Patent landscape of electropolishing technologies for medical implants
OCT 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electropolishing Technology Evolution and Objectives
Electropolishing technology for medical implants has evolved significantly over the past century, with its origins dating back to the early 1900s when basic electrochemical polishing techniques were first developed for metallurgical applications. The fundamental principle of electropolishing—using an electrochemical cell to remove material from a metallic workpiece—has remained consistent, though the precision, control, and applications have advanced dramatically.
The 1950s marked a pivotal turning point when electropolishing began to be applied to medical devices, primarily for stainless steel surgical instruments. By the 1970s, as implantable medical devices gained prominence, researchers recognized the potential benefits of electropolished surfaces for biocompatibility and corrosion resistance. This period saw the development of specialized electrolytes and process parameters specifically designed for medical-grade alloys.
The 1990s witnessed significant technological advancement with the introduction of computer-controlled electropolishing systems, enabling precise parameter control and reproducibility essential for medical applications. Concurrently, the rise of minimally invasive procedures drove demand for electropolished stents and other small, complex implantable devices, further accelerating innovation in the field.
Patent activity in this domain reveals a clear trajectory toward increasingly specialized applications. Early patents focused on basic process improvements, while more recent innovations target specific medical alloys, complex geometries, and enhanced surface properties. Notable patent clusters emerge around cardiovascular implants, orthopedic devices, and dental applications, reflecting the diversification of electropolishing technology across medical specialties.
The current technological objectives in electropolishing for medical implants center on several key areas: enhancing biocompatibility through ultra-smooth surfaces that minimize protein adhesion and bacterial colonization; improving corrosion resistance to extend implant longevity; developing selective polishing techniques for complex geometries; and creating environmentally friendly electrolyte formulations to replace traditional acid mixtures.
Emerging research aims to integrate electropolishing with other surface modification technologies, such as coating applications and nano-texturing, to create multifunctional implant surfaces. Additionally, there is growing interest in developing real-time monitoring systems that can provide immediate feedback during the electropolishing process, ensuring consistent quality across production batches.
The evolution of this technology continues to be driven by increasingly stringent regulatory requirements for medical implants, growing demand for personalized medical devices, and the ongoing push toward minimally invasive therapeutic approaches requiring smaller, more complex implantable components.
The 1950s marked a pivotal turning point when electropolishing began to be applied to medical devices, primarily for stainless steel surgical instruments. By the 1970s, as implantable medical devices gained prominence, researchers recognized the potential benefits of electropolished surfaces for biocompatibility and corrosion resistance. This period saw the development of specialized electrolytes and process parameters specifically designed for medical-grade alloys.
The 1990s witnessed significant technological advancement with the introduction of computer-controlled electropolishing systems, enabling precise parameter control and reproducibility essential for medical applications. Concurrently, the rise of minimally invasive procedures drove demand for electropolished stents and other small, complex implantable devices, further accelerating innovation in the field.
Patent activity in this domain reveals a clear trajectory toward increasingly specialized applications. Early patents focused on basic process improvements, while more recent innovations target specific medical alloys, complex geometries, and enhanced surface properties. Notable patent clusters emerge around cardiovascular implants, orthopedic devices, and dental applications, reflecting the diversification of electropolishing technology across medical specialties.
The current technological objectives in electropolishing for medical implants center on several key areas: enhancing biocompatibility through ultra-smooth surfaces that minimize protein adhesion and bacterial colonization; improving corrosion resistance to extend implant longevity; developing selective polishing techniques for complex geometries; and creating environmentally friendly electrolyte formulations to replace traditional acid mixtures.
Emerging research aims to integrate electropolishing with other surface modification technologies, such as coating applications and nano-texturing, to create multifunctional implant surfaces. Additionally, there is growing interest in developing real-time monitoring systems that can provide immediate feedback during the electropolishing process, ensuring consistent quality across production batches.
The evolution of this technology continues to be driven by increasingly stringent regulatory requirements for medical implants, growing demand for personalized medical devices, and the ongoing push toward minimally invasive therapeutic approaches requiring smaller, more complex implantable components.
Market Analysis for Electropolished Medical Implants
The global market for electropolished medical implants has been experiencing robust growth, driven by increasing prevalence of chronic diseases, aging population demographics, and technological advancements in implantable medical devices. The market size for electropolished medical implants was valued at approximately $5.2 billion in 2022 and is projected to reach $8.7 billion by 2028, representing a compound annual growth rate (CAGR) of 8.9% during the forecast period.
Orthopedic implants constitute the largest segment within the electropolished medical implants market, accounting for roughly 40% of the total market share. This dominance can be attributed to the rising incidence of musculoskeletal disorders and the growing number of joint replacement surgeries worldwide. Cardiovascular implants follow closely, representing about 30% of the market, with dental implants and neurological implants comprising the remaining significant portions.
Geographically, North America holds the largest market share at approximately 42%, followed by Europe at 28% and Asia-Pacific at 22%. The dominance of North America can be attributed to advanced healthcare infrastructure, higher healthcare expenditure, and greater adoption of cutting-edge medical technologies. However, the Asia-Pacific region is expected to witness the fastest growth during the forecast period, with a CAGR of 10.5%, primarily due to improving healthcare access, rising disposable incomes, and increasing healthcare awareness in countries like China and India.
Key market drivers include the growing preference for minimally invasive surgeries, increasing prevalence of chronic diseases requiring implantation, and technological advancements in implant materials and surface treatments. The demand for electropolished implants is particularly strong due to their superior corrosion resistance, reduced bacterial adhesion, and enhanced biocompatibility compared to conventionally finished implants.
Market challenges include stringent regulatory requirements for medical implants, high costs associated with advanced surface treatment technologies, and the complex nature of electropolishing processes for newer implant materials such as titanium alloys and cobalt-chromium alloys.
The competitive landscape is characterized by both established medical device manufacturers and specialized surface treatment companies. Major players include Medtronic, Johnson & Johnson, Stryker Corporation, and Boston Scientific, who collectively hold approximately 65% of the market share. These companies are increasingly focusing on research and development to improve electropolishing techniques and develop proprietary surface treatment technologies to gain competitive advantages.
Orthopedic implants constitute the largest segment within the electropolished medical implants market, accounting for roughly 40% of the total market share. This dominance can be attributed to the rising incidence of musculoskeletal disorders and the growing number of joint replacement surgeries worldwide. Cardiovascular implants follow closely, representing about 30% of the market, with dental implants and neurological implants comprising the remaining significant portions.
Geographically, North America holds the largest market share at approximately 42%, followed by Europe at 28% and Asia-Pacific at 22%. The dominance of North America can be attributed to advanced healthcare infrastructure, higher healthcare expenditure, and greater adoption of cutting-edge medical technologies. However, the Asia-Pacific region is expected to witness the fastest growth during the forecast period, with a CAGR of 10.5%, primarily due to improving healthcare access, rising disposable incomes, and increasing healthcare awareness in countries like China and India.
Key market drivers include the growing preference for minimally invasive surgeries, increasing prevalence of chronic diseases requiring implantation, and technological advancements in implant materials and surface treatments. The demand for electropolished implants is particularly strong due to their superior corrosion resistance, reduced bacterial adhesion, and enhanced biocompatibility compared to conventionally finished implants.
Market challenges include stringent regulatory requirements for medical implants, high costs associated with advanced surface treatment technologies, and the complex nature of electropolishing processes for newer implant materials such as titanium alloys and cobalt-chromium alloys.
The competitive landscape is characterized by both established medical device manufacturers and specialized surface treatment companies. Major players include Medtronic, Johnson & Johnson, Stryker Corporation, and Boston Scientific, who collectively hold approximately 65% of the market share. These companies are increasingly focusing on research and development to improve electropolishing techniques and develop proprietary surface treatment technologies to gain competitive advantages.
Current Electropolishing Techniques and Barriers
Electropolishing represents a critical surface finishing process for medical implants, employing an electrochemical mechanism to remove material from metallic surfaces. The current landscape features several established techniques that have become industry standards. Conventional electropolishing typically utilizes phosphoric and sulfuric acid electrolyte baths, operating at controlled temperatures between 40-80°C with precise current densities. This approach remains dominant for stainless steel implants, particularly for cardiovascular stents and orthopedic components.
Pulse electropolishing has emerged as an advanced variation, applying intermittent current rather than continuous flow. This technique offers superior control over material removal rates and has demonstrated particular efficacy for complex geometries in dental implants and small-dimension components. Research indicates pulse electropolishing can reduce processing time by 15-30% while improving surface uniformity compared to conventional methods.
Vibratory electropolishing combines traditional electrochemical processes with mechanical vibration of either the workpiece or the electrolyte. This hybrid approach has gained traction for addressing internal surfaces of hollow implants, such as spinal fusion cages and dental abutments, where conventional techniques struggle to achieve uniform results.
Despite these advancements, significant barriers persist in electropolishing technologies for medical implants. Material-specific limitations represent a primary challenge, as titanium alloys and cobalt-chromium compositions respond differently to standard electrolyte formulations, necessitating customized approaches that complicate manufacturing standardization. The industry faces substantial difficulties in achieving consistent results across varying implant geometries, particularly for additively manufactured components with internal features and complex lattice structures.
Regulatory hurdles constitute another major barrier, with stringent FDA and international requirements for surface characterization and validation. The correlation between electropolished surfaces and biological responses remains incompletely understood, creating uncertainty in approval pathways. Environmental and worker safety concerns also present challenges, as traditional electrolytes contain hazardous chemicals requiring specialized handling and disposal protocols.
Scale-up limitations further constrain advancement, with many promising techniques demonstrating efficacy in laboratory settings but encountering difficulties in industrial implementation. The high capital investment required for advanced electropolishing equipment, coupled with specialized operator training needs, has slowed adoption rates among smaller medical device manufacturers, creating market fragmentation between technological capabilities.
Pulse electropolishing has emerged as an advanced variation, applying intermittent current rather than continuous flow. This technique offers superior control over material removal rates and has demonstrated particular efficacy for complex geometries in dental implants and small-dimension components. Research indicates pulse electropolishing can reduce processing time by 15-30% while improving surface uniformity compared to conventional methods.
Vibratory electropolishing combines traditional electrochemical processes with mechanical vibration of either the workpiece or the electrolyte. This hybrid approach has gained traction for addressing internal surfaces of hollow implants, such as spinal fusion cages and dental abutments, where conventional techniques struggle to achieve uniform results.
Despite these advancements, significant barriers persist in electropolishing technologies for medical implants. Material-specific limitations represent a primary challenge, as titanium alloys and cobalt-chromium compositions respond differently to standard electrolyte formulations, necessitating customized approaches that complicate manufacturing standardization. The industry faces substantial difficulties in achieving consistent results across varying implant geometries, particularly for additively manufactured components with internal features and complex lattice structures.
Regulatory hurdles constitute another major barrier, with stringent FDA and international requirements for surface characterization and validation. The correlation between electropolished surfaces and biological responses remains incompletely understood, creating uncertainty in approval pathways. Environmental and worker safety concerns also present challenges, as traditional electrolytes contain hazardous chemicals requiring specialized handling and disposal protocols.
Scale-up limitations further constrain advancement, with many promising techniques demonstrating efficacy in laboratory settings but encountering difficulties in industrial implementation. The high capital investment required for advanced electropolishing equipment, coupled with specialized operator training needs, has slowed adoption rates among smaller medical device manufacturers, creating market fragmentation between technological capabilities.
Contemporary Electropolishing Solutions for Implants
01 Electrolyte compositions for electropolishing
Various electrolyte compositions can be used in electropolishing processes to enhance surface finish quality. These compositions may include mixtures of acids (such as phosphoric, sulfuric, or nitric acids), organic compounds, and additives that improve conductivity and polishing performance. The specific formulation affects the dissolution rate of the metal surface, resulting in smoother finishes with reduced roughness and improved corrosion resistance.- Electrolyte compositions for electropolishing: Various electrolyte compositions are used in electropolishing processes to enhance surface finish quality. These compositions may include acids such as phosphoric acid, sulfuric acid, or mixtures with organic compounds. The specific formulation affects the dissolution rate of metal surfaces, resulting in smoother finishes. Advanced electrolytes can be tailored for specific metals and alloys, improving efficiency and reducing environmental impact.
- Pulse and modulated current techniques: Pulse electropolishing techniques involve applying current in controlled pulses rather than continuously. This approach allows for better control of the polishing process, reducing heat generation and improving surface quality. By varying parameters such as pulse frequency, duration, and amplitude, the process can be optimized for different materials and geometries. These techniques are particularly useful for complex shapes and sensitive materials where traditional methods might cause overheating or uneven polishing.
- Electropolishing for semiconductor and microelectronics applications: Specialized electropolishing technologies have been developed for semiconductor manufacturing and microelectronics. These processes focus on achieving ultra-smooth surfaces with nanometer-level precision required for advanced electronic components. The techniques often incorporate specialized fixturing, controlled flow dynamics, and precise temperature regulation to ensure uniform material removal across wafers and components. These methods are critical for improving device performance and reliability in high-tech applications.
- Automated and robotic electropolishing systems: Modern electropolishing technologies incorporate automation and robotics to improve process consistency and efficiency. These systems use computer-controlled movement and positioning to ensure uniform treatment of complex parts. Advanced monitoring systems provide real-time feedback on current density, temperature, and solution chemistry, allowing for process adjustments during operation. Automated systems can handle multiple parts simultaneously and adapt to different geometries, significantly increasing throughput while maintaining quality standards.
- Environmentally friendly electropolishing methods: Recent developments in electropolishing focus on reducing environmental impact through green chemistry approaches. These methods replace traditional hazardous chemicals with more environmentally benign alternatives while maintaining polishing effectiveness. Some innovations include closed-loop systems that recycle electrolytes, reducing waste generation and chemical consumption. Other approaches involve lower operating temperatures and reduced energy consumption, making the overall process more sustainable while still achieving high-quality surface finishes.
02 Pulse and modulated current electropolishing techniques
Advanced electropolishing methods utilize pulse or modulated current patterns rather than continuous direct current. These techniques provide better control over the polishing process by allowing periodic relaxation of the diffusion layer, resulting in more uniform material removal. Pulse electropolishing can reduce surface defects, improve finish quality, and enable processing of complex geometries while minimizing heat generation and electrolyte degradation.Expand Specific Solutions03 Electropolishing for medical and implantable devices
Specialized electropolishing technologies have been developed for medical devices and implants, particularly for stents and surgical instruments. These processes focus on achieving ultra-smooth surfaces that reduce thrombogenicity, bacterial adhesion, and tissue trauma. The techniques often involve custom fixturing, controlled agitation, and precise parameter control to polish complex geometries while maintaining dimensional accuracy and biocompatibility of materials like stainless steel, nitinol, and titanium alloys.Expand Specific Solutions04 Semiconductor and microelectronics electropolishing
Electropolishing technologies for semiconductor manufacturing focus on achieving planarization and precise material removal for wafers, interconnects, and microelectronic components. These processes often integrate with chemical mechanical planarization (CMP) techniques and require extremely tight control of current distribution and electrolyte flow. Advanced monitoring systems ensure uniform polishing across the entire surface while preventing over-etching of critical features and maintaining the electrical properties of the processed materials.Expand Specific Solutions05 Environmentally friendly electropolishing methods
Recent innovations in electropolishing focus on developing more environmentally sustainable processes that reduce or eliminate hazardous chemicals. These green electropolishing technologies utilize biodegradable electrolytes, closed-loop recycling systems, and energy-efficient power delivery methods. Some approaches incorporate ionic liquids, neutral salt solutions, or organic acid blends as alternatives to traditional strong acid mixtures, reducing environmental impact while maintaining or improving surface finish quality.Expand Specific Solutions
Leading Companies in Medical Implant Electropolishing
The electropolishing technology for medical implants market is in a growth phase, characterized by increasing demand for high-quality surface finishing solutions that enhance implant biocompatibility and longevity. The competitive landscape features established medical device manufacturers like Medtronic, Abbott Laboratories, and Boston Scientific alongside specialized surface treatment companies. Major players are actively developing proprietary electropolishing techniques, with companies like Smith & Nephew, Warsaw Orthopedic (Medtronic), and Cook Medical Technologies leading patent activities. Academic institutions including Johns Hopkins University and Beihang University contribute significant research innovations. The technology is reaching maturity in traditional applications while expanding into novel areas such as drug-eluting stents and biodegradable implants, with companies like Biotyx Medical and Solenic Medical introducing specialized electropolishing solutions for next-generation implantable devices.
Abbott Cardiovascular Systems, Inc.
Technical Solution: Abbott Cardiovascular Systems has developed advanced electropolishing technologies specifically for coronary stents and other cardiovascular implants. Their proprietary electropolishing process utilizes a controlled electrolytic removal system with precise current density management to achieve ultra-smooth surfaces on complex stent geometries. The company employs a multi-stage electropolishing protocol that includes pre-treatment surface conditioning, customized electrolyte formulations with organic acid blends, and computer-controlled pulse electropolishing parameters. This approach allows for selective material removal at the microscale level, effectively eliminating surface irregularities while maintaining critical design features. Abbott's technology has demonstrated the ability to achieve surface roughness values below 0.1μm, significantly reducing thrombogenicity and improving hemocompatibility of their implants. Their electropolishing systems incorporate real-time monitoring and feedback controls to ensure consistent results across different implant designs and materials, including their cobalt-chromium and platinum-chromium alloy platforms.
Strengths: Superior surface finish quality with documented reduction in thrombogenicity; highly automated process with excellent reproducibility; integrated quality control systems. Weaknesses: Process requires specialized equipment with high capital costs; limited flexibility for processing diverse implant geometries; relatively slow processing times compared to mechanical polishing methods.
Boston Scientific Scimed, Inc.
Technical Solution: Boston Scientific Scimed has developed sophisticated electropolishing technologies specifically optimized for vascular stents and other minimally invasive medical devices. Their "Precision Electro-Finish" system employs a combination of proprietary electrolyte formulations and precisely controlled electrical parameters to achieve exceptional surface finishes on nitinol and stainless steel components. The company utilizes a multi-stage process that includes pre-electropolishing surface preparation, computer-controlled electropolishing with variable current densities, and post-processing passivation treatments. Boston Scientific's technology incorporates advanced cathode designs that create optimized electric field distributions around complex implant geometries, ensuring uniform material removal even in hard-to-reach areas. Their system includes specialized fixturing that maintains consistent electrical contact while preventing damage to delicate features. The company has also developed in-line quality control methods using optical profilometry and electrochemical testing to verify surface characteristics in real-time. Boston Scientific's electropolishing technology has been documented to reduce surface roughness to below 0.08μm while simultaneously enhancing corrosion resistance and fatigue properties of their implantable devices.
Strengths: Exceptional surface finish quality particularly suited for vascular applications; excellent process consistency with sophisticated monitoring systems; enhanced corrosion resistance properties. Weaknesses: Process optimization required for each new device design; relatively high operational costs; limited throughput for high-volume production.
Key Patent Analysis in Electropolishing Technologies
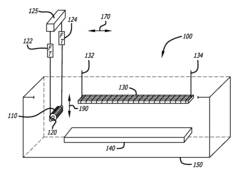
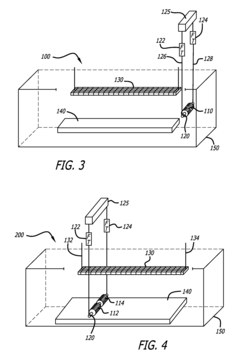
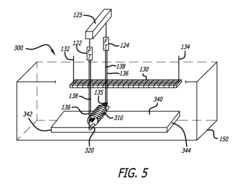
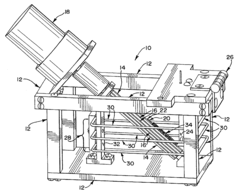
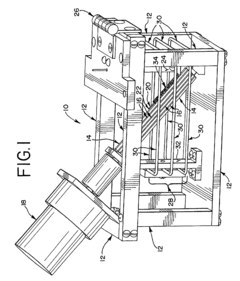
Automated electropolishing process
PatentInactiveUS20130008780A1
Innovation
- An automated electropolishing system with a reservoir, cathode subassembly, roller plate, and anode configuration, utilizing a positioning subassembly with force transducers and various mandrel designs for precise control and contact points to reduce handling and enhance uniformity, allowing continuous motion and polishing without manual intervention.
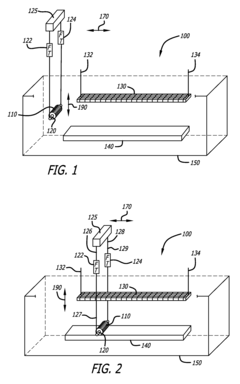
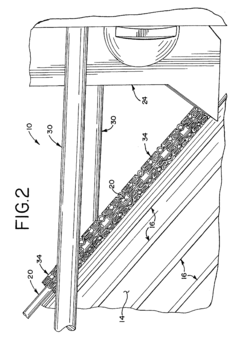
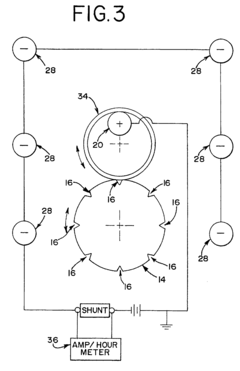
Method of electropolishing medical implants
PatentInactiveUS7799201B2
Innovation
- A method and apparatus that continuously rotate the stent during electropolishing, changing the electrical contact between the anode and cathode, and utilize a small diameter platinum wire anode and multiple cathode loops to ensure uniform current distribution and fluid circulation, minimizing marks and achieving consistent metal removal across the stent surface.
Regulatory Framework for Medical Implant Finishing
The regulatory landscape governing medical implant finishing processes, particularly electropolishing technologies, is complex and multifaceted. The FDA (Food and Drug Administration) in the United States maintains stringent requirements through its 510(k) and Premarket Approval (PMA) pathways, which specifically address surface treatment processes including electropolishing. These regulations emphasize the importance of surface finish quality in preventing corrosion, reducing bacterial adhesion, and ensuring biocompatibility.
In the European Union, the Medical Device Regulation (MDR 2017/745) has significantly strengthened requirements for surface treatments of implantable devices. The regulation specifically addresses electropolishing processes under Annex I, which covers general safety and performance requirements. Manufacturers must demonstrate that their electropolishing methods produce consistent results that meet predetermined specifications for surface roughness and chemical composition.
ISO 10993 series standards play a crucial role globally in evaluating the biocompatibility of medical devices. Particularly relevant to electropolishing are ISO 10993-1 (evaluation and testing) and ISO 10993-15 (identification and quantification of degradation products from metals and alloys). These standards require manufacturers to assess how electropolishing affects material leaching and corrosion resistance.
ASTM F86 specifically addresses surface preparation and marking of metallic surgical implants, providing guidelines for electropolishing processes. This standard details acceptable parameters for current density, electrolyte composition, and process duration to achieve optimal surface finishes for different implant materials.
Regulatory bodies increasingly focus on process validation rather than just final product testing. For electropolishing technologies, this means manufacturers must implement robust quality management systems that document process parameters, verify consistency, and demonstrate correlation between process controls and final surface characteristics. The FDA's Quality System Regulation (21 CFR Part 820) and ISO 13485 both emphasize this approach.
Patent considerations intersect with regulatory requirements, as novel electropolishing methods must not only meet technical specifications but also comply with increasingly stringent regulatory standards. This has led to a trend in patent applications that specifically address regulatory compliance features within electropolishing technology innovations.
Recent regulatory developments show an increasing focus on patient-specific risk assessment for surface treatments. Regulators now expect manufacturers to demonstrate how their electropolishing processes mitigate specific risks associated with different implant types and placement locations, moving beyond one-size-fits-all approaches to surface finishing requirements.
In the European Union, the Medical Device Regulation (MDR 2017/745) has significantly strengthened requirements for surface treatments of implantable devices. The regulation specifically addresses electropolishing processes under Annex I, which covers general safety and performance requirements. Manufacturers must demonstrate that their electropolishing methods produce consistent results that meet predetermined specifications for surface roughness and chemical composition.
ISO 10993 series standards play a crucial role globally in evaluating the biocompatibility of medical devices. Particularly relevant to electropolishing are ISO 10993-1 (evaluation and testing) and ISO 10993-15 (identification and quantification of degradation products from metals and alloys). These standards require manufacturers to assess how electropolishing affects material leaching and corrosion resistance.
ASTM F86 specifically addresses surface preparation and marking of metallic surgical implants, providing guidelines for electropolishing processes. This standard details acceptable parameters for current density, electrolyte composition, and process duration to achieve optimal surface finishes for different implant materials.
Regulatory bodies increasingly focus on process validation rather than just final product testing. For electropolishing technologies, this means manufacturers must implement robust quality management systems that document process parameters, verify consistency, and demonstrate correlation between process controls and final surface characteristics. The FDA's Quality System Regulation (21 CFR Part 820) and ISO 13485 both emphasize this approach.
Patent considerations intersect with regulatory requirements, as novel electropolishing methods must not only meet technical specifications but also comply with increasingly stringent regulatory standards. This has led to a trend in patent applications that specifically address regulatory compliance features within electropolishing technology innovations.
Recent regulatory developments show an increasing focus on patient-specific risk assessment for surface treatments. Regulators now expect manufacturers to demonstrate how their electropolishing processes mitigate specific risks associated with different implant types and placement locations, moving beyond one-size-fits-all approaches to surface finishing requirements.
Biocompatibility Considerations in Electropolishing
Biocompatibility represents a critical factor in the electropolishing of medical implants, as these devices must integrate seamlessly with biological tissues without triggering adverse reactions. The electropolishing process significantly enhances biocompatibility by creating an ultra-smooth surface that minimizes protein adsorption and bacterial adhesion, two primary factors in implant rejection and infection.
The electropolished surface demonstrates superior corrosion resistance compared to mechanically polished alternatives, particularly for implants manufactured from stainless steel and titanium alloys. This resistance is attributed to the formation of a chromium-rich passive layer during the electropolishing process, which acts as a protective barrier against bodily fluids and prevents the release of potentially toxic metal ions into surrounding tissues.
Patent analysis reveals a significant focus on developing electropolishing techniques that specifically enhance biocompatibility parameters. Notable innovations include methods for creating nanoscale surface topographies that promote selective cell adhesion while inhibiting bacterial colonization. These advanced techniques represent a departure from traditional electropolishing approaches that primarily focused on aesthetic improvements rather than biological interactions.
Recent patent filings demonstrate increasing attention to the removal of embedded particles and contaminants during electropolishing, as these can compromise biocompatibility even when surfaces appear visually smooth. Several proprietary electrolyte formulations have been developed specifically to address this challenge, with compositions designed to dissolve or dislodge particulate matter without compromising the base material properties.
The biocompatibility advantages of electropolished surfaces extend beyond reduced infection risk to include improved osseointegration for orthopedic implants. Patents in this domain describe specialized electropolishing protocols that create controlled surface roughness at the microscale while maintaining nanoscale smoothness, creating an optimal environment for bone cell attachment and proliferation.
Regulatory considerations have significantly influenced patent activity in this field, with numerous innovations addressing specific biocompatibility testing requirements established by FDA and ISO standards. These patents often detail methods for achieving consistent surface characteristics that can be validated through standardized biological response testing, reflecting the increasing importance of regulatory compliance in medical device manufacturing.
The electropolished surface demonstrates superior corrosion resistance compared to mechanically polished alternatives, particularly for implants manufactured from stainless steel and titanium alloys. This resistance is attributed to the formation of a chromium-rich passive layer during the electropolishing process, which acts as a protective barrier against bodily fluids and prevents the release of potentially toxic metal ions into surrounding tissues.
Patent analysis reveals a significant focus on developing electropolishing techniques that specifically enhance biocompatibility parameters. Notable innovations include methods for creating nanoscale surface topographies that promote selective cell adhesion while inhibiting bacterial colonization. These advanced techniques represent a departure from traditional electropolishing approaches that primarily focused on aesthetic improvements rather than biological interactions.
Recent patent filings demonstrate increasing attention to the removal of embedded particles and contaminants during electropolishing, as these can compromise biocompatibility even when surfaces appear visually smooth. Several proprietary electrolyte formulations have been developed specifically to address this challenge, with compositions designed to dissolve or dislodge particulate matter without compromising the base material properties.
The biocompatibility advantages of electropolished surfaces extend beyond reduced infection risk to include improved osseointegration for orthopedic implants. Patents in this domain describe specialized electropolishing protocols that create controlled surface roughness at the microscale while maintaining nanoscale smoothness, creating an optimal environment for bone cell attachment and proliferation.
Regulatory considerations have significantly influenced patent activity in this field, with numerous innovations addressing specific biocompatibility testing requirements established by FDA and ISO standards. These patents often detail methods for achieving consistent surface characteristics that can be validated through standardized biological response testing, reflecting the increasing importance of regulatory compliance in medical device manufacturing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!