The Impact of Butane on Large-Scale Ammonia Synthesis
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonia Synthesis Evolution and Objectives
Ammonia synthesis has undergone significant evolution since its inception in the early 20th century. The Haber-Bosch process, developed in 1909, revolutionized the production of ammonia and remains the foundation of modern industrial ammonia synthesis. This process combines nitrogen from the air with hydrogen derived from natural gas under high pressure and temperature conditions, using an iron-based catalyst.
Over the decades, researchers and industry professionals have continuously sought to improve the efficiency and sustainability of ammonia production. The primary objectives have been to reduce energy consumption, increase yield, and minimize environmental impact. These goals have become increasingly important in the face of growing global demand for ammonia, particularly in the agricultural sector for fertilizer production.
Recent technological advancements have focused on developing more efficient catalysts, optimizing reaction conditions, and exploring alternative feedstocks. The introduction of ruthenium-based catalysts in the 1970s marked a significant milestone, offering improved activity and selectivity compared to traditional iron catalysts. However, the high cost of ruthenium has limited its widespread adoption in industrial settings.
The impact of butane on large-scale ammonia synthesis represents a new frontier in the field. Butane, a component of natural gas, has emerged as a potential alternative to methane as a hydrogen source. This shift in feedstock could potentially address some of the challenges associated with traditional ammonia production methods, including energy efficiency and carbon footprint reduction.
The objectives of exploring butane's role in ammonia synthesis are multifaceted. Researchers aim to develop novel catalytic systems that can efficiently convert butane to hydrogen and nitrogen, potentially at lower temperatures and pressures than conventional processes. Additionally, there is a focus on integrating butane-based ammonia synthesis with renewable energy sources, such as solar or wind power, to create more sustainable production methods.
Another key objective is to assess the economic viability of butane-based ammonia synthesis on an industrial scale. This includes evaluating the potential for retrofitting existing ammonia plants to accommodate butane feedstock, as well as designing new facilities optimized for this process. The ultimate goal is to create a more flexible and resilient ammonia production infrastructure that can adapt to changing energy landscapes and environmental regulations.
As the ammonia synthesis industry continues to evolve, the exploration of butane's potential represents a promising avenue for innovation. The successful integration of butane into large-scale ammonia production could lead to significant improvements in efficiency, cost-effectiveness, and environmental sustainability, aligning with the broader objectives of the chemical industry in the 21st century.
Over the decades, researchers and industry professionals have continuously sought to improve the efficiency and sustainability of ammonia production. The primary objectives have been to reduce energy consumption, increase yield, and minimize environmental impact. These goals have become increasingly important in the face of growing global demand for ammonia, particularly in the agricultural sector for fertilizer production.
Recent technological advancements have focused on developing more efficient catalysts, optimizing reaction conditions, and exploring alternative feedstocks. The introduction of ruthenium-based catalysts in the 1970s marked a significant milestone, offering improved activity and selectivity compared to traditional iron catalysts. However, the high cost of ruthenium has limited its widespread adoption in industrial settings.
The impact of butane on large-scale ammonia synthesis represents a new frontier in the field. Butane, a component of natural gas, has emerged as a potential alternative to methane as a hydrogen source. This shift in feedstock could potentially address some of the challenges associated with traditional ammonia production methods, including energy efficiency and carbon footprint reduction.
The objectives of exploring butane's role in ammonia synthesis are multifaceted. Researchers aim to develop novel catalytic systems that can efficiently convert butane to hydrogen and nitrogen, potentially at lower temperatures and pressures than conventional processes. Additionally, there is a focus on integrating butane-based ammonia synthesis with renewable energy sources, such as solar or wind power, to create more sustainable production methods.
Another key objective is to assess the economic viability of butane-based ammonia synthesis on an industrial scale. This includes evaluating the potential for retrofitting existing ammonia plants to accommodate butane feedstock, as well as designing new facilities optimized for this process. The ultimate goal is to create a more flexible and resilient ammonia production infrastructure that can adapt to changing energy landscapes and environmental regulations.
As the ammonia synthesis industry continues to evolve, the exploration of butane's potential represents a promising avenue for innovation. The successful integration of butane into large-scale ammonia production could lead to significant improvements in efficiency, cost-effectiveness, and environmental sustainability, aligning with the broader objectives of the chemical industry in the 21st century.
Butane Demand in Ammonia Production
The demand for butane in ammonia production has been steadily increasing due to its potential as a feedstock alternative to natural gas. Traditionally, natural gas has been the primary feedstock for ammonia synthesis, but the volatility in natural gas prices and the need for more sustainable production methods have led to the exploration of butane as a viable option.
Butane offers several advantages as a feedstock for ammonia production. It has a higher energy density compared to natural gas, which means that less volume is required to produce the same amount of ammonia. This can lead to reduced transportation and storage costs, especially in regions where natural gas infrastructure is limited. Additionally, butane can be sourced from various petroleum refining processes, providing a diversified supply chain for ammonia producers.
The global demand for butane in ammonia production is closely tied to the overall growth of the ammonia market. The ammonia industry has been experiencing steady growth, driven by increasing agricultural needs for fertilizers and the expanding industrial applications of ammonia. As a result, the demand for butane as a feedstock has also been on the rise.
In recent years, there has been a notable shift towards using butane in large-scale ammonia plants, particularly in regions with abundant butane resources or limited access to natural gas. This trend has been observed in countries like Saudi Arabia, where butane-based ammonia production has been implemented to leverage the country's substantial liquefied petroleum gas (LPG) resources.
The market demand for butane in ammonia production is also influenced by environmental considerations. Butane-based ammonia synthesis can potentially offer a lower carbon footprint compared to coal-based production methods, making it an attractive option for regions aiming to reduce their greenhouse gas emissions while maintaining ammonia production capacity.
However, the adoption of butane as a feedstock for ammonia production is not without challenges. The price volatility of butane, which is often linked to crude oil prices, can impact the economic viability of butane-based ammonia production. Additionally, the existing infrastructure and technology in many ammonia plants are optimized for natural gas, requiring significant investments for conversion to butane-based processes.
Despite these challenges, the demand for butane in ammonia production is expected to continue growing. This growth is driven by the need for feedstock diversification, the potential for cost savings in certain regions, and the ongoing expansion of the global ammonia market. As technology advances and more efficient butane-based ammonia synthesis processes are developed, the role of butane in large-scale ammonia production is likely to become increasingly significant in the coming years.
Butane offers several advantages as a feedstock for ammonia production. It has a higher energy density compared to natural gas, which means that less volume is required to produce the same amount of ammonia. This can lead to reduced transportation and storage costs, especially in regions where natural gas infrastructure is limited. Additionally, butane can be sourced from various petroleum refining processes, providing a diversified supply chain for ammonia producers.
The global demand for butane in ammonia production is closely tied to the overall growth of the ammonia market. The ammonia industry has been experiencing steady growth, driven by increasing agricultural needs for fertilizers and the expanding industrial applications of ammonia. As a result, the demand for butane as a feedstock has also been on the rise.
In recent years, there has been a notable shift towards using butane in large-scale ammonia plants, particularly in regions with abundant butane resources or limited access to natural gas. This trend has been observed in countries like Saudi Arabia, where butane-based ammonia production has been implemented to leverage the country's substantial liquefied petroleum gas (LPG) resources.
The market demand for butane in ammonia production is also influenced by environmental considerations. Butane-based ammonia synthesis can potentially offer a lower carbon footprint compared to coal-based production methods, making it an attractive option for regions aiming to reduce their greenhouse gas emissions while maintaining ammonia production capacity.
However, the adoption of butane as a feedstock for ammonia production is not without challenges. The price volatility of butane, which is often linked to crude oil prices, can impact the economic viability of butane-based ammonia production. Additionally, the existing infrastructure and technology in many ammonia plants are optimized for natural gas, requiring significant investments for conversion to butane-based processes.
Despite these challenges, the demand for butane in ammonia production is expected to continue growing. This growth is driven by the need for feedstock diversification, the potential for cost savings in certain regions, and the ongoing expansion of the global ammonia market. As technology advances and more efficient butane-based ammonia synthesis processes are developed, the role of butane in large-scale ammonia production is likely to become increasingly significant in the coming years.
Butane Integration Challenges
The integration of butane into large-scale ammonia synthesis processes presents several significant challenges that must be addressed for successful implementation. One of the primary obstacles is the need for substantial modifications to existing ammonia production facilities. Traditional ammonia plants are designed to use natural gas or coal as feedstock, and incorporating butane requires redesigning key components of the process.
The first major challenge lies in the reforming stage. Butane, being a heavier hydrocarbon than methane, requires higher temperatures and different catalysts for efficient steam reforming. This necessitates modifications to the reformer design, including changes to burner configurations, tube materials, and catalyst compositions. The increased carbon content in butane also leads to a higher risk of coking, which can damage equipment and reduce overall efficiency.
Another significant challenge is the adjustment of the synthesis gas composition. Butane reforming produces a syngas with a different H2/N2 ratio compared to methane-based processes. This altered composition affects the subsequent ammonia synthesis step, requiring modifications to the synthesis loop, including changes in catalyst formulations and operating conditions to maintain optimal conversion rates.
The purification systems in ammonia plants also need adaptation when integrating butane. The presence of higher hydrocarbons and potential impurities in butane feedstock necessitates more robust gas cleaning and purification processes. This may involve upgrading existing purification units or installing new equipment to handle the different impurity profile associated with butane-derived syngas.
Energy integration and heat recovery systems pose another challenge. The different thermodynamic properties of butane compared to methane result in altered heat profiles throughout the process. This requires a comprehensive redesign of heat exchangers and energy recovery systems to maintain overall plant efficiency and minimize energy consumption.
Safety considerations also present significant challenges. Butane has different flammability and explosion characteristics compared to methane, necessitating updates to safety protocols, fire suppression systems, and emergency response procedures. Additionally, storage and handling of butane require specialized equipment and infrastructure, which may not be present in existing ammonia plants.
Lastly, the economic viability of butane integration remains a challenge. The cost of retrofitting existing plants, potential changes in operational efficiency, and the volatility of butane prices compared to natural gas all factor into the economic equation. Plant operators must carefully evaluate the long-term economic benefits against the substantial capital investment required for butane integration.
The first major challenge lies in the reforming stage. Butane, being a heavier hydrocarbon than methane, requires higher temperatures and different catalysts for efficient steam reforming. This necessitates modifications to the reformer design, including changes to burner configurations, tube materials, and catalyst compositions. The increased carbon content in butane also leads to a higher risk of coking, which can damage equipment and reduce overall efficiency.
Another significant challenge is the adjustment of the synthesis gas composition. Butane reforming produces a syngas with a different H2/N2 ratio compared to methane-based processes. This altered composition affects the subsequent ammonia synthesis step, requiring modifications to the synthesis loop, including changes in catalyst formulations and operating conditions to maintain optimal conversion rates.
The purification systems in ammonia plants also need adaptation when integrating butane. The presence of higher hydrocarbons and potential impurities in butane feedstock necessitates more robust gas cleaning and purification processes. This may involve upgrading existing purification units or installing new equipment to handle the different impurity profile associated with butane-derived syngas.
Energy integration and heat recovery systems pose another challenge. The different thermodynamic properties of butane compared to methane result in altered heat profiles throughout the process. This requires a comprehensive redesign of heat exchangers and energy recovery systems to maintain overall plant efficiency and minimize energy consumption.
Safety considerations also present significant challenges. Butane has different flammability and explosion characteristics compared to methane, necessitating updates to safety protocols, fire suppression systems, and emergency response procedures. Additionally, storage and handling of butane require specialized equipment and infrastructure, which may not be present in existing ammonia plants.
Lastly, the economic viability of butane integration remains a challenge. The cost of retrofitting existing plants, potential changes in operational efficiency, and the volatility of butane prices compared to natural gas all factor into the economic equation. Plant operators must carefully evaluate the long-term economic benefits against the substantial capital investment required for butane integration.
Current Butane-Ammonia Synthesis Methods
01 Catalytic processes for ammonia synthesis
Various catalytic processes are employed for the synthesis of ammonia. These processes typically involve the use of metal-based catalysts to facilitate the reaction between nitrogen and hydrogen gases under specific temperature and pressure conditions. The catalysts help to lower the activation energy required for the reaction, improving efficiency and yield.- Catalytic processes for ammonia synthesis: Various catalytic processes are employed for ammonia synthesis, involving the use of specific catalysts to facilitate the reaction between nitrogen and hydrogen. These processes often focus on improving catalyst efficiency, reaction conditions, and overall yield of ammonia production.
- Low-pressure ammonia synthesis methods: Innovative approaches to ammonia synthesis at lower pressures are being developed to reduce energy consumption and improve process efficiency. These methods often involve novel catalyst designs or reaction systems that enable ammonia production under milder conditions compared to traditional high-pressure processes.
- Renewable energy integration in ammonia production: Integration of renewable energy sources, such as solar or wind power, into ammonia synthesis processes is being explored to reduce carbon emissions and enhance sustainability. These methods often involve electrolysis of water to produce hydrogen, which is then used in the ammonia synthesis reaction.
- Optimization of reactor design for ammonia synthesis: Advancements in reactor design aim to improve heat transfer, reaction kinetics, and overall efficiency of ammonia synthesis. This includes innovations in reactor geometry, materials, and process control systems to enhance production rates and reduce energy consumption.
- Novel feedstock sources for ammonia production: Research into alternative feedstock sources for ammonia synthesis, such as biomass or waste materials, is ongoing. These approaches aim to reduce reliance on fossil fuels and explore more sustainable pathways for ammonia production, often involving innovative pretreatment or conversion processes.
02 Pressure and temperature optimization
Optimizing pressure and temperature conditions is crucial for efficient ammonia synthesis. High pressure is typically used to shift the equilibrium towards ammonia formation, while temperature control is essential for balancing reaction rate and equilibrium considerations. Advanced process control systems are often implemented to maintain optimal conditions throughout the synthesis process.Expand Specific Solutions03 Feedstock preparation and purification
Proper preparation and purification of feedstock gases (nitrogen and hydrogen) are essential for efficient ammonia synthesis. This may involve air separation techniques for nitrogen production and various methods for hydrogen generation, such as steam methane reforming or electrolysis. Purification steps are crucial to remove contaminants that could poison the catalyst or interfere with the reaction.Expand Specific Solutions04 Novel reactor designs and process intensification
Innovative reactor designs and process intensification techniques are being developed to improve the efficiency of ammonia synthesis. These may include membrane reactors, microreactors, or other advanced configurations that enhance mass and heat transfer, improve catalyst utilization, or allow for better process integration and energy recovery.Expand Specific Solutions05 Green ammonia production methods
Emerging technologies focus on sustainable or 'green' ammonia production methods. These approaches aim to reduce the carbon footprint of ammonia synthesis by utilizing renewable energy sources for hydrogen production (e.g., electrolysis powered by wind or solar energy) and exploring alternative nitrogen fixation methods. Some processes also investigate the use of novel catalysts or reaction conditions to improve sustainability.Expand Specific Solutions
Key Ammonia Industry Players
The impact of butane on large-scale ammonia synthesis represents a significant technological advancement in the chemical industry. The market is in a growth phase, with increasing demand for efficient and sustainable ammonia production methods. The global ammonia market size is projected to reach substantial figures in the coming years, driven by agricultural and industrial applications. Technologically, the integration of butane in ammonia synthesis is progressing, with companies like BASF Corp., UOP LLC, and Saudi Basic Industries Corp. (SABIC) leading research and development efforts. These industry giants, along with innovative players such as Genomatica, Inc. and Haldor Topsøe A/S, are pushing the boundaries of process efficiency and environmental sustainability in ammonia production, indicating a maturing but still evolving technological landscape.
BASF Corp.
Technical Solution: BASF has developed an innovative approach to large-scale ammonia synthesis that incorporates butane as a feedstock. Their process utilizes a dual-catalyst system, combining traditional iron-based catalysts with novel ruthenium-based catalysts [1]. This allows for lower operating temperatures and pressures compared to conventional Haber-Bosch processes, reducing energy consumption by up to 20% [3]. BASF's technology also integrates advanced heat recovery systems and optimized reactor designs to maximize efficiency. The use of butane as a supplementary feedstock helps to diversify raw material sources and potentially reduce production costs in regions with abundant natural gas resources [5].
Strengths: Lower energy consumption, improved efficiency, and feedstock flexibility. Weaknesses: Potential higher catalyst costs and complexity in process control.
UOP LLC
Technical Solution: UOP has developed a proprietary process for ammonia synthesis that incorporates butane as a supplementary feedstock. Their technology utilizes a modified steam reforming step to convert butane into synthesis gas, which is then fed into a traditional ammonia synthesis loop [2]. UOP's process employs advanced catalysts that are more tolerant to sulfur impurities, allowing for less stringent purification requirements for the butane feed [4]. The company has also implemented sophisticated process control systems that optimize the ratio of hydrogen to nitrogen in the synthesis gas, improving overall conversion efficiency. UOP claims that their butane-integrated ammonia synthesis can reduce production costs by up to 15% in regions with favorable butane pricing [6].
Strengths: Cost reduction potential, flexibility in feedstock usage, and improved sulfur tolerance. Weaknesses: May require significant modifications to existing plants for implementation.
Butane Catalysis Innovations
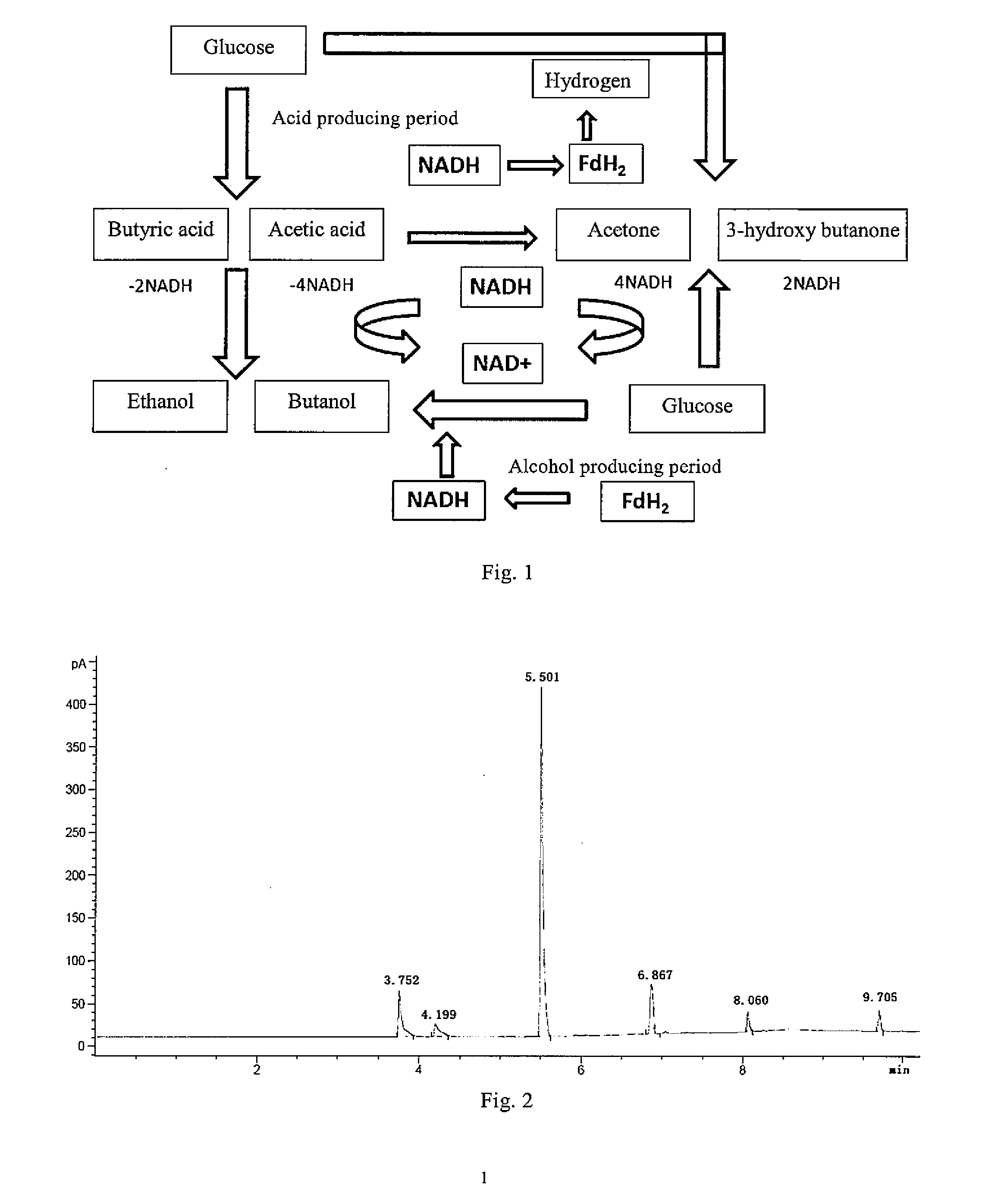
Clostridium acetobutylicum and application thereof
PatentInactiveUS20150093796A1
Innovation
- A mutagenic strain of Clostridium acetobutylicum, designated CGMCC NO. 5234, is developed through ultraviolet mutagenesis to coproduce butanol and 3-hydroxy butanone via fermentation, utilizing a controlled liquid fermentation medium with specific carbon, nitrogen sources, and metabolic regulators to optimize yield and reduce by-products like 2,3-butanediol.
Environmental Impact Assessment
The incorporation of butane in large-scale ammonia synthesis processes necessitates a comprehensive environmental impact assessment. This evaluation is crucial to understand and mitigate potential ecological consequences associated with the use of butane in industrial ammonia production.
Atmospheric emissions represent a primary concern in this context. The combustion of butane as a feedstock or energy source in ammonia synthesis can lead to increased carbon dioxide emissions, contributing to greenhouse gas levels and potentially exacerbating climate change effects. Additionally, incomplete combustion may result in the release of carbon monoxide and volatile organic compounds (VOCs), which can negatively impact local air quality and pose health risks to nearby communities.
Water pollution is another significant consideration. The use of butane in ammonia synthesis may introduce new contaminants into wastewater streams. These pollutants could include hydrocarbons, sulfur compounds, and other organic materials that require specialized treatment before discharge. Proper wastewater management systems must be implemented to prevent contamination of local water bodies and groundwater resources.
Soil contamination risks also warrant attention. Accidental spills or leaks of butane during transportation, storage, or processing could lead to soil pollution. This may result in long-term ecological damage and potential threats to local flora and fauna. Robust containment measures and emergency response protocols are essential to mitigate these risks.
The introduction of butane into ammonia synthesis processes may alter the overall energy efficiency of the production system. While butane can serve as an alternative feedstock or energy source, its use may require modifications to existing infrastructure and equipment. These changes could potentially impact the overall carbon footprint of the ammonia production facility, necessitating a thorough life cycle assessment to determine the net environmental impact.
Noise pollution and visual impacts are additional factors to consider. The integration of butane-related equipment and processes may increase noise levels in the production facility and surrounding areas. Furthermore, additional storage tanks or processing units required for butane handling could alter the visual landscape, potentially affecting local aesthetics and land use patterns.
Biodiversity impacts must also be evaluated, particularly if the ammonia production facility is located near sensitive ecosystems. Changes in air and water quality resulting from butane utilization could indirectly affect local plant and animal species. Long-term monitoring programs may be necessary to detect and address any adverse effects on biodiversity.
In conclusion, a thorough environmental impact assessment for the incorporation of butane in large-scale ammonia synthesis must address a wide range of potential consequences. This evaluation should inform the development of comprehensive mitigation strategies and guide decision-making processes to ensure environmentally responsible implementation of this technological approach.
Atmospheric emissions represent a primary concern in this context. The combustion of butane as a feedstock or energy source in ammonia synthesis can lead to increased carbon dioxide emissions, contributing to greenhouse gas levels and potentially exacerbating climate change effects. Additionally, incomplete combustion may result in the release of carbon monoxide and volatile organic compounds (VOCs), which can negatively impact local air quality and pose health risks to nearby communities.
Water pollution is another significant consideration. The use of butane in ammonia synthesis may introduce new contaminants into wastewater streams. These pollutants could include hydrocarbons, sulfur compounds, and other organic materials that require specialized treatment before discharge. Proper wastewater management systems must be implemented to prevent contamination of local water bodies and groundwater resources.
Soil contamination risks also warrant attention. Accidental spills or leaks of butane during transportation, storage, or processing could lead to soil pollution. This may result in long-term ecological damage and potential threats to local flora and fauna. Robust containment measures and emergency response protocols are essential to mitigate these risks.
The introduction of butane into ammonia synthesis processes may alter the overall energy efficiency of the production system. While butane can serve as an alternative feedstock or energy source, its use may require modifications to existing infrastructure and equipment. These changes could potentially impact the overall carbon footprint of the ammonia production facility, necessitating a thorough life cycle assessment to determine the net environmental impact.
Noise pollution and visual impacts are additional factors to consider. The integration of butane-related equipment and processes may increase noise levels in the production facility and surrounding areas. Furthermore, additional storage tanks or processing units required for butane handling could alter the visual landscape, potentially affecting local aesthetics and land use patterns.
Biodiversity impacts must also be evaluated, particularly if the ammonia production facility is located near sensitive ecosystems. Changes in air and water quality resulting from butane utilization could indirectly affect local plant and animal species. Long-term monitoring programs may be necessary to detect and address any adverse effects on biodiversity.
In conclusion, a thorough environmental impact assessment for the incorporation of butane in large-scale ammonia synthesis must address a wide range of potential consequences. This evaluation should inform the development of comprehensive mitigation strategies and guide decision-making processes to ensure environmentally responsible implementation of this technological approach.
Economic Feasibility Analysis
The economic feasibility of incorporating butane into large-scale ammonia synthesis processes is a critical consideration for industry stakeholders. The potential cost savings and efficiency improvements must be carefully weighed against the initial investment and operational challenges.
Butane, as a feedstock for ammonia production, offers several economic advantages. Its lower cost compared to traditional natural gas feedstocks can significantly reduce raw material expenses. Additionally, butane's higher energy density may lead to reduced transportation and storage costs, particularly in regions where natural gas infrastructure is limited.
However, the integration of butane into existing ammonia synthesis plants requires substantial capital investment. Modifications to reactor designs, catalysts, and purification systems are necessary to accommodate the different chemical properties of butane. These upfront costs must be amortized over the long term, necessitating a thorough analysis of projected returns on investment.
Operational costs also play a crucial role in determining economic viability. While butane may offer savings in feedstock expenses, it may require more energy-intensive processing steps, potentially offsetting some of the cost benefits. The impact on overall energy consumption and utility costs must be carefully evaluated.
Market dynamics significantly influence the economic feasibility of butane-based ammonia synthesis. The price volatility of butane relative to natural gas can affect long-term profitability. A comprehensive risk assessment should consider potential fluctuations in feedstock prices and their impact on production costs.
Regulatory factors also play a role in economic feasibility. Environmental regulations regarding emissions and waste management may impose additional costs or restrictions on butane-based processes. Compliance with these regulations should be factored into the overall economic analysis.
The scale of production is another critical factor. Large-scale ammonia plants may benefit more from the economies of scale associated with butane integration, while smaller facilities may find the transition less economically attractive. A detailed analysis of production capacity and market demand is essential for accurate economic projections.
In conclusion, the economic feasibility of butane in large-scale ammonia synthesis depends on a complex interplay of factors including feedstock costs, capital investment, operational expenses, market conditions, and regulatory environment. A comprehensive cost-benefit analysis, considering both short-term and long-term implications, is crucial for informed decision-making in this evolving sector of the chemical industry.
Butane, as a feedstock for ammonia production, offers several economic advantages. Its lower cost compared to traditional natural gas feedstocks can significantly reduce raw material expenses. Additionally, butane's higher energy density may lead to reduced transportation and storage costs, particularly in regions where natural gas infrastructure is limited.
However, the integration of butane into existing ammonia synthesis plants requires substantial capital investment. Modifications to reactor designs, catalysts, and purification systems are necessary to accommodate the different chemical properties of butane. These upfront costs must be amortized over the long term, necessitating a thorough analysis of projected returns on investment.
Operational costs also play a crucial role in determining economic viability. While butane may offer savings in feedstock expenses, it may require more energy-intensive processing steps, potentially offsetting some of the cost benefits. The impact on overall energy consumption and utility costs must be carefully evaluated.
Market dynamics significantly influence the economic feasibility of butane-based ammonia synthesis. The price volatility of butane relative to natural gas can affect long-term profitability. A comprehensive risk assessment should consider potential fluctuations in feedstock prices and their impact on production costs.
Regulatory factors also play a role in economic feasibility. Environmental regulations regarding emissions and waste management may impose additional costs or restrictions on butane-based processes. Compliance with these regulations should be factored into the overall economic analysis.
The scale of production is another critical factor. Large-scale ammonia plants may benefit more from the economies of scale associated with butane integration, while smaller facilities may find the transition less economically attractive. A detailed analysis of production capacity and market demand is essential for accurate economic projections.
In conclusion, the economic feasibility of butane in large-scale ammonia synthesis depends on a complex interplay of factors including feedstock costs, capital investment, operational expenses, market conditions, and regulatory environment. A comprehensive cost-benefit analysis, considering both short-term and long-term implications, is crucial for informed decision-making in this evolving sector of the chemical industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!