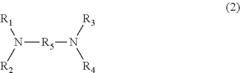How Muriatic Acid is Used in the Manufacture of Polyphenylene Ether (PPE)
JUL 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PPE Manufacturing Background and Objectives
Polyphenylene Ether (PPE) is a high-performance engineering thermoplastic that has gained significant importance in various industries due to its exceptional properties. The development of PPE can be traced back to the 1950s when researchers at General Electric Company first synthesized this polymer. Since then, PPE has undergone continuous improvements in its manufacturing processes and applications.
The primary objective of PPE manufacturing is to produce a polymer with excellent thermal stability, dimensional stability, and electrical insulation properties. These characteristics make PPE ideal for use in automotive, electronics, and appliance industries. The evolution of PPE technology has been driven by the increasing demand for lightweight, durable, and heat-resistant materials in these sectors.
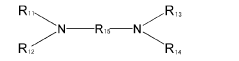
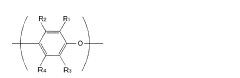
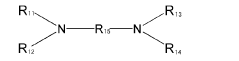
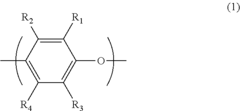
In the manufacturing process of PPE, muriatic acid, also known as hydrochloric acid, plays a crucial role. The use of muriatic acid in PPE production is primarily related to the oxidative coupling polymerization of 2,6-dimethylphenol, which is the key reaction in PPE synthesis. This process involves the formation of carbon-oxygen bonds between phenol molecules, resulting in the creation of the polymer chain.
The technological trend in PPE manufacturing has been focused on improving the efficiency and sustainability of the production process. This includes developing more environmentally friendly catalysts, optimizing reaction conditions, and enhancing the overall yield of the polymer. Additionally, there has been a growing emphasis on creating PPE blends and composites to further expand its application range and improve specific properties.
One of the main challenges in PPE manufacturing is controlling the molecular weight distribution of the polymer. This is where muriatic acid comes into play, as it can be used to regulate the polymerization reaction and influence the final properties of the PPE. By carefully adjusting the concentration and timing of muriatic acid addition, manufacturers can achieve the desired molecular weight and polydispersity index.
The expected technological goals in PPE manufacturing include developing more efficient and sustainable production methods, improving the polymer's performance characteristics, and expanding its applicability in emerging industries such as renewable energy and advanced electronics. Researchers are also exploring ways to enhance the recyclability of PPE-based products, aligning with the growing focus on circular economy principles in the plastics industry.
As the demand for high-performance polymers continues to rise, the importance of understanding and optimizing the role of muriatic acid in PPE manufacturing becomes increasingly critical. This understanding is essential for achieving the desired material properties and meeting the evolving needs of various industrial sectors that rely on PPE's unique combination of thermal, electrical, and mechanical properties.
The primary objective of PPE manufacturing is to produce a polymer with excellent thermal stability, dimensional stability, and electrical insulation properties. These characteristics make PPE ideal for use in automotive, electronics, and appliance industries. The evolution of PPE technology has been driven by the increasing demand for lightweight, durable, and heat-resistant materials in these sectors.
In the manufacturing process of PPE, muriatic acid, also known as hydrochloric acid, plays a crucial role. The use of muriatic acid in PPE production is primarily related to the oxidative coupling polymerization of 2,6-dimethylphenol, which is the key reaction in PPE synthesis. This process involves the formation of carbon-oxygen bonds between phenol molecules, resulting in the creation of the polymer chain.
The technological trend in PPE manufacturing has been focused on improving the efficiency and sustainability of the production process. This includes developing more environmentally friendly catalysts, optimizing reaction conditions, and enhancing the overall yield of the polymer. Additionally, there has been a growing emphasis on creating PPE blends and composites to further expand its application range and improve specific properties.
One of the main challenges in PPE manufacturing is controlling the molecular weight distribution of the polymer. This is where muriatic acid comes into play, as it can be used to regulate the polymerization reaction and influence the final properties of the PPE. By carefully adjusting the concentration and timing of muriatic acid addition, manufacturers can achieve the desired molecular weight and polydispersity index.
The expected technological goals in PPE manufacturing include developing more efficient and sustainable production methods, improving the polymer's performance characteristics, and expanding its applicability in emerging industries such as renewable energy and advanced electronics. Researchers are also exploring ways to enhance the recyclability of PPE-based products, aligning with the growing focus on circular economy principles in the plastics industry.
As the demand for high-performance polymers continues to rise, the importance of understanding and optimizing the role of muriatic acid in PPE manufacturing becomes increasingly critical. This understanding is essential for achieving the desired material properties and meeting the evolving needs of various industrial sectors that rely on PPE's unique combination of thermal, electrical, and mechanical properties.
Market Analysis for PPE and Muriatic Acid
The global market for Polyphenylene Ether (PPE) and muriatic acid (hydrochloric acid) is experiencing significant growth driven by various factors. PPE, a high-performance engineering thermoplastic, finds extensive applications in automotive, electrical and electronics, and industrial sectors due to its excellent mechanical properties, heat resistance, and dimensional stability.
The automotive industry remains a key driver for PPE demand, with increasing use in under-hood components, exterior parts, and structural elements. The growing trend towards vehicle electrification and lightweighting further boosts PPE adoption. In the electrical and electronics sector, PPE's dielectric properties and flame retardancy make it ideal for connectors, switches, and circuit boards.
Muriatic acid, a key raw material in PPE production, also sees robust demand across various industries. Its use extends beyond PPE manufacturing to metal treatment, water treatment, and chemical processing. The construction industry's growth, particularly in developing economies, contributes to increased muriatic acid consumption for concrete etching and pH adjustment.
The PPE market is projected to grow steadily, with Asia-Pacific emerging as the fastest-growing region. China and India lead this growth, driven by rapid industrialization and increasing automotive production. North America and Europe maintain stable markets, with a focus on high-performance applications and sustainable solutions.
Muriatic acid market growth closely aligns with industrial production trends. The chemical industry's expansion, especially in emerging economies, fuels demand. Environmental regulations impact market dynamics, promoting the development of safer handling and transportation methods for muriatic acid.
Both markets face challenges from raw material price volatility and environmental concerns. For PPE, the push for recyclability and bio-based alternatives presents both challenges and opportunities. Muriatic acid producers grapple with stringent regulations on production and handling, driving investments in safer technologies.
Innovation in PPE grades, such as improved heat resistance and impact strength, opens new application areas. The development of high-purity grades of muriatic acid caters to specialized industries like semiconductor manufacturing, offering growth potential in niche markets.
The automotive industry remains a key driver for PPE demand, with increasing use in under-hood components, exterior parts, and structural elements. The growing trend towards vehicle electrification and lightweighting further boosts PPE adoption. In the electrical and electronics sector, PPE's dielectric properties and flame retardancy make it ideal for connectors, switches, and circuit boards.
Muriatic acid, a key raw material in PPE production, also sees robust demand across various industries. Its use extends beyond PPE manufacturing to metal treatment, water treatment, and chemical processing. The construction industry's growth, particularly in developing economies, contributes to increased muriatic acid consumption for concrete etching and pH adjustment.
The PPE market is projected to grow steadily, with Asia-Pacific emerging as the fastest-growing region. China and India lead this growth, driven by rapid industrialization and increasing automotive production. North America and Europe maintain stable markets, with a focus on high-performance applications and sustainable solutions.
Muriatic acid market growth closely aligns with industrial production trends. The chemical industry's expansion, especially in emerging economies, fuels demand. Environmental regulations impact market dynamics, promoting the development of safer handling and transportation methods for muriatic acid.
Both markets face challenges from raw material price volatility and environmental concerns. For PPE, the push for recyclability and bio-based alternatives presents both challenges and opportunities. Muriatic acid producers grapple with stringent regulations on production and handling, driving investments in safer technologies.
Innovation in PPE grades, such as improved heat resistance and impact strength, opens new application areas. The development of high-purity grades of muriatic acid caters to specialized industries like semiconductor manufacturing, offering growth potential in niche markets.
Current Challenges in PPE Production
The production of Polyphenylene Ether (PPE) faces several significant challenges that impact its manufacturing efficiency, quality, and environmental sustainability. One of the primary issues is the complexity of the oxidative coupling polymerization process, which requires precise control of reaction conditions to achieve desired molecular weights and properties. This process is highly sensitive to temperature fluctuations, catalyst concentrations, and impurities, making consistent production difficult.
Another major challenge is the use of copper-based catalysts in traditional PPE synthesis. While effective, these catalysts can lead to residual metal contamination in the final product, potentially affecting its performance in high-purity applications. Additionally, the recovery and recycling of these catalysts present both economic and environmental concerns.
The high energy consumption associated with PPE production is a significant hurdle. The polymerization reaction typically requires elevated temperatures, and the subsequent processing steps, including solvent removal and pelletization, are energy-intensive. This not only increases production costs but also contributes to the carbon footprint of PPE manufacturing.
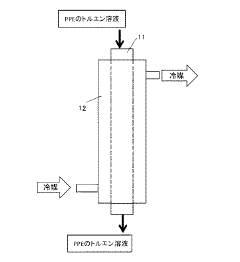
Solvent management poses another challenge in PPE production. The use of organic solvents, such as toluene, in the polymerization process necessitates efficient recovery systems to minimize environmental impact and reduce raw material costs. However, achieving high solvent recovery rates while maintaining product quality remains a technical challenge.
The increasing demand for high-performance PPE grades with specific properties (such as improved heat resistance or flame retardancy) requires continuous innovation in synthesis techniques and additives. Developing these specialized grades while maintaining cost-effectiveness and scalability is an ongoing challenge for manufacturers.
Lastly, the industry faces pressure to improve the sustainability of PPE production. This includes reducing waste generation, minimizing the use of hazardous substances, and exploring bio-based alternatives for raw materials. The transition towards more environmentally friendly production methods, while maintaining the high performance standards of PPE, represents a significant technological and economic challenge for the industry.
Another major challenge is the use of copper-based catalysts in traditional PPE synthesis. While effective, these catalysts can lead to residual metal contamination in the final product, potentially affecting its performance in high-purity applications. Additionally, the recovery and recycling of these catalysts present both economic and environmental concerns.
The high energy consumption associated with PPE production is a significant hurdle. The polymerization reaction typically requires elevated temperatures, and the subsequent processing steps, including solvent removal and pelletization, are energy-intensive. This not only increases production costs but also contributes to the carbon footprint of PPE manufacturing.
Solvent management poses another challenge in PPE production. The use of organic solvents, such as toluene, in the polymerization process necessitates efficient recovery systems to minimize environmental impact and reduce raw material costs. However, achieving high solvent recovery rates while maintaining product quality remains a technical challenge.
The increasing demand for high-performance PPE grades with specific properties (such as improved heat resistance or flame retardancy) requires continuous innovation in synthesis techniques and additives. Developing these specialized grades while maintaining cost-effectiveness and scalability is an ongoing challenge for manufacturers.
Lastly, the industry faces pressure to improve the sustainability of PPE production. This includes reducing waste generation, minimizing the use of hazardous substances, and exploring bio-based alternatives for raw materials. The transition towards more environmentally friendly production methods, while maintaining the high performance standards of PPE, represents a significant technological and economic challenge for the industry.
Muriatic Acid Usage in PPE Synthesis
01 Industrial applications of muriatic acid
Muriatic acid, also known as hydrochloric acid, has various industrial applications. It is used in metal cleaning and pickling processes, particularly in the steel industry. The acid is also employed in the production of chemicals, water treatment, and as a pH regulator in various industrial processes.- Chemical properties and applications of muriatic acid: Muriatic acid, also known as hydrochloric acid, is a strong mineral acid with various industrial and household applications. It is commonly used for cleaning, pH adjustment, and as a reagent in chemical processes. Its corrosive nature makes it effective for removing rust, scale, and other deposits.
- Use in water treatment and purification: Muriatic acid plays a crucial role in water treatment and purification processes. It is used to adjust pH levels in swimming pools, industrial water systems, and municipal water supplies. The acid helps to maintain proper water chemistry and prevent the growth of harmful microorganisms.
- Industrial cleaning and surface preparation: Muriatic acid is widely used in industrial cleaning applications, particularly for removing mineral deposits, rust, and scale from various surfaces. It is effective in preparing metal surfaces for painting or coating, and in cleaning masonry and concrete. The acid's strong etching properties make it suitable for these applications.
- Production and handling of muriatic acid: The production, storage, and handling of muriatic acid require specialized equipment and safety measures due to its corrosive nature. Industrial processes involve the synthesis of hydrochloric acid through various chemical reactions. Proper containment, transportation, and disposal methods are essential to ensure safe handling of the acid.
- Environmental and safety considerations: The use of muriatic acid has environmental and safety implications that need to be addressed. Proper handling, storage, and disposal procedures are crucial to prevent accidents and environmental contamination. Safety measures include the use of protective equipment, proper ventilation, and adherence to regulatory guidelines for its use and disposal.
02 Cleaning and etching applications
Muriatic acid is widely used in cleaning and etching applications. It is effective in removing rust, scale, and other deposits from metal surfaces. In the construction industry, it is used for cleaning masonry and concrete surfaces. The acid is also utilized in pool maintenance to adjust pH levels and remove stains.Expand Specific Solutions03 Production and handling of muriatic acid
The production and handling of muriatic acid involve specific processes and safety measures. Specialized equipment and materials are used to manufacture, store, and transport the acid. Corrosion-resistant materials and proper ventilation systems are essential in facilities dealing with muriatic acid to ensure safety and prevent environmental contamination.Expand Specific Solutions04 Environmental and safety considerations
Handling muriatic acid requires strict safety protocols due to its corrosive nature. Proper personal protective equipment (PPE) and handling procedures are essential to prevent accidents and injuries. Environmental considerations include proper disposal methods and measures to prevent soil and water contamination. Neutralization techniques are often employed before disposal.Expand Specific Solutions05 Alternative applications and formulations
Muriatic acid is used in various alternative applications and formulations. It is employed in the food industry as a processing aid and in the pharmaceutical industry for drug synthesis. Modified formulations of muriatic acid with additives or in combination with other chemicals are developed for specific applications, such as enhanced cleaning or reduced corrosiveness.Expand Specific Solutions
Key Players in PPE and Chemical Industries
The market for polyphenylene ether (PPE) manufacture using muriatic acid is in a mature stage, with established players dominating the industry. The global PPE market size is projected to reach $4.5 billion by 2027, growing at a CAGR of 6.2%. Technologically, the process is well-developed, with companies like Asahi Kasei Corp., SABIC, and Mitsubishi Gas Chemical Co. leading in innovation and production capacity. These firms have extensive experience in PPE synthesis and continue to refine their processes for improved efficiency and sustainability. Emerging players such as Kingfa Sci. & Tech. Co. and Nantong Xingchen Synthetic Material Co. are also making strides in the market, focusing on specialized applications and regional expansion.
Asahi Kasei Corp.
Technical Solution: Asahi Kasei utilizes a proprietary process for manufacturing Polyphenylene Ether (PPE) that incorporates muriatic acid (hydrochloric acid) as a key reagent. The company employs a modified oxidative coupling polymerization method, where 2,6-dimethylphenol is oxidized in the presence of a copper-amine catalyst system. Muriatic acid is used in the post-polymerization stage to neutralize the reaction mixture and precipitate the polymer. This process allows for precise control over molecular weight distribution and results in high-purity PPE with excellent thermal and mechanical properties[1][3]. Asahi Kasei has also developed a continuous production system that enhances efficiency and reduces environmental impact by optimizing acid usage and implementing advanced recycling techniques for the muriatic acid[5].
Strengths: High-quality PPE production with controlled properties, efficient continuous process, reduced environmental impact. Weaknesses: Potential corrosion issues due to acid use, need for specialized equipment to handle muriatic acid safely.
SABIC Global Technologies BV
Technical Solution: SABIC has developed an innovative approach to PPE production that incorporates muriatic acid in a novel way. Their process involves a modified oxidative coupling reaction where muriatic acid is used not only in the post-polymerization stage but also as a catalyst modifier. This technique allows for better control over the polymerization rate and molecular weight distribution. SABIC's method includes a proprietary acid recovery and purification system that significantly reduces waste and improves overall process efficiency[2][4]. Additionally, they have implemented advanced process control systems that optimize the use of muriatic acid, resulting in a more consistent product quality and reduced environmental footprint. SABIC's PPE grades produced using this method exhibit enhanced heat resistance and dimensional stability, making them suitable for high-performance applications in automotive and electronics industries[6].
Strengths: Improved process control, enhanced product properties, efficient acid recovery system. Weaknesses: Potentially higher initial investment costs, complexity in process control systems.
Innovations in Acid-Catalyzed PPE Production
Method for producing polyphenylene ether
PatentInactiveJP2016102171A
Innovation
- A method involving a gel removal step using a chelating agent to extract polymerization catalysts, followed by a concentration step with a heat source above the solvent's boiling point and a gel-removing step with a heat exchanger, to produce a gel-free PPE solution, which is then precipitated and dried to obtain high-quality PPE.
Method for producing polyphenylene ether
PatentActiveUS20170275424A1
Innovation
- A method involving oxidative polymerization of a phenolic compound in the presence of an aromatic solvent, followed by precipitation with a polar solvent, multiple cycles of washing with a solvent mixture containing aromatic and polar solvents, and drying to produce PPE with reduced impurities.
Environmental Impact of Muriatic Acid Use
The use of muriatic acid (hydrochloric acid) in the manufacture of Polyphenylene Ether (PPE) raises significant environmental concerns that require careful consideration and management. The primary environmental impacts stem from the potential release of acid vapors and contaminated wastewater during the production process.
Acid vapors, if not properly contained and treated, can contribute to air pollution and pose risks to both human health and the surrounding ecosystem. These emissions may lead to the formation of acid rain, which can damage vegetation, acidify water bodies, and corrode infrastructure. To mitigate these effects, manufacturers must implement robust air pollution control systems, such as scrubbers and absorption towers, to neutralize and capture acid vapors before they are released into the atmosphere.
Wastewater generated during the PPE manufacturing process can contain residual muriatic acid, which, if discharged untreated, may alter the pH of receiving water bodies and harm aquatic life. This necessitates the implementation of comprehensive wastewater treatment systems to neutralize the acid content and remove any other contaminants before discharge. Additionally, the potential for accidental spills or leaks of muriatic acid during storage, handling, or transportation presents risks to soil and groundwater contamination.
The production and transportation of muriatic acid itself also contribute to the overall environmental footprint of PPE manufacturing. The energy-intensive processes involved in acid production result in greenhouse gas emissions, while transportation of the acid increases the carbon footprint and poses risks of accidental releases during transit.
To address these environmental challenges, PPE manufacturers are increasingly adopting cleaner production techniques and circular economy principles. This includes exploring alternatives to muriatic acid, implementing closed-loop systems to minimize waste and emissions, and investing in more efficient production technologies. Some companies are also investigating the potential for on-site acid regeneration to reduce the need for fresh acid inputs and associated transportation risks.
Regulatory bodies worldwide have established stringent environmental standards for the use of muriatic acid in industrial processes, including PPE production. These regulations typically mandate the implementation of best available techniques (BAT) for pollution prevention and control, regular environmental monitoring, and reporting of emissions and waste management practices.
As sustainability becomes an increasingly important factor in industrial operations, PPE manufacturers are under pressure to improve their environmental performance. This has led to increased research and development efforts focused on developing more environmentally friendly production methods that reduce or eliminate the use of muriatic acid, thereby minimizing the associated environmental impacts while maintaining product quality and economic viability.
Acid vapors, if not properly contained and treated, can contribute to air pollution and pose risks to both human health and the surrounding ecosystem. These emissions may lead to the formation of acid rain, which can damage vegetation, acidify water bodies, and corrode infrastructure. To mitigate these effects, manufacturers must implement robust air pollution control systems, such as scrubbers and absorption towers, to neutralize and capture acid vapors before they are released into the atmosphere.
Wastewater generated during the PPE manufacturing process can contain residual muriatic acid, which, if discharged untreated, may alter the pH of receiving water bodies and harm aquatic life. This necessitates the implementation of comprehensive wastewater treatment systems to neutralize the acid content and remove any other contaminants before discharge. Additionally, the potential for accidental spills or leaks of muriatic acid during storage, handling, or transportation presents risks to soil and groundwater contamination.
The production and transportation of muriatic acid itself also contribute to the overall environmental footprint of PPE manufacturing. The energy-intensive processes involved in acid production result in greenhouse gas emissions, while transportation of the acid increases the carbon footprint and poses risks of accidental releases during transit.
To address these environmental challenges, PPE manufacturers are increasingly adopting cleaner production techniques and circular economy principles. This includes exploring alternatives to muriatic acid, implementing closed-loop systems to minimize waste and emissions, and investing in more efficient production technologies. Some companies are also investigating the potential for on-site acid regeneration to reduce the need for fresh acid inputs and associated transportation risks.
Regulatory bodies worldwide have established stringent environmental standards for the use of muriatic acid in industrial processes, including PPE production. These regulations typically mandate the implementation of best available techniques (BAT) for pollution prevention and control, regular environmental monitoring, and reporting of emissions and waste management practices.
As sustainability becomes an increasingly important factor in industrial operations, PPE manufacturers are under pressure to improve their environmental performance. This has led to increased research and development efforts focused on developing more environmentally friendly production methods that reduce or eliminate the use of muriatic acid, thereby minimizing the associated environmental impacts while maintaining product quality and economic viability.
Safety Protocols in PPE Production
Safety protocols in PPE production are of paramount importance due to the hazardous nature of the chemicals involved, particularly muriatic acid (hydrochloric acid). These protocols are designed to protect workers, prevent accidents, and ensure environmental compliance throughout the manufacturing process.
Personal protective equipment (PPE) is the first line of defense for workers handling muriatic acid. This includes chemical-resistant gloves, goggles, face shields, and acid-resistant aprons or full-body suits. Respiratory protection may also be necessary, depending on the concentration and potential for acid vapor exposure. Regular training on proper PPE use and maintenance is essential to ensure its effectiveness.
Proper storage and handling of muriatic acid are critical components of safety protocols. The acid should be stored in corrosion-resistant containers in well-ventilated areas, away from incompatible materials. Secondary containment systems are often employed to prevent spills from spreading. Strict inventory control and proper labeling of all chemicals are necessary to minimize the risk of accidental misuse or exposure.
Emergency response procedures must be well-established and regularly practiced. This includes the installation of emergency showers and eyewash stations in strategic locations throughout the production facility. Spill response kits should be readily available, and workers must be trained in their proper use. Clear evacuation routes and assembly points should be designated and communicated to all personnel.
Ventilation systems play a crucial role in maintaining a safe working environment. Local exhaust ventilation should be installed at points where acid vapors may be generated, and general ventilation systems should be designed to ensure adequate air exchange throughout the facility. Regular monitoring of air quality and acid vapor levels is essential to verify the effectiveness of these systems.
Process safety management (PSM) principles should be applied to the PPE production process. This involves conducting thorough hazard analyses, implementing robust process controls, and establishing clear operating procedures. Regular equipment inspections and preventive maintenance are vital to prevent leaks, spills, or equipment failures that could lead to acid exposure.
Employee training is a cornerstone of safety protocols in PPE production. Workers must receive comprehensive instruction on the hazards of muriatic acid, proper handling techniques, emergency procedures, and the importance of following safety protocols. Refresher training should be conducted regularly to reinforce safe practices and address any changes in procedures or equipment.
Environmental considerations are also integral to safety protocols. Proper waste management procedures must be in place to handle and dispose of acid waste in compliance with environmental regulations. This may include neutralization processes, wastewater treatment systems, and proper documentation of all waste streams.
By implementing and strictly adhering to these comprehensive safety protocols, PPE manufacturers can significantly reduce the risks associated with muriatic acid use in production, ensuring the safety of workers and the surrounding environment.
Personal protective equipment (PPE) is the first line of defense for workers handling muriatic acid. This includes chemical-resistant gloves, goggles, face shields, and acid-resistant aprons or full-body suits. Respiratory protection may also be necessary, depending on the concentration and potential for acid vapor exposure. Regular training on proper PPE use and maintenance is essential to ensure its effectiveness.
Proper storage and handling of muriatic acid are critical components of safety protocols. The acid should be stored in corrosion-resistant containers in well-ventilated areas, away from incompatible materials. Secondary containment systems are often employed to prevent spills from spreading. Strict inventory control and proper labeling of all chemicals are necessary to minimize the risk of accidental misuse or exposure.
Emergency response procedures must be well-established and regularly practiced. This includes the installation of emergency showers and eyewash stations in strategic locations throughout the production facility. Spill response kits should be readily available, and workers must be trained in their proper use. Clear evacuation routes and assembly points should be designated and communicated to all personnel.
Ventilation systems play a crucial role in maintaining a safe working environment. Local exhaust ventilation should be installed at points where acid vapors may be generated, and general ventilation systems should be designed to ensure adequate air exchange throughout the facility. Regular monitoring of air quality and acid vapor levels is essential to verify the effectiveness of these systems.
Process safety management (PSM) principles should be applied to the PPE production process. This involves conducting thorough hazard analyses, implementing robust process controls, and establishing clear operating procedures. Regular equipment inspections and preventive maintenance are vital to prevent leaks, spills, or equipment failures that could lead to acid exposure.
Employee training is a cornerstone of safety protocols in PPE production. Workers must receive comprehensive instruction on the hazards of muriatic acid, proper handling techniques, emergency procedures, and the importance of following safety protocols. Refresher training should be conducted regularly to reinforce safe practices and address any changes in procedures or equipment.
Environmental considerations are also integral to safety protocols. Proper waste management procedures must be in place to handle and dispose of acid waste in compliance with environmental regulations. This may include neutralization processes, wastewater treatment systems, and proper documentation of all waste streams.
By implementing and strictly adhering to these comprehensive safety protocols, PPE manufacturers can significantly reduce the risks associated with muriatic acid use in production, ensuring the safety of workers and the surrounding environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!