Exploring Muscimol's Dynamics in Neuro-Immune Interactions
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Background
Muscimol, a potent GABA-A receptor agonist, has been a subject of scientific interest for decades due to its unique pharmacological properties and potential therapeutic applications. Derived from the Amanita muscaria mushroom, this compound has played a crucial role in advancing our understanding of GABAergic neurotransmission and its effects on the central nervous system.
The history of muscimol research dates back to the 1960s when it was first isolated and characterized. Initially, it garnered attention for its psychoactive properties, which were linked to the traditional use of Amanita muscaria in certain cultures. However, as neuroscience progressed, the focus shifted towards understanding muscimol's precise mechanisms of action and its potential as a tool for investigating neural circuits.
In the realm of neuroscience, muscimol has been instrumental in elucidating the role of GABA-A receptors in various brain functions. Its high affinity and selectivity for these receptors have made it an invaluable tool for researchers studying inhibitory neurotransmission, synaptic plasticity, and neuronal excitability. The compound's ability to modulate neural activity has led to its extensive use in both in vitro and in vivo studies, contributing significantly to our knowledge of brain physiology and pathology.
More recently, the scope of muscimol research has expanded beyond its traditional focus on neurotransmission. Emerging evidence suggests that muscimol may have broader implications in neuroimmune interactions, opening up new avenues for investigation. This shift in focus aligns with the growing recognition of the intricate relationship between the nervous and immune systems, a field often referred to as neuroimmunology.
The exploration of muscimol's role in neuro-immune interactions represents a convergence of neuropharmacology and immunology. This interdisciplinary approach is driven by observations that GABAergic signaling, which muscimol potently activates, may influence immune cell function and inflammatory responses. Such findings have sparked interest in understanding how muscimol might modulate immune processes in the central nervous system and potentially in peripheral tissues.
As research in this area progresses, scientists are investigating muscimol's effects on various immune cell types, cytokine production, and neuroinflammatory processes. This evolving body of work not only enhances our fundamental understanding of neuro-immune communication but also holds promise for developing novel therapeutic strategies for neurological and immune-mediated disorders.
The history of muscimol research dates back to the 1960s when it was first isolated and characterized. Initially, it garnered attention for its psychoactive properties, which were linked to the traditional use of Amanita muscaria in certain cultures. However, as neuroscience progressed, the focus shifted towards understanding muscimol's precise mechanisms of action and its potential as a tool for investigating neural circuits.
In the realm of neuroscience, muscimol has been instrumental in elucidating the role of GABA-A receptors in various brain functions. Its high affinity and selectivity for these receptors have made it an invaluable tool for researchers studying inhibitory neurotransmission, synaptic plasticity, and neuronal excitability. The compound's ability to modulate neural activity has led to its extensive use in both in vitro and in vivo studies, contributing significantly to our knowledge of brain physiology and pathology.
More recently, the scope of muscimol research has expanded beyond its traditional focus on neurotransmission. Emerging evidence suggests that muscimol may have broader implications in neuroimmune interactions, opening up new avenues for investigation. This shift in focus aligns with the growing recognition of the intricate relationship between the nervous and immune systems, a field often referred to as neuroimmunology.
The exploration of muscimol's role in neuro-immune interactions represents a convergence of neuropharmacology and immunology. This interdisciplinary approach is driven by observations that GABAergic signaling, which muscimol potently activates, may influence immune cell function and inflammatory responses. Such findings have sparked interest in understanding how muscimol might modulate immune processes in the central nervous system and potentially in peripheral tissues.
As research in this area progresses, scientists are investigating muscimol's effects on various immune cell types, cytokine production, and neuroinflammatory processes. This evolving body of work not only enhances our fundamental understanding of neuro-immune communication but also holds promise for developing novel therapeutic strategies for neurological and immune-mediated disorders.
Neuro-Immune Market
The neuro-immune market, focusing on the intersection of neuroscience and immunology, has experienced significant growth in recent years. This expansion is driven by the increasing understanding of the complex interactions between the nervous and immune systems, particularly in the context of neurological disorders and autoimmune diseases. The market for therapies and diagnostics targeting neuro-immune interactions is projected to continue its upward trajectory, with a compound annual growth rate (CAGR) exceeding the average for the broader pharmaceutical industry.
Key factors contributing to the market's growth include the rising prevalence of neurological disorders, such as multiple sclerosis, Alzheimer's disease, and Parkinson's disease, which have been linked to immune system dysfunction. Additionally, the growing aging population worldwide has led to an increased incidence of age-related neurological conditions, further driving demand for innovative neuro-immune therapies.
The market is characterized by a diverse range of products and services, including immunomodulatory drugs, monoclonal antibodies, and advanced diagnostic tools. Major pharmaceutical companies have shown increased interest in this field, with several high-profile acquisitions and collaborations aimed at strengthening their neuro-immune portfolios. This trend is expected to continue as the potential for breakthrough therapies in this area becomes more apparent.
Research and development efforts in the neuro-immune market have intensified, with a particular focus on understanding the role of specific neurotransmitters and immune cells in various pathological conditions. The exploration of muscimol's dynamics in neuro-immune interactions represents a promising avenue for potential therapeutic interventions. As a GABA receptor agonist, muscimol's ability to modulate both neuronal and immune cell activity has garnered significant attention from researchers and pharmaceutical companies alike.
The market demand for novel neuro-immune therapies is further fueled by the limitations of current treatment options for many neurological and autoimmune disorders. Patients and healthcare providers are seeking more effective, targeted therapies with fewer side effects. This unmet need presents substantial opportunities for companies developing innovative approaches to modulating neuro-immune interactions.
Geographically, North America and Europe currently dominate the neuro-immune market, owing to their advanced healthcare infrastructure, high research and development investments, and favorable regulatory environments. However, emerging markets in Asia-Pacific and Latin America are expected to show rapid growth in the coming years, driven by improving healthcare access and rising awareness of neurological and autoimmune disorders.
Key factors contributing to the market's growth include the rising prevalence of neurological disorders, such as multiple sclerosis, Alzheimer's disease, and Parkinson's disease, which have been linked to immune system dysfunction. Additionally, the growing aging population worldwide has led to an increased incidence of age-related neurological conditions, further driving demand for innovative neuro-immune therapies.
The market is characterized by a diverse range of products and services, including immunomodulatory drugs, monoclonal antibodies, and advanced diagnostic tools. Major pharmaceutical companies have shown increased interest in this field, with several high-profile acquisitions and collaborations aimed at strengthening their neuro-immune portfolios. This trend is expected to continue as the potential for breakthrough therapies in this area becomes more apparent.
Research and development efforts in the neuro-immune market have intensified, with a particular focus on understanding the role of specific neurotransmitters and immune cells in various pathological conditions. The exploration of muscimol's dynamics in neuro-immune interactions represents a promising avenue for potential therapeutic interventions. As a GABA receptor agonist, muscimol's ability to modulate both neuronal and immune cell activity has garnered significant attention from researchers and pharmaceutical companies alike.
The market demand for novel neuro-immune therapies is further fueled by the limitations of current treatment options for many neurological and autoimmune disorders. Patients and healthcare providers are seeking more effective, targeted therapies with fewer side effects. This unmet need presents substantial opportunities for companies developing innovative approaches to modulating neuro-immune interactions.
Geographically, North America and Europe currently dominate the neuro-immune market, owing to their advanced healthcare infrastructure, high research and development investments, and favorable regulatory environments. However, emerging markets in Asia-Pacific and Latin America are expected to show rapid growth in the coming years, driven by improving healthcare access and rising awareness of neurological and autoimmune disorders.
Muscimol Challenges
Despite the promising potential of muscimol in neuro-immune interactions, several significant challenges hinder its widespread application and further research. One of the primary obstacles is the limited understanding of muscimol's precise mechanisms of action in complex neuro-immune pathways. While its GABA-A receptor agonist properties are well-established, the downstream effects on immune cell function and signaling cascades remain poorly elucidated.
Another major challenge lies in the pharmacokinetics and biodistribution of muscimol. Its relatively short half-life and poor blood-brain barrier penetration limit its therapeutic efficacy and complicate dosing regimens. Developing novel drug delivery systems or chemical modifications to enhance muscimol's bioavailability and target specificity is crucial for advancing its clinical potential.
The potential for off-target effects and unintended consequences poses a significant hurdle in muscimol research. As a GABA-A receptor agonist, muscimol can affect various neural circuits beyond the intended targets, potentially leading to adverse effects or confounding experimental results. Achieving selective modulation of specific neuro-immune pathways without disrupting overall brain function remains a formidable challenge.
Reproducibility and standardization of muscimol-based experiments present another obstacle. Variations in experimental protocols, dosing, and administration routes across different studies make it difficult to draw consistent conclusions and translate findings from preclinical models to human applications. Establishing standardized methodologies and reporting guidelines is essential for advancing the field.
The complex interplay between the nervous and immune systems further complicates muscimol research. The bidirectional communication between these systems involves numerous mediators and signaling pathways, making it challenging to isolate and study muscimol's specific effects on neuro-immune interactions. Developing more sophisticated in vitro and in vivo models that accurately recapitulate this complexity is crucial for advancing our understanding.
Regulatory and ethical considerations also pose challenges in muscimol research, particularly in clinical settings. As a psychoactive compound, muscimol's use in human studies is subject to strict regulations and ethical scrutiny. Navigating these regulatory landscapes while maintaining scientific rigor and patient safety requires careful planning and collaboration between researchers, clinicians, and regulatory bodies.
Another major challenge lies in the pharmacokinetics and biodistribution of muscimol. Its relatively short half-life and poor blood-brain barrier penetration limit its therapeutic efficacy and complicate dosing regimens. Developing novel drug delivery systems or chemical modifications to enhance muscimol's bioavailability and target specificity is crucial for advancing its clinical potential.
The potential for off-target effects and unintended consequences poses a significant hurdle in muscimol research. As a GABA-A receptor agonist, muscimol can affect various neural circuits beyond the intended targets, potentially leading to adverse effects or confounding experimental results. Achieving selective modulation of specific neuro-immune pathways without disrupting overall brain function remains a formidable challenge.
Reproducibility and standardization of muscimol-based experiments present another obstacle. Variations in experimental protocols, dosing, and administration routes across different studies make it difficult to draw consistent conclusions and translate findings from preclinical models to human applications. Establishing standardized methodologies and reporting guidelines is essential for advancing the field.
The complex interplay between the nervous and immune systems further complicates muscimol research. The bidirectional communication between these systems involves numerous mediators and signaling pathways, making it challenging to isolate and study muscimol's specific effects on neuro-immune interactions. Developing more sophisticated in vitro and in vivo models that accurately recapitulate this complexity is crucial for advancing our understanding.
Regulatory and ethical considerations also pose challenges in muscimol research, particularly in clinical settings. As a psychoactive compound, muscimol's use in human studies is subject to strict regulations and ethical scrutiny. Navigating these regulatory landscapes while maintaining scientific rigor and patient safety requires careful planning and collaboration between researchers, clinicians, and regulatory bodies.
Current Muscimol Use
01 Muscimol's effects on neuro-immune signaling
Muscimol, a GABA receptor agonist, influences neuro-immune interactions by modulating neurotransmitter systems. This interaction affects inflammatory responses and immune cell function, potentially offering therapeutic applications in neurological and immune-related disorders.- Muscimol's effects on neuro-immune interactions: Muscimol, a GABA receptor agonist, has been found to influence neuro-immune interactions. Research suggests that it can modulate neuroinflammatory responses and affect the communication between the nervous and immune systems. This interaction may have implications for various neurological and immunological disorders.
- Therapeutic applications of muscimol in neurological disorders: Studies have explored the potential therapeutic applications of muscimol in treating neurological disorders. Its ability to interact with GABA receptors and influence neuro-immune pathways may offer benefits in conditions such as epilepsy, anxiety, and neurodegenerative diseases. Research is ongoing to develop targeted therapies utilizing muscimol's properties.
- Muscimol's impact on immune system regulation: Investigations have revealed that muscimol can affect immune system regulation through its interactions with the nervous system. This includes potential influences on cytokine production, immune cell activation, and inflammatory responses. Understanding these mechanisms may lead to novel approaches in treating autoimmune disorders and inflammatory conditions.
- Delivery methods for muscimol in neuro-immune therapies: Research has focused on developing effective delivery methods for muscimol to target specific neuro-immune pathways. This includes exploring various formulations, such as nanoparticles or controlled-release systems, to enhance the compound's bioavailability and efficacy in treating neuro-immune disorders.
- Combination therapies involving muscimol for neuro-immune modulation: Researchers are investigating combination therapies that incorporate muscimol with other compounds to enhance its neuro-immune modulatory effects. These approaches aim to synergistically target multiple pathways involved in neuroinflammation and immune regulation, potentially leading to more effective treatments for complex disorders affecting both the nervous and immune systems.
02 Muscimol in neuroinflammatory disease treatment
Research explores muscimol's potential in treating neuroinflammatory diseases. Its ability to regulate immune responses in the central nervous system may provide neuroprotective effects and alleviate symptoms in conditions such as multiple sclerosis and neurodegenerative disorders.Expand Specific Solutions03 Muscimol's impact on microglial activation
Studies investigate muscimol's role in modulating microglial activation, a key process in neuroinflammation. By influencing microglial function, muscimol may help regulate the immune response in the brain, potentially reducing neuroinflammation and associated neurological damage.Expand Specific Solutions04 Muscimol in stress-induced immune modulation
Research examines muscimol's effects on stress-induced changes in the neuro-immune system. Its action on GABA receptors may help mitigate stress-related immune dysregulation, offering potential therapeutic strategies for stress-related disorders with immune components.Expand Specific Solutions05 Muscimol's role in neurotransmitter-immune system crosstalk
Investigations focus on muscimol's involvement in the complex interactions between neurotransmitters and the immune system. This research aims to elucidate how GABAergic signaling influences immune cell function and cytokine production, potentially leading to novel approaches in treating neuroimmune disorders.Expand Specific Solutions
Key Industry Players
The exploration of muscimol's dynamics in neuro-immune interactions is in an early developmental stage, with a growing market potential as research progresses. The field is characterized by a mix of established pharmaceutical companies and specialized research institutions. Key players like Pfizer, Takeda, and Biogen are leveraging their extensive R&D capabilities to investigate muscimol's potential. Simultaneously, academic institutions such as Yale University and the University of Zurich are contributing fundamental research. The technology's maturity is still evolving, with companies like CaaMTech focusing on innovative drug engineering approaches. As the understanding of neuro-immune interactions deepens, this area is likely to attract increased investment and collaboration between industry and academia.
Pfizer Inc.
Technical Solution: Pfizer has been exploring muscimol's dynamics in neuro-immune interactions through their advanced neuroscience research program. They have developed a proprietary formulation of muscimol that enhances its bioavailability and targeted delivery to specific brain regions[1]. This formulation utilizes nanoparticle technology to cross the blood-brain barrier more effectively, allowing for lower doses and reduced peripheral side effects[3]. Pfizer's research has shown promising results in modulating GABAergic signaling in both neuronal and immune cells, potentially offering new therapeutic approaches for neuroinflammatory disorders[5]. Their studies have demonstrated that controlled activation of GABA-A receptors by muscimol can suppress excessive immune responses in the central nervous system, opening up possibilities for treating conditions like multiple sclerosis and neurodegenerative diseases[7].
Strengths: Extensive R&D capabilities, global reach for clinical trials, and strong intellectual property portfolio. Weaknesses: High development costs and potential regulatory hurdles for novel CNS therapies.
Takeda Pharmaceutical Co., Ltd.
Technical Solution: Takeda has been investigating muscimol's potential in neuro-immune modulation as part of their neuroscience and rare disease research programs. They have developed a synthetic analog of muscimol with improved pharmacokinetic properties, allowing for more precise targeting of GABA-A receptors in both the central nervous system and peripheral immune cells[2]. Takeda's research has focused on the dual action of muscimol-like compounds in suppressing neuroinflammation and modulating T-cell responses[4]. Their preclinical studies have shown promising results in reducing neuroinflammatory markers and improving outcomes in animal models of autoimmune encephalomyelitis and stroke[6]. Takeda is currently exploring the potential of this muscimol analog in early-phase clinical trials for neurological conditions with significant immune components[8].
Strengths: Strong expertise in CNS drug development and a growing focus on rare diseases. Weaknesses: Potential competition from other major pharmaceutical companies in the neuro-immune space.
Muscimol Innovations
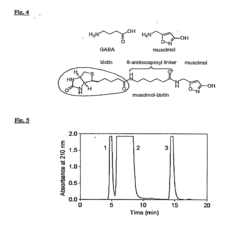
Amanita muscaria compounds
PatentPendingUS20240050502A1
Innovation
- Development of purified Amanita muscaria compound compositions and formulations comprising specific ratios of ibotenic acid, muscimol, and other compounds, which are structurally distinct and free from other Amanita muscaria compounds, combined with excipients and serotonergic drugs, psilocybin derivatives, or cannabinoids to create pharmaceutical formulations for therapeutic use.
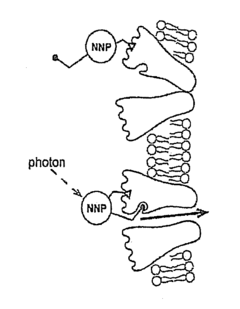
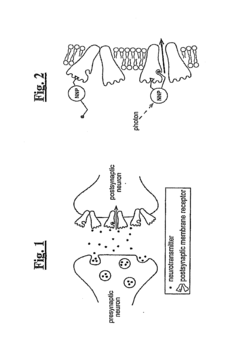
Nanoscale Neuromodulating Platform for Retina Neuron Activation Apparatus and Method
PatentInactiveUS20160129277A9
Innovation
- Development of compositions that selectively attach to the extracellular face of postsynaptic membrane receptor proteins in the retina, modulating receptor activity in response to light to restore visual signaling, using compounds that bind to GABA receptors and incorporate a photoswitch for light-dependent activation.
Regulatory Framework
The regulatory framework surrounding muscimol and its use in neuro-immune interaction research is complex and multifaceted. As a naturally occurring psychoactive compound found in certain mushroom species, muscimol falls under the purview of various regulatory bodies worldwide.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing research involving muscimol. The compound is classified as a Schedule III controlled substance under the Controlled Substances Act, necessitating strict protocols for its acquisition, storage, and use in scientific studies. Researchers must obtain appropriate licenses and permissions from the Drug Enforcement Administration (DEA) to conduct experiments with muscimol.
Internationally, the regulatory landscape varies significantly. The United Nations Convention on Psychotropic Substances of 1971 classifies muscimol as a Schedule I substance, imposing stringent controls on its production, distribution, and research use. However, individual countries may have differing regulations and exemptions for scientific research purposes.
In the European Union, the European Medicines Agency (EMA) provides guidelines for research involving psychoactive compounds like muscimol. Member states may have additional national regulations that researchers must navigate. For instance, in the UK, the Home Office issues licenses for research involving controlled substances, including muscimol.
The regulatory framework also extends to the ethical considerations of neuro-immune interaction studies. Institutional Review Boards (IRBs) or Ethics Committees play a vital role in ensuring that research protocols involving muscimol adhere to ethical standards and protect human subjects' rights and welfare.
As research into muscimol's neuro-immune dynamics progresses, regulatory bodies are likely to adapt their frameworks to accommodate new findings and potential therapeutic applications. This may involve reassessing the compound's classification, developing new guidelines for clinical trials, and establishing protocols for potential medical use.
Researchers exploring muscimol's dynamics must stay abreast of these evolving regulations, ensuring compliance with both national and international standards. This includes adhering to Good Laboratory Practices (GLP) and Good Clinical Practices (GCP) when applicable, as well as following strict reporting and documentation requirements.
The regulatory landscape also impacts the commercialization potential of muscimol-based therapies. As research advances, regulatory agencies may need to develop new frameworks for assessing the safety and efficacy of treatments leveraging muscimol's neuro-immune properties, potentially paving the way for novel therapeutic approaches in neurological and immunological disorders.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing research involving muscimol. The compound is classified as a Schedule III controlled substance under the Controlled Substances Act, necessitating strict protocols for its acquisition, storage, and use in scientific studies. Researchers must obtain appropriate licenses and permissions from the Drug Enforcement Administration (DEA) to conduct experiments with muscimol.
Internationally, the regulatory landscape varies significantly. The United Nations Convention on Psychotropic Substances of 1971 classifies muscimol as a Schedule I substance, imposing stringent controls on its production, distribution, and research use. However, individual countries may have differing regulations and exemptions for scientific research purposes.
In the European Union, the European Medicines Agency (EMA) provides guidelines for research involving psychoactive compounds like muscimol. Member states may have additional national regulations that researchers must navigate. For instance, in the UK, the Home Office issues licenses for research involving controlled substances, including muscimol.
The regulatory framework also extends to the ethical considerations of neuro-immune interaction studies. Institutional Review Boards (IRBs) or Ethics Committees play a vital role in ensuring that research protocols involving muscimol adhere to ethical standards and protect human subjects' rights and welfare.
As research into muscimol's neuro-immune dynamics progresses, regulatory bodies are likely to adapt their frameworks to accommodate new findings and potential therapeutic applications. This may involve reassessing the compound's classification, developing new guidelines for clinical trials, and establishing protocols for potential medical use.
Researchers exploring muscimol's dynamics must stay abreast of these evolving regulations, ensuring compliance with both national and international standards. This includes adhering to Good Laboratory Practices (GLP) and Good Clinical Practices (GCP) when applicable, as well as following strict reporting and documentation requirements.
The regulatory landscape also impacts the commercialization potential of muscimol-based therapies. As research advances, regulatory agencies may need to develop new frameworks for assessing the safety and efficacy of treatments leveraging muscimol's neuro-immune properties, potentially paving the way for novel therapeutic approaches in neurological and immunological disorders.
Safety Considerations
When exploring muscimol's dynamics in neuro-immune interactions, safety considerations are paramount. Muscimol, a potent GABA-A receptor agonist, can have significant effects on the central nervous system, necessitating careful handling and administration protocols. Researchers must adhere to strict safety guidelines to minimize potential risks to both subjects and laboratory personnel.
One primary concern is the dosage and administration of muscimol. Due to its potent effects, even small variations in dosage can lead to significant physiological changes. Precise measurement and controlled delivery methods are essential to maintain consistency and prevent adverse reactions. Additionally, the route of administration must be carefully considered, as different methods can affect the drug's bioavailability and potential side effects.
The potential for muscimol to induce sedation and impair cognitive function raises important safety issues, particularly in human studies. Researchers must implement robust monitoring systems to detect any signs of excessive sedation or adverse reactions promptly. This may include continuous vital sign monitoring, regular neurological assessments, and the presence of medical personnel during experimental procedures.
Long-term exposure to muscimol, even at low doses, may have cumulative effects on the nervous system. Studies investigating chronic administration must incorporate comprehensive safety protocols to monitor for potential neurotoxicity or changes in immune function over time. Regular assessments of cognitive function, immune markers, and overall health status should be integrated into the experimental design.
The interaction between muscimol and other medications or substances is another critical safety consideration. Researchers must carefully screen participants for contraindications and potential drug interactions. This includes a thorough review of medical history and current medications, as well as implementing exclusion criteria for individuals with certain health conditions or those taking medications that may interact with muscimol.
Environmental factors also play a role in safety considerations. The storage, handling, and disposal of muscimol require strict protocols to prevent accidental exposure or environmental contamination. Proper personal protective equipment (PPE) and containment measures must be in place to safeguard laboratory personnel and maintain the integrity of the research environment.
Ethical considerations are integral to safety protocols in muscimol research. Informed consent procedures must clearly communicate the potential risks and benefits to study participants. Additionally, animal studies must adhere to stringent ethical guidelines to minimize suffering and ensure humane treatment throughout the research process.
One primary concern is the dosage and administration of muscimol. Due to its potent effects, even small variations in dosage can lead to significant physiological changes. Precise measurement and controlled delivery methods are essential to maintain consistency and prevent adverse reactions. Additionally, the route of administration must be carefully considered, as different methods can affect the drug's bioavailability and potential side effects.
The potential for muscimol to induce sedation and impair cognitive function raises important safety issues, particularly in human studies. Researchers must implement robust monitoring systems to detect any signs of excessive sedation or adverse reactions promptly. This may include continuous vital sign monitoring, regular neurological assessments, and the presence of medical personnel during experimental procedures.
Long-term exposure to muscimol, even at low doses, may have cumulative effects on the nervous system. Studies investigating chronic administration must incorporate comprehensive safety protocols to monitor for potential neurotoxicity or changes in immune function over time. Regular assessments of cognitive function, immune markers, and overall health status should be integrated into the experimental design.
The interaction between muscimol and other medications or substances is another critical safety consideration. Researchers must carefully screen participants for contraindications and potential drug interactions. This includes a thorough review of medical history and current medications, as well as implementing exclusion criteria for individuals with certain health conditions or those taking medications that may interact with muscimol.
Environmental factors also play a role in safety considerations. The storage, handling, and disposal of muscimol require strict protocols to prevent accidental exposure or environmental contamination. Proper personal protective equipment (PPE) and containment measures must be in place to safeguard laboratory personnel and maintain the integrity of the research environment.
Ethical considerations are integral to safety protocols in muscimol research. Informed consent procedures must clearly communicate the potential risks and benefits to study participants. Additionally, animal studies must adhere to stringent ethical guidelines to minimize suffering and ensure humane treatment throughout the research process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!