How electrolyte composition influences micro-level material removal in electropolishing
OCT 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electropolishing Technology Background and Objectives
Electropolishing has evolved significantly since its inception in the early 20th century, transforming from a rudimentary surface treatment technique to a sophisticated process critical in numerous high-precision industries. Initially developed for metallographic sample preparation, electropolishing now serves as an essential finishing method in medical device manufacturing, semiconductor production, aerospace components, and pharmaceutical equipment fabrication where surface quality directly impacts functionality and performance.
The fundamental principle of electropolishing involves the controlled dissolution of metal surfaces through an electrochemical process. When a workpiece is immersed in an electrolyte solution and subjected to an electric current as an anode, microscopic peaks on the surface dissolve preferentially compared to valleys, resulting in a smoothed, polished surface. This process not only enhances aesthetic appearance but also improves corrosion resistance, reduces bacterial adhesion, and eliminates microscopic defects.
The electrolyte composition represents one of the most critical yet complex aspects of the electropolishing process. Traditional electrolytes typically consist of concentrated acid mixtures, such as phosphoric and sulfuric acids, often with additives that modify viscosity, conductivity, and dissolution characteristics. Recent technological advancements have introduced more environmentally friendly formulations and specialized compositions tailored for specific alloys and applications.
Understanding the micro-level material removal mechanisms during electropolishing presents significant scientific and industrial value. The interaction between electrolyte components and metal surfaces occurs at the nanoscale, involving complex electrochemical reactions, diffusion processes, and mass transport phenomena that collectively determine the quality and efficiency of the polishing process. Despite decades of industrial application, many aspects of these interactions remain empirically driven rather than theoretically understood.
The primary objective of current research in this field is to establish a comprehensive model that correlates electrolyte composition parameters with material removal mechanisms at the microscopic level. This includes investigating how specific electrolyte components influence the formation and behavior of the viscous layer at the metal-electrolyte interface, the role of additives in modifying anodic dissolution kinetics, and the impact of electrolyte aging on process stability and repeatability.
Additionally, there is growing interest in developing predictive capabilities that would enable precise control over surface characteristics through deliberate manipulation of electrolyte composition. Such advancements would transform electropolishing from an experience-based art to a precisely controlled science, potentially revolutionizing surface engineering across multiple industries and enabling unprecedented surface quality for next-generation applications in medical implants, microelectronics, and advanced manufacturing.
The fundamental principle of electropolishing involves the controlled dissolution of metal surfaces through an electrochemical process. When a workpiece is immersed in an electrolyte solution and subjected to an electric current as an anode, microscopic peaks on the surface dissolve preferentially compared to valleys, resulting in a smoothed, polished surface. This process not only enhances aesthetic appearance but also improves corrosion resistance, reduces bacterial adhesion, and eliminates microscopic defects.
The electrolyte composition represents one of the most critical yet complex aspects of the electropolishing process. Traditional electrolytes typically consist of concentrated acid mixtures, such as phosphoric and sulfuric acids, often with additives that modify viscosity, conductivity, and dissolution characteristics. Recent technological advancements have introduced more environmentally friendly formulations and specialized compositions tailored for specific alloys and applications.
Understanding the micro-level material removal mechanisms during electropolishing presents significant scientific and industrial value. The interaction between electrolyte components and metal surfaces occurs at the nanoscale, involving complex electrochemical reactions, diffusion processes, and mass transport phenomena that collectively determine the quality and efficiency of the polishing process. Despite decades of industrial application, many aspects of these interactions remain empirically driven rather than theoretically understood.
The primary objective of current research in this field is to establish a comprehensive model that correlates electrolyte composition parameters with material removal mechanisms at the microscopic level. This includes investigating how specific electrolyte components influence the formation and behavior of the viscous layer at the metal-electrolyte interface, the role of additives in modifying anodic dissolution kinetics, and the impact of electrolyte aging on process stability and repeatability.
Additionally, there is growing interest in developing predictive capabilities that would enable precise control over surface characteristics through deliberate manipulation of electrolyte composition. Such advancements would transform electropolishing from an experience-based art to a precisely controlled science, potentially revolutionizing surface engineering across multiple industries and enabling unprecedented surface quality for next-generation applications in medical implants, microelectronics, and advanced manufacturing.
Market Analysis of Electropolishing Applications
The electropolishing market has witnessed substantial growth in recent years, driven by increasing demand across multiple industries including medical devices, aerospace, automotive, and semiconductor manufacturing. The global electropolishing services market was valued at approximately $1.2 billion in 2022 and is projected to reach $1.8 billion by 2028, growing at a CAGR of around 6.5% during the forecast period.
Medical device manufacturing represents the largest application segment, accounting for nearly 35% of the market share. This dominance is attributed to stringent regulatory requirements for surface finish quality and biocompatibility. The healthcare sector's expansion, particularly in implantable devices and surgical instruments, continues to fuel demand for electropolishing services that can deliver ultra-smooth, corrosion-resistant surfaces.
Aerospace and defense applications constitute the second-largest market segment at 28%, where electropolishing is critical for components requiring high precision, fatigue resistance, and performance under extreme conditions. The increasing production of commercial aircraft and defense equipment has significantly contributed to market growth in this sector.
The semiconductor industry represents the fastest-growing application segment with an estimated growth rate of 8.2% annually. As chip manufacturers continue to develop increasingly miniaturized components, the demand for precision electropolishing that can achieve nanometer-level surface modifications has intensified.
Geographically, North America leads the market with approximately 38% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the highest growth rate due to rapid industrialization in countries like China, India, and South Korea, coupled with increasing investment in high-tech manufacturing facilities.
The market is witnessing a shift toward customized electrolyte formulations designed for specific material applications. Companies are increasingly investing in R&D to develop proprietary electrolyte compositions that can achieve superior surface finish quality while reducing processing time and environmental impact.
Environmental regulations are significantly influencing market dynamics, with growing emphasis on developing eco-friendly electrolyte compositions that reduce or eliminate hazardous chemicals such as chromic acid. This trend has created new market opportunities for green electropolishing solutions, which are expected to grow at twice the rate of conventional methods over the next five years.
Customer demand is increasingly focused on process consistency and reproducibility, driving adoption of automated electropolishing systems with precise electrolyte composition control. This shift is particularly evident in industries with zero-defect requirements, where electrolyte composition directly impacts micro-level material removal uniformity.
Medical device manufacturing represents the largest application segment, accounting for nearly 35% of the market share. This dominance is attributed to stringent regulatory requirements for surface finish quality and biocompatibility. The healthcare sector's expansion, particularly in implantable devices and surgical instruments, continues to fuel demand for electropolishing services that can deliver ultra-smooth, corrosion-resistant surfaces.
Aerospace and defense applications constitute the second-largest market segment at 28%, where electropolishing is critical for components requiring high precision, fatigue resistance, and performance under extreme conditions. The increasing production of commercial aircraft and defense equipment has significantly contributed to market growth in this sector.
The semiconductor industry represents the fastest-growing application segment with an estimated growth rate of 8.2% annually. As chip manufacturers continue to develop increasingly miniaturized components, the demand for precision electropolishing that can achieve nanometer-level surface modifications has intensified.
Geographically, North America leads the market with approximately 38% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the highest growth rate due to rapid industrialization in countries like China, India, and South Korea, coupled with increasing investment in high-tech manufacturing facilities.
The market is witnessing a shift toward customized electrolyte formulations designed for specific material applications. Companies are increasingly investing in R&D to develop proprietary electrolyte compositions that can achieve superior surface finish quality while reducing processing time and environmental impact.
Environmental regulations are significantly influencing market dynamics, with growing emphasis on developing eco-friendly electrolyte compositions that reduce or eliminate hazardous chemicals such as chromic acid. This trend has created new market opportunities for green electropolishing solutions, which are expected to grow at twice the rate of conventional methods over the next five years.
Customer demand is increasingly focused on process consistency and reproducibility, driving adoption of automated electropolishing systems with precise electrolyte composition control. This shift is particularly evident in industries with zero-defect requirements, where electrolyte composition directly impacts micro-level material removal uniformity.
Current Electrolyte Technology Challenges
Despite significant advancements in electropolishing technology, several critical challenges persist in current electrolyte compositions that limit process efficiency and material removal precision at the micro level. Traditional electrolyte formulations often suffer from inconsistent performance across different material substrates, particularly when processing complex alloys or advanced materials with varying electrochemical properties.
The stability of electrolyte solutions remains a major concern, as many compositions experience degradation during extended operation periods. This degradation manifests as changes in conductivity, pH shifts, and accumulation of dissolved metal ions, all of which can significantly alter the electropolishing kinetics and compromise surface finish quality. The limited operational lifespan of many electrolytes necessitates frequent replacement, increasing operational costs and environmental impact.
Temperature sensitivity presents another substantial challenge, as most conventional electrolytes exhibit dramatic changes in performance characteristics across even narrow temperature ranges. This sensitivity requires precise temperature control systems, adding complexity and cost to electropolishing operations. Furthermore, the optimal temperature window for many electrolytes is quite narrow, restricting process flexibility.
Environmental and health concerns have intensified scrutiny on traditional electrolyte compositions containing hazardous components such as chromic acid, phosphoric acid, and hydrofluoric acid. Regulatory pressures are driving the industry toward greener alternatives, yet environmentally friendly formulations frequently demonstrate inferior performance in terms of material removal rates and surface finish quality.
The lack of standardization in electrolyte composition across the industry creates significant reproducibility challenges. Proprietary formulations dominate the market, making systematic research and development difficult and hindering knowledge transfer between academic research and industrial applications.
Current electrolytes also struggle with selectivity issues, often removing material indiscriminately rather than targeting specific microstructural features. This limitation becomes particularly problematic when processing components with complex geometries or heterogeneous microstructures, where preferential dissolution of certain phases or grain boundaries is desired.
The fundamental understanding of electrolyte-material interactions at the micro and nano scales remains incomplete. While empirical approaches have yielded workable solutions, the absence of comprehensive theoretical models linking electrolyte composition to material removal mechanisms hampers the development of next-generation formulations optimized for specific applications and materials.
The stability of electrolyte solutions remains a major concern, as many compositions experience degradation during extended operation periods. This degradation manifests as changes in conductivity, pH shifts, and accumulation of dissolved metal ions, all of which can significantly alter the electropolishing kinetics and compromise surface finish quality. The limited operational lifespan of many electrolytes necessitates frequent replacement, increasing operational costs and environmental impact.
Temperature sensitivity presents another substantial challenge, as most conventional electrolytes exhibit dramatic changes in performance characteristics across even narrow temperature ranges. This sensitivity requires precise temperature control systems, adding complexity and cost to electropolishing operations. Furthermore, the optimal temperature window for many electrolytes is quite narrow, restricting process flexibility.
Environmental and health concerns have intensified scrutiny on traditional electrolyte compositions containing hazardous components such as chromic acid, phosphoric acid, and hydrofluoric acid. Regulatory pressures are driving the industry toward greener alternatives, yet environmentally friendly formulations frequently demonstrate inferior performance in terms of material removal rates and surface finish quality.
The lack of standardization in electrolyte composition across the industry creates significant reproducibility challenges. Proprietary formulations dominate the market, making systematic research and development difficult and hindering knowledge transfer between academic research and industrial applications.
Current electrolytes also struggle with selectivity issues, often removing material indiscriminately rather than targeting specific microstructural features. This limitation becomes particularly problematic when processing components with complex geometries or heterogeneous microstructures, where preferential dissolution of certain phases or grain boundaries is desired.
The fundamental understanding of electrolyte-material interactions at the micro and nano scales remains incomplete. While empirical approaches have yielded workable solutions, the absence of comprehensive theoretical models linking electrolyte composition to material removal mechanisms hampers the development of next-generation formulations optimized for specific applications and materials.
Current Electrolyte Formulation Approaches
01 Electrolyte compositions for lithium-ion batteries
Specialized electrolyte compositions designed for lithium-ion batteries that enhance performance and stability. These compositions typically include lithium salts, organic solvents, and additives that improve conductivity and electrochemical stability. The formulations help prevent material degradation during charge-discharge cycles and extend battery life while maintaining high energy density.- Electrolyte compositions for lithium-ion batteries: Specialized electrolyte compositions designed for lithium-ion batteries that enhance performance and stability. These compositions typically include lithium salts, organic solvents, and additives that improve ionic conductivity, reduce unwanted side reactions, and extend battery life. The formulations are engineered to maintain stability during charging and discharging cycles while preventing material degradation within the battery system.
- Electrolyte systems for material removal processes: Electrolyte compositions specifically formulated for electrochemical material removal applications such as electropolishing, electroetching, and electromachining. These systems contain carefully balanced ionic components that facilitate controlled dissolution of target materials while maintaining process stability. The electrolytes are designed to achieve uniform material removal rates, superior surface finish, and precise dimensional control during processing.
- Advanced electrolyte additives for performance enhancement: Specialized additives incorporated into electrolyte compositions to enhance specific performance characteristics. These additives can include film-forming compounds, stabilizers, wetting agents, and conductivity enhancers that modify the behavior of the base electrolyte. By incorporating these additives, the electrolyte systems can achieve improved material removal uniformity, reduced side reactions, enhanced conductivity, or better surface quality depending on the specific application requirements.
- Solid-state and gel electrolyte compositions: Non-liquid electrolyte systems including solid-state and gel-based compositions that offer advantages in safety, stability, and form factor flexibility. These electrolytes typically incorporate polymers, ceramic materials, or composite structures that enable ion transport while maintaining structural integrity. The solid or semi-solid nature of these electrolytes can prevent leakage issues and allow for novel device architectures while still facilitating the necessary electrochemical reactions for material processing or energy storage.
- Environmentally friendly electrolyte formulations: Electrolyte compositions designed with environmental sustainability in mind, reducing or eliminating toxic components while maintaining performance. These formulations often replace traditional hazardous materials with more benign alternatives that achieve similar functional properties. The environmentally friendly electrolytes can reduce waste treatment requirements, workplace hazards, and environmental impact while still enabling effective material removal or electrochemical processing.
02 Solid-state electrolyte materials
Advanced solid-state electrolyte materials that facilitate ion transport while preventing material degradation. These solid electrolytes eliminate the need for liquid components, reducing leakage risks and improving safety. The compositions often incorporate ceramic or polymer materials that maintain structural integrity while allowing efficient ion movement between electrodes.Expand Specific Solutions03 Electrolyte additives for improved performance
Specialized additives incorporated into electrolyte compositions to enhance specific properties such as conductivity, stability, or material protection. These additives can form protective films on electrode surfaces, prevent unwanted side reactions, and improve the overall electrochemical performance. They play a crucial role in preventing material removal during battery operation and extending cycle life.Expand Specific Solutions04 Electrolyte systems for material processing
Electrolyte compositions specifically designed for controlled material removal processes such as electrochemical machining, polishing, or etching. These systems utilize precise electrolyte formulations to facilitate selective material removal while maintaining dimensional accuracy and surface quality. The compositions typically include conductive salts, buffers, and inhibitors that control the dissolution rate and pattern.Expand Specific Solutions05 Novel electrolyte formulations for next-generation energy storage
Innovative electrolyte compositions developed for emerging energy storage technologies beyond traditional lithium-ion batteries. These formulations may incorporate ionic liquids, deep eutectic solvents, or hybrid electrolyte systems that offer advantages in terms of safety, environmental impact, and performance. The compositions are designed to minimize material degradation while enabling higher energy densities and faster charging capabilities.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The electropolishing market is currently in a growth phase, driven by increasing demand for precision surface finishing in semiconductor, medical, and aerospace industries. The global market size is estimated to exceed $500 million, with a CAGR of 6-8%. Regarding technical maturity, established players like Applied Materials and Lam Research lead with advanced electrolyte composition technologies, while POLIGRAT GmbH and Drylyte SL offer specialized solutions. Academic institutions such as Dalian University of Technology and University College Dublin are advancing fundamental research on micro-level material removal mechanisms. Companies like CHEMEON Surface Technology and Fujimi Corp. are developing innovative electrolyte formulations that enhance removal rates and surface quality, indicating a technology landscape that balances mature industrial applications with ongoing innovation.
Applied Materials, Inc.
Technical Solution: Applied Materials has developed advanced electropolishing solutions that precisely control electrolyte composition to optimize material removal at the micro-level. Their technology utilizes a proprietary blend of acids, inhibitors, and additives that work synergistically to create a diffusion-limited electrochemical cell. This approach enables selective dissolution of microscopic surface asperities while preserving the base material. Their research has demonstrated that specific organic additives in the electrolyte can form a protective film on lower areas of the surface, directing the dissolution process primarily to protruding features[1]. By manipulating the concentration of complexing agents in the electrolyte, they've achieved control over the thickness of the diffusion layer, which directly influences the material removal rate and surface finish quality[3]. Applied Materials' systems also incorporate real-time monitoring of electrolyte conductivity and pH, automatically adjusting composition to maintain optimal polishing conditions throughout the process lifecycle.
Strengths: Precise control over surface roughness down to nanometer scale; highly reproducible results across large surface areas; reduced processing time compared to traditional methods. Weaknesses: Requires sophisticated control systems; some proprietary electrolyte formulations have limited shelf life; process optimization can be material-specific and require extensive development work.
Cabot Microelectronics Corporation
Technical Solution: Cabot Microelectronics has pioneered electrolyte formulations specifically designed for semiconductor and advanced materials electropolishing. Their approach focuses on the relationship between electrolyte viscosity and diffusion layer characteristics during electropolishing. By engineering electrolytes with carefully controlled viscosity profiles, they've developed solutions that create optimized diffusion barriers at the material surface. Their research shows that electrolyte compositions with specific ratios of phosphoric and sulfuric acids, combined with proprietary organic additives, can significantly enhance material removal selectivity[2]. The company has demonstrated that temperature-dependent viscosity changes in their electrolytes can be leveraged to achieve different removal rates and surface finishes. Their latest generation of electrolytes incorporates nano-sized particles that modify the electrical double layer at the material interface, allowing for unprecedented control over the dissolution kinetics[4]. Cabot's systems also utilize pulsed current techniques synchronized with electrolyte composition to further enhance micro-level material removal precision.
Strengths: Exceptional surface finish quality with Ra values below 0.02μm; excellent for complex geometries where mechanical polishing is challenging; highly specialized formulations for different metal alloys. Weaknesses: Higher cost compared to conventional electrolytes; some formulations require careful handling due to environmental concerns; process sensitivity to temperature fluctuations.
Key Mechanisms of Material Removal Processes
Electrolyte for polishing stainless steels, containing a pyridinecarboxylic acid
PatentInactiveEP3186417A1
Innovation
- An electrolyte composition based on a mixture of phosphoric acid and sulfuric acid with the addition of pyridine carboxylic acids, such as nicotinic acid, which improves leveling behavior, reduces processing time, and lowers minimum current density, while being safe and compatible with all stainless steel grades.
Electropolishing method and electrolyte for same
PatentWO2018102845A1
Innovation
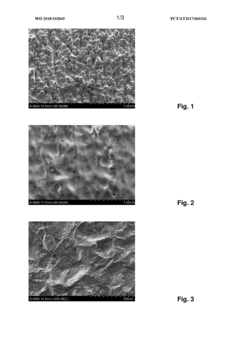
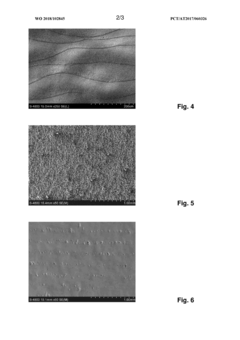
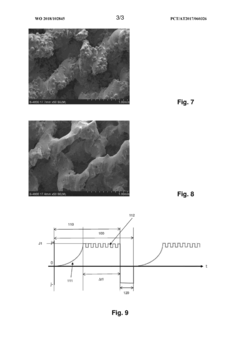
- An electrolyte containing methanesulfonic acid and at least one phosphonic acid, along with polyhydric alcohols and other additives, is used in an electropolishing process with controlled pulse sequences to effectively smooth out large roughness, utilizing repetitive anodic pulses with increasing current intensity and micropulses to enhance material removal and surface finishing.
Environmental Impact of Electropolishing Solutions
Electropolishing processes, while effective for surface finishing, present significant environmental challenges due to the chemical composition of their electrolyte solutions. These solutions typically contain concentrated acids such as phosphoric, sulfuric, and chromic acids, along with various additives that can pose serious environmental hazards when improperly managed. The environmental footprint of electropolishing operations extends across multiple dimensions, including water pollution, air quality degradation, and waste management concerns.
The discharge of spent electropolishing solutions into water systems introduces heavy metals and toxic compounds that can severely impact aquatic ecosystems. Metals removed during the electropolishing process, including nickel, chromium, copper, and zinc, accumulate in the electrolyte and can persist in the environment for extended periods. These metals may bioaccumulate in aquatic organisms and potentially enter the human food chain, presenting long-term ecological and public health risks.
Volatile organic compounds (VOCs) and acid mists generated during electropolishing operations contribute to air pollution and can cause respiratory issues for workers and surrounding communities. The evaporation of solution components, particularly when heated electrolytes are used, releases harmful substances that may contribute to smog formation and ozone depletion depending on their chemical nature.
Waste management challenges are equally significant, as spent electropolishing solutions require specialized treatment before disposal. The high acidity and metal content of these wastes classify them as hazardous materials in most regulatory frameworks, necessitating costly treatment processes. Traditional neutralization methods often generate substantial quantities of metal-laden sludge that requires secure landfilling, creating additional environmental burdens.
Recent regulatory trends worldwide have increasingly focused on restricting the use of particularly harmful components in electropolishing solutions, such as hexavalent chromium compounds. This has driven research into more environmentally benign alternatives that maintain effective material removal capabilities while reducing ecological impact. Green electropolishing solutions utilizing organic acids, ionic liquids, or deep eutectic solvents represent promising developments in this field.
The environmental impact also varies significantly based on electrolyte composition choices. For instance, phosphoric acid-based electrolytes generally present lower environmental hazards compared to chromic acid formulations, though they may require different operational parameters to achieve comparable material removal rates. The selection of additives similarly influences both process efficiency and environmental footprint, creating complex trade-offs that manufacturers must navigate.
Implementing closed-loop recycling systems for electropolishing solutions can substantially reduce environmental impact by extending electrolyte lifespan and minimizing waste generation. Advanced treatment technologies such as membrane filtration, ion exchange, and electrochemical recovery methods enable the removal of accumulated metals while preserving the functional components of the electrolyte for reuse.
The discharge of spent electropolishing solutions into water systems introduces heavy metals and toxic compounds that can severely impact aquatic ecosystems. Metals removed during the electropolishing process, including nickel, chromium, copper, and zinc, accumulate in the electrolyte and can persist in the environment for extended periods. These metals may bioaccumulate in aquatic organisms and potentially enter the human food chain, presenting long-term ecological and public health risks.
Volatile organic compounds (VOCs) and acid mists generated during electropolishing operations contribute to air pollution and can cause respiratory issues for workers and surrounding communities. The evaporation of solution components, particularly when heated electrolytes are used, releases harmful substances that may contribute to smog formation and ozone depletion depending on their chemical nature.
Waste management challenges are equally significant, as spent electropolishing solutions require specialized treatment before disposal. The high acidity and metal content of these wastes classify them as hazardous materials in most regulatory frameworks, necessitating costly treatment processes. Traditional neutralization methods often generate substantial quantities of metal-laden sludge that requires secure landfilling, creating additional environmental burdens.
Recent regulatory trends worldwide have increasingly focused on restricting the use of particularly harmful components in electropolishing solutions, such as hexavalent chromium compounds. This has driven research into more environmentally benign alternatives that maintain effective material removal capabilities while reducing ecological impact. Green electropolishing solutions utilizing organic acids, ionic liquids, or deep eutectic solvents represent promising developments in this field.
The environmental impact also varies significantly based on electrolyte composition choices. For instance, phosphoric acid-based electrolytes generally present lower environmental hazards compared to chromic acid formulations, though they may require different operational parameters to achieve comparable material removal rates. The selection of additives similarly influences both process efficiency and environmental footprint, creating complex trade-offs that manufacturers must navigate.
Implementing closed-loop recycling systems for electropolishing solutions can substantially reduce environmental impact by extending electrolyte lifespan and minimizing waste generation. Advanced treatment technologies such as membrane filtration, ion exchange, and electrochemical recovery methods enable the removal of accumulated metals while preserving the functional components of the electrolyte for reuse.
Quality Control Standards for Electropolished Surfaces
Quality control standards for electropolished surfaces are critical to ensure consistent performance and reliability in industries where surface finish directly impacts functionality. These standards typically encompass several key parameters that quantify the quality of electropolished surfaces in relation to electrolyte composition effects.
Surface roughness measurements, including Ra (average roughness), Rz (maximum height), and Rq (root mean square roughness), serve as primary indicators of electropolishing quality. The American Society for Testing and Materials (ASTM) has established specific standards such as ASTM B912 for stainless steel electropolishing, which defines acceptable roughness values based on application requirements.
Reflectivity and gloss measurements provide quantitative assessment of the visual appearance of electropolished surfaces. These parameters are particularly important in decorative applications and are measured using glossmeters at standardized angles (typically 20°, 60°, and 85°). The correlation between electrolyte composition and achievable gloss levels is well-documented in industry specifications.
Material removal uniformity represents another critical quality parameter. Standards typically specify acceptable thickness variation across the workpiece, with tolerances ranging from ±2.5μm for precision applications to ±10μm for general industrial use. Advanced measurement techniques such as X-ray fluorescence (XRF) and eddy current testing enable non-destructive verification of this parameter.
Surface cleanliness standards address residual chemical contamination from electrolyte components. Techniques such as X-ray photoelectron spectroscopy (XPS) and secondary ion mass spectrometry (SIMS) can detect trace contaminants at parts-per-billion levels. Medical and semiconductor industries typically adhere to standards like ASTM F3127 for cleanliness verification.
Corrosion resistance testing, including salt spray testing (ASTM B117) and electrochemical impedance spectroscopy (EIS), evaluates the protective passive layer formed during electropolishing. The quality of this layer is directly influenced by electrolyte composition, with standards specifying minimum exposure times without corrosion onset.
Biocompatibility standards, particularly relevant for medical implants, include ISO 10993 series tests that evaluate how surface properties affected by electropolishing influence cell adhesion and biofilm formation. These standards recognize the critical relationship between electrolyte composition, resulting surface chemistry, and biological response.
Documentation requirements complete the quality control framework, mandating detailed records of electrolyte composition, operating parameters, and verification test results for traceability and process validation purposes.
Surface roughness measurements, including Ra (average roughness), Rz (maximum height), and Rq (root mean square roughness), serve as primary indicators of electropolishing quality. The American Society for Testing and Materials (ASTM) has established specific standards such as ASTM B912 for stainless steel electropolishing, which defines acceptable roughness values based on application requirements.
Reflectivity and gloss measurements provide quantitative assessment of the visual appearance of electropolished surfaces. These parameters are particularly important in decorative applications and are measured using glossmeters at standardized angles (typically 20°, 60°, and 85°). The correlation between electrolyte composition and achievable gloss levels is well-documented in industry specifications.
Material removal uniformity represents another critical quality parameter. Standards typically specify acceptable thickness variation across the workpiece, with tolerances ranging from ±2.5μm for precision applications to ±10μm for general industrial use. Advanced measurement techniques such as X-ray fluorescence (XRF) and eddy current testing enable non-destructive verification of this parameter.
Surface cleanliness standards address residual chemical contamination from electrolyte components. Techniques such as X-ray photoelectron spectroscopy (XPS) and secondary ion mass spectrometry (SIMS) can detect trace contaminants at parts-per-billion levels. Medical and semiconductor industries typically adhere to standards like ASTM F3127 for cleanliness verification.
Corrosion resistance testing, including salt spray testing (ASTM B117) and electrochemical impedance spectroscopy (EIS), evaluates the protective passive layer formed during electropolishing. The quality of this layer is directly influenced by electrolyte composition, with standards specifying minimum exposure times without corrosion onset.
Biocompatibility standards, particularly relevant for medical implants, include ISO 10993 series tests that evaluate how surface properties affected by electropolishing influence cell adhesion and biofilm formation. These standards recognize the critical relationship between electrolyte composition, resulting surface chemistry, and biological response.
Documentation requirements complete the quality control framework, mandating detailed records of electrolyte composition, operating parameters, and verification test results for traceability and process validation purposes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!