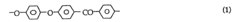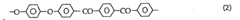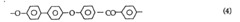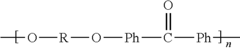How Muriatic Acid is Used in the Manufacture of Polyetheretherketone (PEEK)
JUL 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEEK Synthesis Background and Objectives
Polyetheretherketone (PEEK) synthesis has a rich history dating back to the late 1970s when it was first developed by Imperial Chemical Industries (ICI). The primary objective of PEEK synthesis is to create a high-performance thermoplastic polymer with exceptional mechanical, thermal, and chemical resistance properties. This advanced material has found widespread applications in various industries, including aerospace, automotive, and medical sectors.
The evolution of PEEK synthesis techniques has been driven by the need for more efficient and cost-effective production methods. Initially, PEEK was synthesized using a step-growth polymerization process, which involved the reaction of aromatic dihalides with aromatic diphenols in the presence of a base. However, this method had limitations in terms of molecular weight control and production scale.
As research progressed, new synthesis routes were explored to overcome these challenges. One significant development was the nucleophilic aromatic substitution reaction, which allowed for better control over the polymer's molecular weight and improved scalability. This method typically involves the reaction of 4,4'-difluorobenzophenone with hydroquinone in the presence of a base, such as potassium carbonate.
The role of muriatic acid, also known as hydrochloric acid, in PEEK synthesis has become increasingly important. While not directly involved in the polymerization reaction, muriatic acid plays a crucial role in the purification and post-processing stages of PEEK production. It is used to neutralize excess base, remove impurities, and adjust the pH of the reaction mixture, ensuring the final product meets stringent quality standards.
The primary technical objectives in PEEK synthesis include achieving high molecular weight, maintaining consistent polymer properties, and minimizing the formation of unwanted by-products. Researchers and manufacturers continually strive to optimize reaction conditions, such as temperature, pressure, and catalyst systems, to enhance the efficiency and quality of PEEK production.
Recent advancements in PEEK synthesis have focused on developing more environmentally friendly processes, reducing energy consumption, and exploring alternative raw materials. These efforts align with the growing emphasis on sustainable manufacturing practices and the circular economy concept in the polymer industry.
As PEEK continues to gain prominence in high-performance applications, the synthesis techniques are expected to evolve further. Future research directions may include the development of novel catalysts, the exploration of continuous flow reactors for improved process control, and the integration of advanced monitoring technologies to ensure consistent product quality.
The evolution of PEEK synthesis techniques has been driven by the need for more efficient and cost-effective production methods. Initially, PEEK was synthesized using a step-growth polymerization process, which involved the reaction of aromatic dihalides with aromatic diphenols in the presence of a base. However, this method had limitations in terms of molecular weight control and production scale.
As research progressed, new synthesis routes were explored to overcome these challenges. One significant development was the nucleophilic aromatic substitution reaction, which allowed for better control over the polymer's molecular weight and improved scalability. This method typically involves the reaction of 4,4'-difluorobenzophenone with hydroquinone in the presence of a base, such as potassium carbonate.
The role of muriatic acid, also known as hydrochloric acid, in PEEK synthesis has become increasingly important. While not directly involved in the polymerization reaction, muriatic acid plays a crucial role in the purification and post-processing stages of PEEK production. It is used to neutralize excess base, remove impurities, and adjust the pH of the reaction mixture, ensuring the final product meets stringent quality standards.
The primary technical objectives in PEEK synthesis include achieving high molecular weight, maintaining consistent polymer properties, and minimizing the formation of unwanted by-products. Researchers and manufacturers continually strive to optimize reaction conditions, such as temperature, pressure, and catalyst systems, to enhance the efficiency and quality of PEEK production.
Recent advancements in PEEK synthesis have focused on developing more environmentally friendly processes, reducing energy consumption, and exploring alternative raw materials. These efforts align with the growing emphasis on sustainable manufacturing practices and the circular economy concept in the polymer industry.
As PEEK continues to gain prominence in high-performance applications, the synthesis techniques are expected to evolve further. Future research directions may include the development of novel catalysts, the exploration of continuous flow reactors for improved process control, and the integration of advanced monitoring technologies to ensure consistent product quality.
Market Analysis for PEEK Applications
The market for Polyetheretherketone (PEEK) applications has been experiencing significant growth due to its exceptional properties and versatility across various industries. PEEK's high-performance characteristics, including excellent mechanical strength, chemical resistance, and thermal stability, have positioned it as a preferred material in demanding applications.
In the aerospace sector, PEEK has gained substantial traction for its lightweight nature and ability to withstand extreme temperatures. It is increasingly used in aircraft components, replacing traditional metals and reducing overall weight, which translates to improved fuel efficiency. The automotive industry has also embraced PEEK for similar reasons, utilizing it in engine components, transmission systems, and other high-stress areas.
The medical industry represents another key market for PEEK applications. Its biocompatibility and resistance to sterilization processes make it ideal for implants, surgical instruments, and dental applications. The growing demand for minimally invasive procedures and long-lasting medical devices has further boosted PEEK's adoption in this sector.
In the electronics industry, PEEK finds applications in semiconductor manufacturing equipment, connectors, and insulation components. Its excellent electrical properties and resistance to high temperatures make it suitable for use in harsh environments and miniaturized electronic devices.
The oil and gas industry has also recognized PEEK's potential, particularly in offshore and subsea applications. Its resistance to corrosion, high pressures, and extreme temperatures makes it valuable for seals, bearings, and other critical components in oil extraction and processing equipment.
The global PEEK market size was valued at several billion dollars in recent years, with projections indicating continued growth. The Asia-Pacific region, particularly China and India, is expected to witness the highest growth rate due to rapid industrialization and increasing adoption of high-performance polymers in manufacturing processes.
However, the high cost of PEEK compared to conventional polymers remains a challenge for wider adoption in price-sensitive applications. This has led to ongoing research and development efforts to optimize production processes and reduce costs, which could potentially expand PEEK's market reach in the coming years.
As environmental concerns gain prominence, PEEK's recyclability and potential for sustainable production are becoming increasingly important factors in market growth. Industries are exploring ways to incorporate recycled PEEK into new products, aligning with circular economy principles and potentially opening new market opportunities.
In the aerospace sector, PEEK has gained substantial traction for its lightweight nature and ability to withstand extreme temperatures. It is increasingly used in aircraft components, replacing traditional metals and reducing overall weight, which translates to improved fuel efficiency. The automotive industry has also embraced PEEK for similar reasons, utilizing it in engine components, transmission systems, and other high-stress areas.
The medical industry represents another key market for PEEK applications. Its biocompatibility and resistance to sterilization processes make it ideal for implants, surgical instruments, and dental applications. The growing demand for minimally invasive procedures and long-lasting medical devices has further boosted PEEK's adoption in this sector.
In the electronics industry, PEEK finds applications in semiconductor manufacturing equipment, connectors, and insulation components. Its excellent electrical properties and resistance to high temperatures make it suitable for use in harsh environments and miniaturized electronic devices.
The oil and gas industry has also recognized PEEK's potential, particularly in offshore and subsea applications. Its resistance to corrosion, high pressures, and extreme temperatures makes it valuable for seals, bearings, and other critical components in oil extraction and processing equipment.
The global PEEK market size was valued at several billion dollars in recent years, with projections indicating continued growth. The Asia-Pacific region, particularly China and India, is expected to witness the highest growth rate due to rapid industrialization and increasing adoption of high-performance polymers in manufacturing processes.
However, the high cost of PEEK compared to conventional polymers remains a challenge for wider adoption in price-sensitive applications. This has led to ongoing research and development efforts to optimize production processes and reduce costs, which could potentially expand PEEK's market reach in the coming years.
As environmental concerns gain prominence, PEEK's recyclability and potential for sustainable production are becoming increasingly important factors in market growth. Industries are exploring ways to incorporate recycled PEEK into new products, aligning with circular economy principles and potentially opening new market opportunities.
Current Challenges in PEEK Manufacturing
The manufacture of Polyetheretherketone (PEEK) faces several significant challenges, primarily due to the complexity of the production process and the stringent quality requirements of this high-performance polymer. One of the main hurdles is the precise control of the polymerization reaction, which is critical for achieving the desired molecular weight and properties of PEEK.
The use of muriatic acid (hydrochloric acid) in PEEK production introduces additional complexities. While it plays a crucial role in the synthesis process, managing its corrosive nature and ensuring worker safety remain ongoing concerns. The acid's interaction with other reactants and catalysts must be carefully monitored to prevent unwanted side reactions that could compromise the final product's quality.
Another significant challenge lies in the purification of PEEK after synthesis. Residual acid and other impurities must be thoroughly removed to meet the high purity standards required for applications in aerospace, medical, and automotive industries. This purification process is often time-consuming and energy-intensive, contributing to the overall cost of PEEK production.
The high processing temperatures required for PEEK manufacturing also present technical difficulties. Maintaining consistent heat distribution throughout the reaction vessel and subsequent processing stages is crucial for ensuring uniform polymer properties. Any temperature fluctuations can lead to variations in crystallinity, affecting the mechanical and thermal properties of the final product.
Environmental concerns associated with PEEK production are becoming increasingly prominent. The use of muriatic acid and other chemicals necessitates stringent waste management protocols. Developing more environmentally friendly production methods, including the potential recycling of acid and other reagents, is an ongoing challenge for manufacturers.
Scale-up of PEEK production from laboratory to industrial scale presents its own set of challenges. Maintaining reaction kinetics and product quality while increasing batch sizes requires significant engineering expertise. The design of large-scale reactors that can withstand the corrosive environment and high temperatures is a complex task that demands continuous innovation.
Lastly, the high cost of PEEK production remains a significant barrier to its wider adoption in various industries. The expensive raw materials, energy-intensive processes, and specialized equipment all contribute to the elevated price point of PEEK. Finding ways to optimize the production process and reduce costs without compromising quality is a persistent challenge for manufacturers in this field.
The use of muriatic acid (hydrochloric acid) in PEEK production introduces additional complexities. While it plays a crucial role in the synthesis process, managing its corrosive nature and ensuring worker safety remain ongoing concerns. The acid's interaction with other reactants and catalysts must be carefully monitored to prevent unwanted side reactions that could compromise the final product's quality.
Another significant challenge lies in the purification of PEEK after synthesis. Residual acid and other impurities must be thoroughly removed to meet the high purity standards required for applications in aerospace, medical, and automotive industries. This purification process is often time-consuming and energy-intensive, contributing to the overall cost of PEEK production.
The high processing temperatures required for PEEK manufacturing also present technical difficulties. Maintaining consistent heat distribution throughout the reaction vessel and subsequent processing stages is crucial for ensuring uniform polymer properties. Any temperature fluctuations can lead to variations in crystallinity, affecting the mechanical and thermal properties of the final product.
Environmental concerns associated with PEEK production are becoming increasingly prominent. The use of muriatic acid and other chemicals necessitates stringent waste management protocols. Developing more environmentally friendly production methods, including the potential recycling of acid and other reagents, is an ongoing challenge for manufacturers.
Scale-up of PEEK production from laboratory to industrial scale presents its own set of challenges. Maintaining reaction kinetics and product quality while increasing batch sizes requires significant engineering expertise. The design of large-scale reactors that can withstand the corrosive environment and high temperatures is a complex task that demands continuous innovation.
Lastly, the high cost of PEEK production remains a significant barrier to its wider adoption in various industries. The expensive raw materials, energy-intensive processes, and specialized equipment all contribute to the elevated price point of PEEK. Finding ways to optimize the production process and reduce costs without compromising quality is a persistent challenge for manufacturers in this field.
Muriatic Acid Role in PEEK Synthesis
01 Industrial applications of muriatic acid
Muriatic acid, also known as hydrochloric acid, has various industrial applications. It is used in metal cleaning and pickling processes, particularly in the steel industry. The acid is also employed in the production of chemicals, water treatment, and as a pH regulator in various industrial processes.- Production and purification of muriatic acid: Muriatic acid, also known as hydrochloric acid, can be produced and purified through various industrial processes. These methods often involve the reaction of chlorine with hydrogen or the treatment of chloride salts with sulfuric acid. Purification techniques may include distillation or membrane separation to remove impurities and achieve desired concentrations.
- Applications in metal treatment and surface cleaning: Muriatic acid is widely used in metal treatment processes, such as pickling, etching, and surface cleaning. It effectively removes rust, scale, and other contaminants from metal surfaces, preparing them for further processing or coating. The acid's strong reactivity makes it suitable for various industrial cleaning applications.
- Use in construction and building materials: In the construction industry, muriatic acid is utilized for cleaning masonry, concrete, and other building materials. It can remove efflorescence, mortar residues, and stains from surfaces. Additionally, it is used in the production of certain construction materials and for adjusting the pH of cement mixtures.
- Environmental and safety considerations: Handling and disposal of muriatic acid require careful consideration of environmental and safety factors. Proper storage, transportation, and neutralization methods are essential to prevent accidents and minimize environmental impact. Specialized equipment and procedures are often employed to ensure safe handling and use of the acid in various applications.
- Alternative formulations and substitutes: Research has been conducted to develop alternative formulations or substitutes for muriatic acid in certain applications. These alternatives aim to reduce the environmental impact, improve safety, or enhance performance in specific use cases. Some formulations may include additives or modified chemical compositions to achieve desired properties while mitigating the drawbacks of traditional muriatic acid.
02 Cleaning and etching applications
Muriatic acid is widely used in cleaning and etching applications. It is effective in removing rust, scale, and other deposits from metal surfaces. In the construction industry, it is used for cleaning masonry and concrete surfaces. The acid is also utilized in pool maintenance to balance pH levels and remove stains.Expand Specific Solutions03 Production and handling of muriatic acid
The production of muriatic acid involves various processes, including the reaction of sodium chloride with sulfuric acid. Specialized equipment and safety measures are required for its production, storage, and handling due to its corrosive nature. Innovations in production methods focus on improving efficiency and reducing environmental impact.Expand Specific Solutions04 Environmental and safety considerations
Due to its corrosive nature, the use of muriatic acid requires strict safety protocols and environmental considerations. This includes proper storage, handling, and disposal methods to prevent accidents and environmental contamination. Innovations in this area focus on developing safer formulations and improved containment systems.Expand Specific Solutions05 Alternative applications and formulations
Research is ongoing to develop alternative applications and formulations of muriatic acid. This includes its use in specialized chemical processes, such as the production of chlorine dioxide for water treatment. Additionally, efforts are being made to create less hazardous formulations that maintain the acid's effectiveness while reducing its corrosive properties.Expand Specific Solutions
Key Players in PEEK Industry
The competitive landscape for the manufacture of Polyetheretherketone (PEEK) using muriatic acid is characterized by a mature market with established players and growing demand. The global PEEK market is projected to reach $1 billion by 2025, driven by increasing applications in aerospace, automotive, and medical industries. Key players like Victrex Manufacturing, Solvay Specialty Polymers, and Evonik Industries dominate the market with advanced manufacturing capabilities. Emerging companies such as Jilin Joinature Polymer and DiFusion Technologies are focusing on innovative PEEK formulations for specific applications. The technology is well-established, but ongoing research at institutions like Sichuan University and Changsha University of Science & Technology aims to improve production efficiency and explore new applications.
Victrex Manufacturing Ltd.
Technical Solution: Victrex utilizes a proprietary synthesis process for PEEK production involving the reaction of hydroquinone with 4,4'-difluorobenzophenone in the presence of diphenyl sulphone as a solvent and potassium carbonate as a base[1]. Muriatic acid (hydrochloric acid) is used in the post-polymerization process to neutralize the alkaline reaction mixture and precipitate the PEEK polymer[2]. The company employs a continuous polymerization process, allowing for precise control of molecular weight and consistent product quality[3]. Victrex has also developed advanced purification techniques to remove trace impurities, resulting in high-performance PEEK grades suitable for demanding applications in aerospace and medical industries[4].
Strengths: Proprietary synthesis process, precise molecular weight control, and advanced purification techniques. Weaknesses: Potential environmental concerns due to the use of strong acids and the need for careful waste management.
Solvay Specialty Polymers USA LLC
Technical Solution: Solvay's PEEK manufacturing process involves a nucleophilic aromatic substitution reaction between hydroquinone and 4,4'-difluorobenzophenone[5]. Muriatic acid is used in the polymer isolation stage to neutralize the reaction mixture and precipitate the PEEK polymer[6]. The company has developed a unique continuous polymerization technology that allows for the production of high molecular weight PEEK with excellent thermal stability[7]. Solvay has also implemented a solvent recovery system to minimize environmental impact and improve process efficiency[8]. Their PEEK grades are tailored for specific applications, including oil and gas, automotive, and electronics industries[9].
Strengths: Continuous polymerization technology, solvent recovery system, and application-specific PEEK grades. Weaknesses: High energy consumption in the polymerization process and potential for by-product formation.
Critical Patents in PEEK Manufacturing
Process for preparing polyether ether ketone membrane
PatentInactiveEP0796649B1
Innovation
- A process using concentrated sulfuric acid to dissolve PEEK, maintaining the solution at low temperatures, and employing a coagulation liquid to form a non-sulfonated PEEK membrane with added thickening agents like polyvinylpyrrolidone, followed by heat treatment to enhance crystallinity and stability.
Process for preparing a polyether ether ketone
PatentActiveUS20110218315A1
Innovation
- A method utilizing Na2CO3 as the only condensing agent, with step-by-step addition of reactants, including pre-dried anhydrous sodium carbonate and hydroquinone, to control viscosity and enhance molecular weight distribution, resulting in a PEEK product with high molecular weight and narrow distribution.
Environmental Impact of PEEK Synthesis
The synthesis of Polyetheretherketone (PEEK) involves several chemical processes that can have significant environmental implications. The use of muriatic acid, also known as hydrochloric acid, in PEEK production contributes to these environmental concerns. One of the primary issues is the potential for acid emissions during the manufacturing process. These emissions can lead to air pollution and contribute to acid rain formation if not properly controlled and treated.
Water pollution is another critical environmental concern associated with PEEK synthesis. The production process generates wastewater containing acidic residues and other chemical byproducts. If not adequately treated before discharge, this wastewater can harm aquatic ecosystems and contaminate water sources. Proper wastewater treatment facilities are essential to mitigate these risks and ensure compliance with environmental regulations.
The production of PEEK also involves the use of various organic solvents and reagents, which can contribute to volatile organic compound (VOC) emissions. These emissions not only pose potential health risks to workers but also contribute to the formation of ground-level ozone and smog when released into the atmosphere. Implementing effective emission control technologies and adopting best practices in chemical handling are crucial for minimizing these environmental impacts.
Energy consumption is a significant factor in the environmental footprint of PEEK synthesis. The high-temperature processes required for polymerization and the energy-intensive nature of chemical production contribute to greenhouse gas emissions. Improving energy efficiency in manufacturing processes and exploring renewable energy sources can help reduce the carbon footprint associated with PEEK production.
Waste management is another critical aspect of the environmental impact of PEEK synthesis. The production process generates various solid and liquid wastes, including unreacted monomers, catalysts, and other chemical residues. Proper disposal and recycling of these wastes are essential to prevent soil and groundwater contamination. Additionally, implementing circular economy principles in PEEK production can help minimize waste generation and improve resource efficiency.
The long-term environmental persistence of PEEK is also a consideration. While PEEK is known for its durability and chemical resistance, these properties also make it challenging to degrade naturally in the environment. Developing sustainable end-of-life solutions for PEEK products, such as recycling technologies or biodegradable alternatives, is crucial for reducing the long-term environmental impact of this material.
Water pollution is another critical environmental concern associated with PEEK synthesis. The production process generates wastewater containing acidic residues and other chemical byproducts. If not adequately treated before discharge, this wastewater can harm aquatic ecosystems and contaminate water sources. Proper wastewater treatment facilities are essential to mitigate these risks and ensure compliance with environmental regulations.
The production of PEEK also involves the use of various organic solvents and reagents, which can contribute to volatile organic compound (VOC) emissions. These emissions not only pose potential health risks to workers but also contribute to the formation of ground-level ozone and smog when released into the atmosphere. Implementing effective emission control technologies and adopting best practices in chemical handling are crucial for minimizing these environmental impacts.
Energy consumption is a significant factor in the environmental footprint of PEEK synthesis. The high-temperature processes required for polymerization and the energy-intensive nature of chemical production contribute to greenhouse gas emissions. Improving energy efficiency in manufacturing processes and exploring renewable energy sources can help reduce the carbon footprint associated with PEEK production.
Waste management is another critical aspect of the environmental impact of PEEK synthesis. The production process generates various solid and liquid wastes, including unreacted monomers, catalysts, and other chemical residues. Proper disposal and recycling of these wastes are essential to prevent soil and groundwater contamination. Additionally, implementing circular economy principles in PEEK production can help minimize waste generation and improve resource efficiency.
The long-term environmental persistence of PEEK is also a consideration. While PEEK is known for its durability and chemical resistance, these properties also make it challenging to degrade naturally in the environment. Developing sustainable end-of-life solutions for PEEK products, such as recycling technologies or biodegradable alternatives, is crucial for reducing the long-term environmental impact of this material.
Regulatory Framework for PEEK Manufacturing
The regulatory framework for PEEK manufacturing is a complex and multifaceted system designed to ensure the safety, quality, and environmental compliance of the production process. At the core of this framework are the regulations set forth by governmental agencies such as the U.S. Food and Drug Administration (FDA) and the Environmental Protection Agency (EPA). These agencies establish guidelines for the use of chemicals, including muriatic acid, in industrial processes.
For PEEK manufacturing, compliance with Good Manufacturing Practices (GMP) is essential. GMP guidelines cover all aspects of production, from raw materials to equipment, facilities, and staff training. Manufacturers must demonstrate adherence to these practices through rigorous documentation and quality control measures. The use of muriatic acid in PEEK production falls under these regulations, requiring careful handling, storage, and disposal protocols.
Environmental regulations play a significant role in the regulatory framework. The Clean Air Act and Clean Water Act in the United States, along with similar legislation in other countries, set strict limits on emissions and effluents from manufacturing processes. PEEK producers must implement pollution control measures and obtain necessary permits to operate within these environmental constraints.
Occupational health and safety regulations are another critical component of the framework. Organizations such as the Occupational Safety and Health Administration (OSHA) in the U.S. mandate specific safety protocols for handling hazardous chemicals like muriatic acid. This includes requirements for personal protective equipment, emergency response procedures, and employee training programs.
International standards and certifications also form part of the regulatory landscape. ISO 9001 for quality management systems and ISO 14001 for environmental management systems are often required or strongly recommended for PEEK manufacturers. These standards ensure consistent quality and environmental performance across global supply chains.
Product-specific regulations are particularly relevant for PEEK, given its applications in critical industries such as aerospace and medical devices. For instance, PEEK components used in medical implants must meet biocompatibility standards set by ISO 10993 and undergo rigorous testing and approval processes by regulatory bodies like the FDA.
The transportation of raw materials and finished PEEK products is subject to hazardous materials transportation regulations. These rules govern packaging, labeling, and shipping procedures to minimize risks during transit. Compliance with these regulations is crucial for maintaining supply chain integrity and safety.
As the industry evolves, regulatory frameworks continue to adapt. Emerging concerns, such as the environmental impact of microplastics and the push for more sustainable manufacturing processes, are likely to influence future regulations. PEEK manufacturers must stay informed of these developments and proactively adjust their practices to maintain compliance and competitive advantage in a rapidly changing regulatory landscape.
For PEEK manufacturing, compliance with Good Manufacturing Practices (GMP) is essential. GMP guidelines cover all aspects of production, from raw materials to equipment, facilities, and staff training. Manufacturers must demonstrate adherence to these practices through rigorous documentation and quality control measures. The use of muriatic acid in PEEK production falls under these regulations, requiring careful handling, storage, and disposal protocols.
Environmental regulations play a significant role in the regulatory framework. The Clean Air Act and Clean Water Act in the United States, along with similar legislation in other countries, set strict limits on emissions and effluents from manufacturing processes. PEEK producers must implement pollution control measures and obtain necessary permits to operate within these environmental constraints.
Occupational health and safety regulations are another critical component of the framework. Organizations such as the Occupational Safety and Health Administration (OSHA) in the U.S. mandate specific safety protocols for handling hazardous chemicals like muriatic acid. This includes requirements for personal protective equipment, emergency response procedures, and employee training programs.
International standards and certifications also form part of the regulatory landscape. ISO 9001 for quality management systems and ISO 14001 for environmental management systems are often required or strongly recommended for PEEK manufacturers. These standards ensure consistent quality and environmental performance across global supply chains.
Product-specific regulations are particularly relevant for PEEK, given its applications in critical industries such as aerospace and medical devices. For instance, PEEK components used in medical implants must meet biocompatibility standards set by ISO 10993 and undergo rigorous testing and approval processes by regulatory bodies like the FDA.
The transportation of raw materials and finished PEEK products is subject to hazardous materials transportation regulations. These rules govern packaging, labeling, and shipping procedures to minimize risks during transit. Compliance with these regulations is crucial for maintaining supply chain integrity and safety.
As the industry evolves, regulatory frameworks continue to adapt. Emerging concerns, such as the environmental impact of microplastics and the push for more sustainable manufacturing processes, are likely to influence future regulations. PEEK manufacturers must stay informed of these developments and proactively adjust their practices to maintain compliance and competitive advantage in a rapidly changing regulatory landscape.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!