of carbonyl groups, regulatory bodies are adapting their guidelines to address emerging challenges. For instance, the development of carbonyl-based prodrugs and targeted drug delivery systems has prompted regulatory agencies to update their guidance on bioequivalence studies and pharmacokinetic assessments.
Compliance with these regulations requires pharmaceutical companies to implement robust quality management systems and maintain detailed documentation throughout the drug development process. This includes comprehensive analytical methods for detecting and quantifying carbonyl-containing compounds, as well as validated stability-indicating assays to monitor potential degradation products.
In conclusion, regulatory compliance in the exploration of carbonyl group applications in pharmaceuticals is a complex and evolving field. As research continues to uncover new potential uses for carbonyl-containing compounds, regulatory bodies will likely refine and expand their guidelines to ensure the safety and efficacy of these innovative pharmaceutical products.
Carbonyl Safety Profiles
The safety profiles of carbonyl groups in pharmaceuticals are of paramount importance in drug development and regulatory approval processes. These functional groups, while essential for many therapeutic compounds, can pose potential risks that must be carefully evaluated and mitigated.
Carbonyl-containing compounds often exhibit reactivity that can lead to various safety concerns. One primary issue is their potential to form covalent bonds with proteins and other biomolecules, which may result in allergic reactions or toxicity. This reactivity is particularly pronounced in aldehydes and ketones, which can form Schiff bases with amino groups in proteins, potentially altering their structure and function.
Another significant safety consideration is the metabolic fate of carbonyl-containing drugs. Many of these compounds undergo oxidation or reduction reactions in the body, potentially forming reactive intermediates or toxic metabolites. For instance, acetaminophen, a common analgesic, can be metabolized to form N-acetyl-p-benzoquinone imine (NAPQI), a highly reactive compound that can cause hepatotoxicity if not properly detoxified.
The stability of carbonyl groups under various physiological conditions is also a critical factor in their safety profile. Some carbonyl-containing drugs may be susceptible to hydrolysis or other degradation pathways, which can affect their shelf life, bioavailability, and potential for generating harmful byproducts. This instability can lead to challenges in formulation and storage, requiring careful consideration of packaging and storage conditions.
Regulatory agencies, such as the FDA and EMA, have established guidelines for assessing the safety of carbonyl-containing compounds in pharmaceuticals. These guidelines often require extensive toxicological studies, including genotoxicity assessments, carcinogenicity evaluations, and reproductive toxicity studies. The results of these studies are crucial in determining the overall safety profile of a drug candidate and its suitability for human use.
To address safety concerns, pharmaceutical companies employ various strategies. These may include structural modifications to reduce reactivity, the use of pro-drug approaches to mask reactive carbonyl groups until the drug reaches its target, or the development of formulations that enhance stability and reduce the formation of harmful byproducts. Additionally, advanced analytical techniques are employed to detect and characterize potential impurities and degradation products that may arise from carbonyl-containing compounds during manufacturing and storage.
In conclusion, while carbonyl groups are essential in many pharmaceuticals, their safety profiles require careful consideration throughout the drug development process. Balancing the therapeutic benefits with potential risks is crucial, and ongoing research continues to refine our understanding and management of carbonyl-related safety issues in drug design and development.
Carbonyl-containing compounds often exhibit reactivity that can lead to various safety concerns. One primary issue is their potential to form covalent bonds with proteins and other biomolecules, which may result in allergic reactions or toxicity. This reactivity is particularly pronounced in aldehydes and ketones, which can form Schiff bases with amino groups in proteins, potentially altering their structure and function.
Another significant safety consideration is the metabolic fate of carbonyl-containing drugs. Many of these compounds undergo oxidation or reduction reactions in the body, potentially forming reactive intermediates or toxic metabolites. For instance, acetaminophen, a common analgesic, can be metabolized to form N-acetyl-p-benzoquinone imine (NAPQI), a highly reactive compound that can cause hepatotoxicity if not properly detoxified.
The stability of carbonyl groups under various physiological conditions is also a critical factor in their safety profile. Some carbonyl-containing drugs may be susceptible to hydrolysis or other degradation pathways, which can affect their shelf life, bioavailability, and potential for generating harmful byproducts. This instability can lead to challenges in formulation and storage, requiring careful consideration of packaging and storage conditions.
Regulatory agencies, such as the FDA and EMA, have established guidelines for assessing the safety of carbonyl-containing compounds in pharmaceuticals. These guidelines often require extensive toxicological studies, including genotoxicity assessments, carcinogenicity evaluations, and reproductive toxicity studies. The results of these studies are crucial in determining the overall safety profile of a drug candidate and its suitability for human use.
To address safety concerns, pharmaceutical companies employ various strategies. These may include structural modifications to reduce reactivity, the use of pro-drug approaches to mask reactive carbonyl groups until the drug reaches its target, or the development of formulations that enhance stability and reduce the formation of harmful byproducts. Additionally, advanced analytical techniques are employed to detect and characterize potential impurities and degradation products that may arise from carbonyl-containing compounds during manufacturing and storage.
In conclusion, while carbonyl groups are essential in many pharmaceuticals, their safety profiles require careful consideration throughout the drug development process. Balancing the therapeutic benefits with potential risks is crucial, and ongoing research continues to refine our understanding and management of carbonyl-related safety issues in drug design and development.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!