How Butane-Ethylene Interfacial Dynamics Optimize Industrial Polymerization
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Butane-Ethylene Polymerization Background
The polymerization of butane and ethylene represents a critical process in the industrial production of various polymers, particularly polyethylene. This process has evolved significantly since its inception in the early 20th century, driven by the increasing demand for versatile and durable plastic materials across multiple sectors.
The interfacial dynamics between butane and ethylene play a crucial role in optimizing the polymerization process. Butane, a saturated hydrocarbon, acts as a diluent and chain transfer agent, while ethylene serves as the primary monomer. The interaction between these two components at the molecular level influences the reaction kinetics, polymer chain growth, and ultimately, the properties of the final product.
Historically, the development of this polymerization technique can be traced back to the discovery of high-pressure polyethylene by ICI chemists in 1933. However, it was the introduction of Ziegler-Natta catalysts in the 1950s that revolutionized the field, enabling the production of high-density polyethylene (HDPE) under milder conditions.
The optimization of butane-ethylene interfacial dynamics has been a focus of research and industrial innovation for decades. This optimization aims to enhance reaction efficiency, improve product quality, and reduce energy consumption in the polymerization process. Key factors influencing these dynamics include temperature, pressure, catalyst systems, and the ratio of butane to ethylene in the reaction mixture.
Recent advancements in computational modeling and in-situ characterization techniques have provided deeper insights into the molecular-level interactions occurring at the butane-ethylene interface. These developments have led to more precise control over the polymerization process, allowing for the tailoring of polymer properties to meet specific application requirements.
The industrial significance of optimizing butane-ethylene interfacial dynamics extends beyond the production of conventional polyethylene. It has implications for the synthesis of specialized copolymers, the development of more environmentally friendly production methods, and the enhancement of overall process efficiency in polymer manufacturing.
As global demand for polyethylene and related polymers continues to grow, driven by sectors such as packaging, construction, and automotive industries, the importance of refining and optimizing the butane-ethylene polymerization process remains paramount. This ongoing pursuit of optimization not only aims to meet increasing production demands but also addresses the growing emphasis on sustainability and resource efficiency in industrial processes.
The interfacial dynamics between butane and ethylene play a crucial role in optimizing the polymerization process. Butane, a saturated hydrocarbon, acts as a diluent and chain transfer agent, while ethylene serves as the primary monomer. The interaction between these two components at the molecular level influences the reaction kinetics, polymer chain growth, and ultimately, the properties of the final product.
Historically, the development of this polymerization technique can be traced back to the discovery of high-pressure polyethylene by ICI chemists in 1933. However, it was the introduction of Ziegler-Natta catalysts in the 1950s that revolutionized the field, enabling the production of high-density polyethylene (HDPE) under milder conditions.
The optimization of butane-ethylene interfacial dynamics has been a focus of research and industrial innovation for decades. This optimization aims to enhance reaction efficiency, improve product quality, and reduce energy consumption in the polymerization process. Key factors influencing these dynamics include temperature, pressure, catalyst systems, and the ratio of butane to ethylene in the reaction mixture.
Recent advancements in computational modeling and in-situ characterization techniques have provided deeper insights into the molecular-level interactions occurring at the butane-ethylene interface. These developments have led to more precise control over the polymerization process, allowing for the tailoring of polymer properties to meet specific application requirements.
The industrial significance of optimizing butane-ethylene interfacial dynamics extends beyond the production of conventional polyethylene. It has implications for the synthesis of specialized copolymers, the development of more environmentally friendly production methods, and the enhancement of overall process efficiency in polymer manufacturing.
As global demand for polyethylene and related polymers continues to grow, driven by sectors such as packaging, construction, and automotive industries, the importance of refining and optimizing the butane-ethylene polymerization process remains paramount. This ongoing pursuit of optimization not only aims to meet increasing production demands but also addresses the growing emphasis on sustainability and resource efficiency in industrial processes.
Market Analysis for Industrial Polymers
The industrial polymer market has experienced significant growth in recent years, driven by increasing demand across various sectors such as packaging, automotive, construction, and electronics. The global industrial polymer market was valued at approximately $456 billion in 2020 and is projected to reach $698 billion by 2027, growing at a CAGR of 6.2% during the forecast period.
The market for polyethylene, particularly high-density polyethylene (HDPE) and linear low-density polyethylene (LLDPE), has shown robust growth due to their versatile applications in packaging and construction industries. The optimization of butane-ethylene interfacial dynamics in industrial polymerization processes has played a crucial role in enhancing the production efficiency and quality of these polymers.
Asia-Pacific region dominates the industrial polymer market, accounting for over 40% of the global market share. This is primarily due to the rapid industrialization, urbanization, and increasing disposable income in countries like China and India. North America and Europe follow, with significant market shares driven by technological advancements and growing demand for sustainable polymer solutions.
The automotive industry has emerged as a key consumer of industrial polymers, with an increasing focus on lightweight materials to improve fuel efficiency and reduce emissions. This trend is expected to continue, with the automotive polymer market projected to grow at a CAGR of 8.5% from 2021 to 2028.
Environmental concerns and stringent regulations have led to a growing demand for bio-based and recyclable polymers. This shift towards sustainable materials is reshaping the industrial polymer landscape, with major players investing heavily in research and development of eco-friendly alternatives.
The COVID-19 pandemic initially disrupted the industrial polymer supply chain but also led to increased demand in sectors such as healthcare and packaging. As economies recover, the market is expected to witness accelerated growth, driven by pent-up demand and ongoing technological advancements in polymer production processes.
The optimization of butane-ethylene interfacial dynamics in industrial polymerization is expected to play a crucial role in meeting the growing demand for high-performance polymers. This technological advancement will likely contribute to improved product quality, reduced production costs, and enhanced sustainability in the industrial polymer sector.
The market for polyethylene, particularly high-density polyethylene (HDPE) and linear low-density polyethylene (LLDPE), has shown robust growth due to their versatile applications in packaging and construction industries. The optimization of butane-ethylene interfacial dynamics in industrial polymerization processes has played a crucial role in enhancing the production efficiency and quality of these polymers.
Asia-Pacific region dominates the industrial polymer market, accounting for over 40% of the global market share. This is primarily due to the rapid industrialization, urbanization, and increasing disposable income in countries like China and India. North America and Europe follow, with significant market shares driven by technological advancements and growing demand for sustainable polymer solutions.
The automotive industry has emerged as a key consumer of industrial polymers, with an increasing focus on lightweight materials to improve fuel efficiency and reduce emissions. This trend is expected to continue, with the automotive polymer market projected to grow at a CAGR of 8.5% from 2021 to 2028.
Environmental concerns and stringent regulations have led to a growing demand for bio-based and recyclable polymers. This shift towards sustainable materials is reshaping the industrial polymer landscape, with major players investing heavily in research and development of eco-friendly alternatives.
The COVID-19 pandemic initially disrupted the industrial polymer supply chain but also led to increased demand in sectors such as healthcare and packaging. As economies recover, the market is expected to witness accelerated growth, driven by pent-up demand and ongoing technological advancements in polymer production processes.
The optimization of butane-ethylene interfacial dynamics in industrial polymerization is expected to play a crucial role in meeting the growing demand for high-performance polymers. This technological advancement will likely contribute to improved product quality, reduced production costs, and enhanced sustainability in the industrial polymer sector.
Interfacial Dynamics Challenges
The interfacial dynamics between butane and ethylene in industrial polymerization processes present several significant challenges that researchers and engineers must address to optimize production efficiency and product quality. One of the primary obstacles is the complex nature of the interface itself, where the two components interact in a highly dynamic environment. This interaction is influenced by various factors, including temperature, pressure, and the presence of catalysts, making it difficult to predict and control the behavior at the molecular level.
A major challenge lies in understanding and manipulating the mass transfer phenomena at the butane-ethylene interface. The rate at which molecules move across this boundary can significantly impact the polymerization kinetics and, consequently, the properties of the final polymer product. Researchers must develop accurate models and measurement techniques to quantify and optimize this mass transfer, considering the effects of turbulence, diffusion, and interfacial tension.
Another critical issue is the potential for phase separation or inhomogeneous mixing at the interface. This can lead to inconsistencies in the polymerization process, resulting in variations in polymer composition and molecular weight distribution. Overcoming this challenge requires innovative approaches to maintain a stable and well-mixed interface throughout the reaction vessel, which may involve advanced reactor designs or the use of surfactants and stabilizers.
The presence of impurities or byproducts at the butane-ethylene interface poses an additional challenge. These contaminants can accumulate at the interface, potentially inhibiting the desired reactions or introducing unwanted side reactions. Developing effective purification methods and in-situ monitoring techniques is crucial to maintain the integrity of the interfacial dynamics and ensure consistent product quality.
Furthermore, the high-pressure and high-temperature conditions typical in industrial polymerization processes add complexity to the interfacial dynamics. These extreme conditions can alter the physical and chemical properties of both butane and ethylene, affecting their interaction at the interface. Engineers must design robust systems that can withstand these conditions while still allowing for precise control of the interfacial phenomena.
Lastly, scaling up laboratory findings to industrial-scale operations presents its own set of challenges. The behavior of the butane-ethylene interface in large reactors may differ significantly from that observed in smaller-scale experiments. Researchers must develop reliable scaling laws and predictive models to bridge this gap and ensure that the optimized interfacial dynamics can be effectively implemented in full-scale production environments.
A major challenge lies in understanding and manipulating the mass transfer phenomena at the butane-ethylene interface. The rate at which molecules move across this boundary can significantly impact the polymerization kinetics and, consequently, the properties of the final polymer product. Researchers must develop accurate models and measurement techniques to quantify and optimize this mass transfer, considering the effects of turbulence, diffusion, and interfacial tension.
Another critical issue is the potential for phase separation or inhomogeneous mixing at the interface. This can lead to inconsistencies in the polymerization process, resulting in variations in polymer composition and molecular weight distribution. Overcoming this challenge requires innovative approaches to maintain a stable and well-mixed interface throughout the reaction vessel, which may involve advanced reactor designs or the use of surfactants and stabilizers.
The presence of impurities or byproducts at the butane-ethylene interface poses an additional challenge. These contaminants can accumulate at the interface, potentially inhibiting the desired reactions or introducing unwanted side reactions. Developing effective purification methods and in-situ monitoring techniques is crucial to maintain the integrity of the interfacial dynamics and ensure consistent product quality.
Furthermore, the high-pressure and high-temperature conditions typical in industrial polymerization processes add complexity to the interfacial dynamics. These extreme conditions can alter the physical and chemical properties of both butane and ethylene, affecting their interaction at the interface. Engineers must design robust systems that can withstand these conditions while still allowing for precise control of the interfacial phenomena.
Lastly, scaling up laboratory findings to industrial-scale operations presents its own set of challenges. The behavior of the butane-ethylene interface in large reactors may differ significantly from that observed in smaller-scale experiments. Researchers must develop reliable scaling laws and predictive models to bridge this gap and ensure that the optimized interfacial dynamics can be effectively implemented in full-scale production environments.
Current Interfacial Optimization Methods
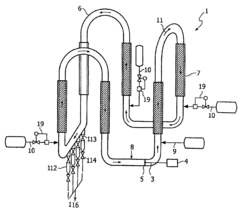
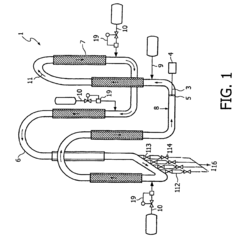
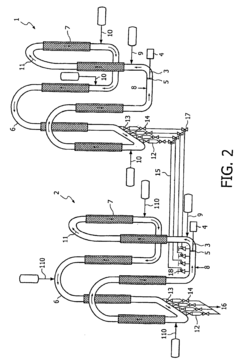
01 Molecular dynamics simulation of butane-ethylene interface
Advanced molecular dynamics simulations are employed to study the interfacial behavior between butane and ethylene. These simulations provide insights into the molecular interactions, diffusion processes, and structural properties at the interface, which are crucial for optimizing the dynamics of the system.- Molecular dynamics simulation of butane-ethylene interface: Advanced molecular dynamics simulations are employed to study the interfacial behavior between butane and ethylene. These simulations provide insights into the molecular interactions, diffusion processes, and structural properties at the interface, which are crucial for optimizing the dynamics of the system.
- Interfacial tension reduction techniques: Various methods are developed to reduce the interfacial tension between butane and ethylene. These techniques may include the use of surfactants, temperature control, or pressure manipulation to enhance the mixing and interaction of the two components at their interface.
- Catalytic processes for butane-ethylene reactions: Catalytic systems are designed to optimize the reactions occurring at the butane-ethylene interface. These catalysts can enhance selectivity, increase reaction rates, and improve overall process efficiency in applications such as alkylation or dehydrogenation.
- Mass transfer enhancement at the interface: Techniques are developed to enhance mass transfer between butane and ethylene phases. This may involve the design of specialized contacting devices, the use of micro- or nano-structured materials, or the application of external fields to promote mixing and increase interfacial area.
- Process control and optimization strategies: Advanced control systems and optimization algorithms are implemented to manage the complex dynamics of butane-ethylene interfacial processes. These strategies may involve real-time monitoring, predictive modeling, and adaptive control to maintain optimal operating conditions and improve overall system performance.
02 Interfacial tension reduction techniques
Various methods are developed to reduce the interfacial tension between butane and ethylene. These techniques may include the use of surfactants, temperature control, or pressure manipulation to enhance the mixing and interaction of the two components, leading to improved interfacial dynamics.Expand Specific Solutions03 Catalytic systems for butane-ethylene reactions
Novel catalytic systems are designed to facilitate and optimize the reactions between butane and ethylene at their interface. These catalysts can enhance reaction rates, improve selectivity, and contribute to the overall optimization of the interfacial dynamics.Expand Specific Solutions04 Process control and monitoring systems
Advanced process control and monitoring systems are developed to optimize the butane-ethylene interfacial dynamics in real-time. These systems may utilize sensors, data analytics, and machine learning algorithms to adjust process parameters and maintain optimal conditions for interfacial interactions.Expand Specific Solutions05 Nanostructured materials for interface modification
Innovative nanostructured materials are designed and synthesized to modify the butane-ethylene interface. These materials can enhance the surface area for interaction, alter the interfacial properties, and improve the overall dynamics of the system.Expand Specific Solutions
Key Industrial Polymer Producers
The competitive landscape for butane-ethylene interfacial dynamics in industrial polymerization is characterized by a mature market with established players and ongoing technological advancements. Major petrochemical companies like China Petroleum & Chemical Corp., PetroChina, and ExxonMobil are at the forefront, leveraging their extensive R&D capabilities. The market is substantial, driven by the growing demand for polyethylene products across various industries. Technological maturity is high, with companies like Dow Global Technologies, BASF, and LyondellBasell continuously innovating to optimize the polymerization process. Academic institutions such as MIT and the University of Florida contribute to fundamental research, while specialized firms like NOVA Chemicals and Versalis focus on developing novel catalysts and process improvements to enhance efficiency and product quality.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced catalytic systems for butane-ethylene polymerization. Their approach involves using zirconium-based metallocene catalysts with modified methylaluminoxane (MAO) cocatalysts to optimize the interfacial dynamics between butane and ethylene[1]. This system allows for precise control of molecular weight distribution and comonomer incorporation, resulting in improved polymer properties. Sinopec has also implemented a proprietary gas-phase reactor design that enhances heat and mass transfer at the butane-ethylene interface, leading to more efficient polymerization[2]. Additionally, they have developed in-line monitoring techniques using Raman spectroscopy to provide real-time feedback on the interfacial dynamics and polymer composition during the production process[3].
Strengths: Advanced catalyst technology, proprietary reactor design, and real-time monitoring capabilities. Weaknesses: Potential high costs associated with specialized catalysts and monitoring equipment.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies has pioneered a solution-phase polymerization process that leverages the unique interfacial dynamics between butane and ethylene. Their approach utilizes a proprietary constrained geometry catalyst (CGC) system that promotes efficient comonomer incorporation at the butane-ethylene interface[4]. This technology enables the production of ethylene-butene copolymers with tailored molecular architectures and enhanced physical properties. Dow has also developed a novel solvent recovery and recycling system that minimizes waste and improves overall process efficiency[5]. Furthermore, they have implemented advanced computational fluid dynamics (CFD) modeling to optimize reactor design and operating conditions, ensuring optimal interfacial contact between butane and ethylene throughout the polymerization process[6].
Strengths: Proprietary catalyst technology, efficient solvent recovery system, and advanced modeling capabilities. Weaknesses: Potential limitations in scaling up the solution-phase process for very high-volume production.
Innovative Interfacial Control Techniques
Ethylene/Butene Multi-Block Copolymer and Process for Producing Same
PatentPendingUS20230074326A1
Innovation
- A process involving the polymerization of ethylene and butene under conditions greater than 125°C with a catalyst system comprising a first polymerization catalyst, a second polymerization catalyst, and a chain shuttling agent, resulting in an ethylene/butene multi-block copolymer with long chain branching (LCB/1000C) greater than or equal to 0.06.
Process for improving the polymerization of ethylene and one or more optional comonomer(s) in a polymerization loop reactor
PatentActiveUS9162204B2
Innovation
- The process involves controlling the hydrogen/ethylene ratio by feeding hydrogen at multiple, spatially separated points along the loop reactor path, which helps maintain a consistent ratio and minimize fluctuations, thereby improving molecular weight distribution and compositional homogeneity of the polymer.
Environmental Impact Assessment
The environmental impact of butane-ethylene interfacial dynamics in industrial polymerization processes is a critical consideration for sustainable manufacturing practices. This assessment focuses on the potential environmental consequences associated with the optimization of these processes.
The use of butane and ethylene in polymerization reactions can lead to the release of volatile organic compounds (VOCs) into the atmosphere. These emissions contribute to air pollution and the formation of ground-level ozone, which can have detrimental effects on human health and ecosystems. However, optimizing the interfacial dynamics between butane and ethylene can potentially reduce VOC emissions by improving reaction efficiency and minimizing waste.
Water pollution is another concern in industrial polymerization processes. Wastewater generated during production may contain trace amounts of unreacted monomers, catalysts, and other chemicals. Proper treatment and disposal of this wastewater are essential to prevent contamination of local water bodies and groundwater resources. Optimized interfacial dynamics can help reduce the volume of wastewater produced and the concentration of pollutants, thereby mitigating the environmental impact.
Energy consumption is a significant factor in the environmental footprint of industrial polymerization. The optimization of butane-ethylene interfacial dynamics can lead to improved energy efficiency by reducing reaction times and lowering the energy requirements for heating and cooling. This, in turn, can result in decreased greenhouse gas emissions associated with energy production.
The production and disposal of polymer products also have environmental implications. By enhancing the efficiency of the polymerization process through optimized interfacial dynamics, manufacturers can potentially reduce material waste and improve the quality of the final product. This can lead to longer-lasting products and a reduction in the overall environmental impact of the polymer lifecycle.
Biodegradability and recyclability of polymers are increasingly important considerations in environmental assessments. While the optimization of butane-ethylene interfacial dynamics primarily focuses on process efficiency, it may indirectly contribute to the development of more environmentally friendly polymers by enabling better control over polymer properties and composition.
Land use and ecosystem impacts should also be considered in the environmental assessment. Industrial polymerization facilities require significant land area and can potentially disrupt local ecosystems. By optimizing processes and improving efficiency, the land footprint of these facilities may be reduced, minimizing habitat destruction and preserving biodiversity.
In conclusion, the optimization of butane-ethylene interfacial dynamics in industrial polymerization has the potential to yield significant environmental benefits. These include reduced emissions, improved energy efficiency, decreased water pollution, and minimized waste generation. However, a comprehensive life cycle assessment is necessary to fully quantify the environmental impact and ensure that improvements in one area do not lead to unintended consequences in others.
The use of butane and ethylene in polymerization reactions can lead to the release of volatile organic compounds (VOCs) into the atmosphere. These emissions contribute to air pollution and the formation of ground-level ozone, which can have detrimental effects on human health and ecosystems. However, optimizing the interfacial dynamics between butane and ethylene can potentially reduce VOC emissions by improving reaction efficiency and minimizing waste.
Water pollution is another concern in industrial polymerization processes. Wastewater generated during production may contain trace amounts of unreacted monomers, catalysts, and other chemicals. Proper treatment and disposal of this wastewater are essential to prevent contamination of local water bodies and groundwater resources. Optimized interfacial dynamics can help reduce the volume of wastewater produced and the concentration of pollutants, thereby mitigating the environmental impact.
Energy consumption is a significant factor in the environmental footprint of industrial polymerization. The optimization of butane-ethylene interfacial dynamics can lead to improved energy efficiency by reducing reaction times and lowering the energy requirements for heating and cooling. This, in turn, can result in decreased greenhouse gas emissions associated with energy production.
The production and disposal of polymer products also have environmental implications. By enhancing the efficiency of the polymerization process through optimized interfacial dynamics, manufacturers can potentially reduce material waste and improve the quality of the final product. This can lead to longer-lasting products and a reduction in the overall environmental impact of the polymer lifecycle.
Biodegradability and recyclability of polymers are increasingly important considerations in environmental assessments. While the optimization of butane-ethylene interfacial dynamics primarily focuses on process efficiency, it may indirectly contribute to the development of more environmentally friendly polymers by enabling better control over polymer properties and composition.
Land use and ecosystem impacts should also be considered in the environmental assessment. Industrial polymerization facilities require significant land area and can potentially disrupt local ecosystems. By optimizing processes and improving efficiency, the land footprint of these facilities may be reduced, minimizing habitat destruction and preserving biodiversity.
In conclusion, the optimization of butane-ethylene interfacial dynamics in industrial polymerization has the potential to yield significant environmental benefits. These include reduced emissions, improved energy efficiency, decreased water pollution, and minimized waste generation. However, a comprehensive life cycle assessment is necessary to fully quantify the environmental impact and ensure that improvements in one area do not lead to unintended consequences in others.
Regulatory Framework for Polymer Production
The regulatory framework for polymer production plays a crucial role in ensuring the safety, quality, and environmental sustainability of industrial polymerization processes. In the context of butane-ethylene interfacial dynamics, regulatory bodies have established comprehensive guidelines to govern the production, handling, and disposal of polymers and their precursors.
At the international level, organizations such as the International Organization for Standardization (ISO) and the American Society for Testing and Materials (ASTM) have developed standards that address various aspects of polymer production. These standards encompass specifications for raw materials, testing methods, and quality control procedures, ensuring consistency and reliability across the industry.
In the United States, the Environmental Protection Agency (EPA) oversees the regulation of polymer production under the Toxic Substances Control Act (TSCA). The TSCA requires manufacturers to submit premanufacture notices for new chemical substances, including polymers, and to comply with reporting, record-keeping, and testing requirements. Additionally, the Occupational Safety and Health Administration (OSHA) sets standards for workplace safety in polymer production facilities, addressing issues such as exposure limits to chemical substances and proper handling procedures.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to the production and use of chemical substances, including those used in polymer manufacturing. REACH mandates that companies register chemical substances and assess their potential risks to human health and the environment. The regulation also promotes the development of alternative methods for hazard assessment of substances.
Specific to the optimization of industrial polymerization through butane-ethylene interfacial dynamics, regulatory frameworks often focus on process safety management and emissions control. For instance, the U.S. Chemical Safety Board (CSB) investigates industrial chemical accidents and provides recommendations for improving process safety in polymerization plants.
Environmental regulations also play a significant role in shaping polymer production practices. Many countries have implemented strict emissions standards to minimize the release of volatile organic compounds (VOCs) and other pollutants associated with polymer manufacturing. These regulations often require the use of best available technologies for emissions control and monitoring.
As the industry continues to evolve, regulatory frameworks are adapting to address emerging challenges and technologies. This includes the development of regulations for bio-based polymers, recycling processes, and the use of advanced catalysts in polymerization. The ongoing dialogue between industry stakeholders, regulatory bodies, and research institutions ensures that the regulatory framework remains responsive to technological advancements while maintaining high standards for safety and environmental protection.
At the international level, organizations such as the International Organization for Standardization (ISO) and the American Society for Testing and Materials (ASTM) have developed standards that address various aspects of polymer production. These standards encompass specifications for raw materials, testing methods, and quality control procedures, ensuring consistency and reliability across the industry.
In the United States, the Environmental Protection Agency (EPA) oversees the regulation of polymer production under the Toxic Substances Control Act (TSCA). The TSCA requires manufacturers to submit premanufacture notices for new chemical substances, including polymers, and to comply with reporting, record-keeping, and testing requirements. Additionally, the Occupational Safety and Health Administration (OSHA) sets standards for workplace safety in polymer production facilities, addressing issues such as exposure limits to chemical substances and proper handling procedures.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to the production and use of chemical substances, including those used in polymer manufacturing. REACH mandates that companies register chemical substances and assess their potential risks to human health and the environment. The regulation also promotes the development of alternative methods for hazard assessment of substances.
Specific to the optimization of industrial polymerization through butane-ethylene interfacial dynamics, regulatory frameworks often focus on process safety management and emissions control. For instance, the U.S. Chemical Safety Board (CSB) investigates industrial chemical accidents and provides recommendations for improving process safety in polymerization plants.
Environmental regulations also play a significant role in shaping polymer production practices. Many countries have implemented strict emissions standards to minimize the release of volatile organic compounds (VOCs) and other pollutants associated with polymer manufacturing. These regulations often require the use of best available technologies for emissions control and monitoring.
As the industry continues to evolve, regulatory frameworks are adapting to address emerging challenges and technologies. This includes the development of regulations for bio-based polymers, recycling processes, and the use of advanced catalysts in polymerization. The ongoing dialogue between industry stakeholders, regulatory bodies, and research institutions ensures that the regulatory framework remains responsive to technological advancements while maintaining high standards for safety and environmental protection.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!