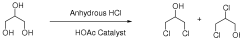
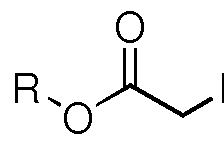
How Muriatic Acid Reacts with Different Types of Metals
JUL 18, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muriatic Acid-Metal Reaction Background
Muriatic acid, also known as hydrochloric acid (HCl), has been a subject of scientific interest and industrial application for centuries. Its interaction with metals forms the basis for numerous chemical processes and has significant implications across various sectors. The reaction between muriatic acid and metals is fundamentally a redox process, where the acid acts as an oxidizing agent and the metal as a reducing agent.
The history of studying these reactions dates back to the alchemists of the Middle Ages, who observed the dissolution of metals in strong acids. However, it wasn't until the 18th century that the true nature of these reactions began to be understood. Antoine Lavoisier's work on oxygen and combustion laid the groundwork for modern understanding of acid-metal reactions.
In the context of metal reactivity, muriatic acid plays a crucial role in the reactivity series of metals. This series, also known as the activity series, ranks metals according to their tendency to lose electrons in reactions. The position of a metal in this series largely determines its reaction with muriatic acid, with more reactive metals higher in the series reacting more vigorously.
The general reaction between muriatic acid and a metal can be represented as: 2HCl + M → MCl2 + H2, where M represents the metal. However, the actual reaction mechanism and products can vary significantly depending on the specific metal involved. Factors such as the metal's position in the periodic table, its oxidation state, and the concentration of the acid all influence the reaction outcome.
Understanding these reactions is crucial in various industrial applications. In metal processing, muriatic acid is used for pickling steel and other metals to remove rust and prepare surfaces for further treatment. In the mining industry, it's employed in ore processing and metal extraction. The semiconductor industry utilizes high-purity hydrochloric acid in the production of silicon wafers.
The environmental impact of these reactions is also a significant consideration. The hydrogen gas produced can be a valuable resource when harnessed properly, but it also poses safety risks if not managed correctly. Additionally, the metal chlorides formed as products can have environmental implications, necessitating proper waste management practices.
Recent technological advancements have focused on optimizing these reactions for specific applications while minimizing environmental impact. This includes developing more efficient acid recovery systems, exploring alternative pickling methods, and investigating the use of inhibitors to control reaction rates in specific scenarios.
The history of studying these reactions dates back to the alchemists of the Middle Ages, who observed the dissolution of metals in strong acids. However, it wasn't until the 18th century that the true nature of these reactions began to be understood. Antoine Lavoisier's work on oxygen and combustion laid the groundwork for modern understanding of acid-metal reactions.
In the context of metal reactivity, muriatic acid plays a crucial role in the reactivity series of metals. This series, also known as the activity series, ranks metals according to their tendency to lose electrons in reactions. The position of a metal in this series largely determines its reaction with muriatic acid, with more reactive metals higher in the series reacting more vigorously.
The general reaction between muriatic acid and a metal can be represented as: 2HCl + M → MCl2 + H2, where M represents the metal. However, the actual reaction mechanism and products can vary significantly depending on the specific metal involved. Factors such as the metal's position in the periodic table, its oxidation state, and the concentration of the acid all influence the reaction outcome.
Understanding these reactions is crucial in various industrial applications. In metal processing, muriatic acid is used for pickling steel and other metals to remove rust and prepare surfaces for further treatment. In the mining industry, it's employed in ore processing and metal extraction. The semiconductor industry utilizes high-purity hydrochloric acid in the production of silicon wafers.
The environmental impact of these reactions is also a significant consideration. The hydrogen gas produced can be a valuable resource when harnessed properly, but it also poses safety risks if not managed correctly. Additionally, the metal chlorides formed as products can have environmental implications, necessitating proper waste management practices.
Recent technological advancements have focused on optimizing these reactions for specific applications while minimizing environmental impact. This includes developing more efficient acid recovery systems, exploring alternative pickling methods, and investigating the use of inhibitors to control reaction rates in specific scenarios.
Industrial Applications and Market Demand
The market demand for muriatic acid, also known as hydrochloric acid, in metal-related applications is substantial and continues to grow across various industries. The reaction between muriatic acid and different types of metals plays a crucial role in numerous industrial processes, driving the demand for this versatile chemical compound.
In the metal manufacturing and processing sector, muriatic acid is extensively used for metal cleaning, pickling, and surface preparation. The ability of muriatic acid to react with and remove rust, scale, and other impurities from metal surfaces makes it indispensable in steel production, galvanizing operations, and metal finishing industries. As global steel production and metal manufacturing activities expand, the demand for muriatic acid in these applications is expected to rise correspondingly.
The construction industry represents another significant market for muriatic acid in metal-related applications. It is commonly used for cleaning masonry surfaces, removing efflorescence, and etching concrete. The acid's reaction with metal oxides and carbonates makes it effective in removing stains and preparing surfaces for further treatment or coating. With the growth of the construction sector, particularly in developing economies, the demand for muriatic acid in this segment is projected to increase.
In the mining and mineral processing industry, muriatic acid finds applications in ore processing, metal extraction, and pH adjustment. Its ability to react with various metals and minerals makes it valuable in leaching processes and hydrometallurgical operations. As the demand for metals and minerals continues to rise due to technological advancements and urbanization, the use of muriatic acid in these processes is expected to grow.
The electronics industry also contributes to the market demand for muriatic acid in metal-related applications. It is used in the production of printed circuit boards, semiconductor manufacturing, and metal etching processes. The ongoing expansion of the electronics sector, driven by the increasing adoption of smart devices and emerging technologies, is likely to fuel the demand for muriatic acid in these specialized applications.
Environmental regulations and sustainability concerns have led to the development of alternative technologies and processes in some industries. However, the unique properties and cost-effectiveness of muriatic acid in metal-related applications continue to make it a preferred choice in many sectors. Manufacturers are focusing on developing high-purity grades and safer handling methods to address environmental and safety concerns, further supporting the market growth.
The global market for muriatic acid in metal-related applications is characterized by regional variations in demand patterns. Developing economies in Asia-Pacific, particularly China and India, are expected to be the primary drivers of market growth due to rapid industrialization and infrastructure development. Mature markets in North America and Europe are likely to see steady demand, primarily driven by replacement and maintenance activities in existing industrial facilities.
In the metal manufacturing and processing sector, muriatic acid is extensively used for metal cleaning, pickling, and surface preparation. The ability of muriatic acid to react with and remove rust, scale, and other impurities from metal surfaces makes it indispensable in steel production, galvanizing operations, and metal finishing industries. As global steel production and metal manufacturing activities expand, the demand for muriatic acid in these applications is expected to rise correspondingly.
The construction industry represents another significant market for muriatic acid in metal-related applications. It is commonly used for cleaning masonry surfaces, removing efflorescence, and etching concrete. The acid's reaction with metal oxides and carbonates makes it effective in removing stains and preparing surfaces for further treatment or coating. With the growth of the construction sector, particularly in developing economies, the demand for muriatic acid in this segment is projected to increase.
In the mining and mineral processing industry, muriatic acid finds applications in ore processing, metal extraction, and pH adjustment. Its ability to react with various metals and minerals makes it valuable in leaching processes and hydrometallurgical operations. As the demand for metals and minerals continues to rise due to technological advancements and urbanization, the use of muriatic acid in these processes is expected to grow.
The electronics industry also contributes to the market demand for muriatic acid in metal-related applications. It is used in the production of printed circuit boards, semiconductor manufacturing, and metal etching processes. The ongoing expansion of the electronics sector, driven by the increasing adoption of smart devices and emerging technologies, is likely to fuel the demand for muriatic acid in these specialized applications.
Environmental regulations and sustainability concerns have led to the development of alternative technologies and processes in some industries. However, the unique properties and cost-effectiveness of muriatic acid in metal-related applications continue to make it a preferred choice in many sectors. Manufacturers are focusing on developing high-purity grades and safer handling methods to address environmental and safety concerns, further supporting the market growth.
The global market for muriatic acid in metal-related applications is characterized by regional variations in demand patterns. Developing economies in Asia-Pacific, particularly China and India, are expected to be the primary drivers of market growth due to rapid industrialization and infrastructure development. Mature markets in North America and Europe are likely to see steady demand, primarily driven by replacement and maintenance activities in existing industrial facilities.
Current Challenges in Acid-Metal Reactions
The reaction between muriatic acid (hydrochloric acid) and different types of metals presents several ongoing challenges in both industrial applications and scientific research. One of the primary issues is the varying reactivity rates among different metals, which can lead to unpredictable outcomes in corrosion processes and chemical manufacturing.
For highly reactive metals such as magnesium and zinc, the reaction with muriatic acid is often too rapid and exothermic, posing safety risks and making it difficult to control the process. This rapid reaction can result in excessive heat generation, potentially leading to equipment damage or even explosions in industrial settings. Conversely, less reactive metals like copper and silver react slowly or not at all with muriatic acid under normal conditions, limiting their use in certain applications where acid-metal reactions are desired.
Another significant challenge is the formation of unwanted byproducts during acid-metal reactions. For instance, when iron reacts with muriatic acid, it produces ferrous chloride and hydrogen gas. The hydrogen gas can create safety hazards if not properly vented, while the ferrous chloride may interfere with subsequent processes or contaminate the final product.
The corrosion of metal surfaces due to acid exposure remains a persistent problem across various industries. This is particularly evident in sectors such as oil and gas, where pipelines and storage tanks are constantly exposed to acidic environments. The challenge lies in developing effective corrosion-resistant materials or protective coatings that can withstand long-term exposure to muriatic acid without compromising structural integrity.
Environmental concerns also pose challenges in acid-metal reactions. The disposal of waste products from these reactions, including metal salts and unreacted acid, requires careful management to prevent environmental contamination. Additionally, the production of hydrogen gas as a byproduct contributes to greenhouse gas emissions, necessitating the development of more environmentally friendly alternatives or improved capture and utilization methods.
The precise control of reaction kinetics in acid-metal systems remains a challenge, particularly in applications requiring specific reaction rates or product yields. Factors such as temperature, concentration, and surface area of the metal all influence the reaction rate, making it difficult to achieve consistent results across different batches or scales of production.
Lastly, the development of novel materials that can selectively react with muriatic acid while resisting corrosion from other acids is an ongoing challenge. This is particularly important in industries where multiple acids are used in sequence, such as in metal surface treatment processes. Creating materials with tailored reactivity profiles could significantly enhance efficiency and reduce costs in various industrial applications.
For highly reactive metals such as magnesium and zinc, the reaction with muriatic acid is often too rapid and exothermic, posing safety risks and making it difficult to control the process. This rapid reaction can result in excessive heat generation, potentially leading to equipment damage or even explosions in industrial settings. Conversely, less reactive metals like copper and silver react slowly or not at all with muriatic acid under normal conditions, limiting their use in certain applications where acid-metal reactions are desired.
Another significant challenge is the formation of unwanted byproducts during acid-metal reactions. For instance, when iron reacts with muriatic acid, it produces ferrous chloride and hydrogen gas. The hydrogen gas can create safety hazards if not properly vented, while the ferrous chloride may interfere with subsequent processes or contaminate the final product.
The corrosion of metal surfaces due to acid exposure remains a persistent problem across various industries. This is particularly evident in sectors such as oil and gas, where pipelines and storage tanks are constantly exposed to acidic environments. The challenge lies in developing effective corrosion-resistant materials or protective coatings that can withstand long-term exposure to muriatic acid without compromising structural integrity.
Environmental concerns also pose challenges in acid-metal reactions. The disposal of waste products from these reactions, including metal salts and unreacted acid, requires careful management to prevent environmental contamination. Additionally, the production of hydrogen gas as a byproduct contributes to greenhouse gas emissions, necessitating the development of more environmentally friendly alternatives or improved capture and utilization methods.
The precise control of reaction kinetics in acid-metal systems remains a challenge, particularly in applications requiring specific reaction rates or product yields. Factors such as temperature, concentration, and surface area of the metal all influence the reaction rate, making it difficult to achieve consistent results across different batches or scales of production.
Lastly, the development of novel materials that can selectively react with muriatic acid while resisting corrosion from other acids is an ongoing challenge. This is particularly important in industries where multiple acids are used in sequence, such as in metal surface treatment processes. Creating materials with tailored reactivity profiles could significantly enhance efficiency and reduce costs in various industrial applications.
Existing Methodologies for Studying Acid-Metal Reactions
01 Chemical reactions involving muriatic acid
Muriatic acid, also known as hydrochloric acid, is involved in various chemical reactions. These reactions can include neutralization with bases, corrosion of metals, and the production of chlorine gas. The acid's reactivity makes it useful in industrial processes, cleaning applications, and laboratory experiments.- Reaction with metals: Muriatic acid, also known as hydrochloric acid, reacts with various metals to produce hydrogen gas and metal chlorides. This reaction is commonly used in metal cleaning, etching, and processing applications. The rate and intensity of the reaction depend on the reactivity of the metal and the concentration of the acid.
- Use in chemical analysis: Muriatic acid is utilized in various chemical analysis techniques, including titration, pH adjustment, and sample preparation. It plays a crucial role in analytical chemistry for detecting and quantifying different substances. The acid's strong proton-donating properties make it valuable for these applications.
- Application in water treatment: Muriatic acid is employed in water treatment processes for pH adjustment, scale removal, and disinfection. It helps maintain proper water chemistry in swimming pools, industrial cooling systems, and municipal water treatment plants. The acid's ability to neutralize alkaline substances and dissolve mineral deposits makes it effective for these purposes.
- Reaction with carbonates and bicarbonates: Muriatic acid reacts vigorously with carbonate and bicarbonate compounds, producing carbon dioxide gas and water. This reaction is utilized in various industrial and laboratory applications, such as limestone dissolution, scale removal, and gas generation. The effervescence observed during this reaction is characteristic of carbonate-acid interactions.
- Use in organic synthesis: Muriatic acid serves as a reagent and catalyst in various organic synthesis reactions. It is used for hydrolysis, dehydration, and chlorination processes in the production of pharmaceuticals, polymers, and other organic compounds. The acid's strong proton-donating ability and nucleophilic chloride ion make it versatile in organic chemistry applications.
02 Muriatic acid in water treatment and purification
Muriatic acid is utilized in water treatment processes for pH adjustment and disinfection. It can help remove scale buildup in pipes and equipment, and is effective in treating swimming pool water. The acid's ability to neutralize alkaline substances makes it valuable in maintaining proper water chemistry.Expand Specific Solutions03 Industrial applications of muriatic acid reactions
Muriatic acid reactions are employed in various industrial processes, including metal pickling, oil well acidizing, and the production of organic and inorganic compounds. Its strong acidic properties make it useful for cleaning and etching metals, as well as in the manufacturing of pharmaceuticals and food additives.Expand Specific Solutions04 Safety considerations and handling of muriatic acid
Due to its corrosive nature, proper safety measures are crucial when handling muriatic acid. This includes using appropriate personal protective equipment, ensuring proper ventilation, and following specific storage and disposal protocols. Understanding the acid's reactivity is essential for preventing accidents and minimizing environmental impact.Expand Specific Solutions05 Analytical methods involving muriatic acid
Muriatic acid is used in various analytical techniques and laboratory procedures. It can be employed in titrations, spectroscopic analyses, and as a reagent in chemical tests. The acid's reactions with different substances can help identify and quantify compounds in samples, making it valuable in research and quality control applications.Expand Specific Solutions
Key Players in Chemical Industry and Research
The competitive landscape for research on muriatic acid reactions with different metals is in a mature stage, with a well-established market and advanced technological capabilities. The global market size for this research area is estimated to be substantial, driven by applications in various industries such as chemical manufacturing, metallurgy, and materials science. Key players in this field include UOP LLC, Hoechst AG, and FormFactor, Inc., who have demonstrated significant expertise in chemical reactions and materials analysis. Universities like Shanghai Jiao Tong University and Shandong University contribute to the academic research aspect, while companies such as Daicel Corp. and China Petroleum & Chemical Corp. bring industrial perspectives. The technology's maturity is evident from the involvement of diverse stakeholders, ranging from specialized chemical companies to large conglomerates, indicating a well-developed ecosystem for research and application in this domain.
UOP LLC
Technical Solution: UOP LLC, a Honeywell company, has developed innovative technologies to address the challenges of muriatic acid reactions with metals in industrial processes. Their approach focuses on process optimization and material selection to minimize acid-metal contact. UOP has introduced advanced reactor designs with specialized linings that resist muriatic acid corrosion, significantly extending equipment life in petrochemical applications[2]. They have also developed proprietary metal alloys with enhanced resistance to chloride stress corrosion cracking, a common issue in muriatic acid environments[4]. Additionally, UOP has implemented smart monitoring systems that use real-time corrosion sensors to detect and mitigate acid-induced metal degradation before it becomes critical[6]. Their holistic approach also includes the development of acid-resistant catalysts that reduce the need for direct metal-acid contact in certain processes[8].
Strengths: Integrated solutions combining equipment design, material science, and process control; extensive experience in industrial-scale applications. Weaknesses: Solutions may be complex and costly to implement; some technologies may be specific to certain industries or processes.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced corrosion-resistant alloys and protective coatings to mitigate the effects of muriatic acid on metal equipment in oil refining processes. Their approach involves using nickel-based alloys with high chromium content, which form a passive oxide layer when exposed to muriatic acid, significantly reducing corrosion rates[1]. Additionally, they have implemented a novel electrochemical protection system that uses sacrificial anodes to further protect metal surfaces in acid environments[3]. Sinopec has also invested in research on acid-resistant polymeric coatings that can be applied to metal surfaces, creating a barrier against muriatic acid attack[5].
Strengths: Comprehensive approach combining material science and electrochemistry; extensive real-world application in oil and gas industry. Weaknesses: High implementation costs; may require frequent maintenance and monitoring.
Innovative Approaches in Reaction Mechanism Analysis
Conversion of a multihydroxylated-aliphatic hydrocarbon or ester thereof to a chlorohydrin
PatentWO2008128011A2
Innovation
- The process involves controlling the temperature of hydrochlorination products to minimize hydrogen chloride liberation, using non-resistant materials where possible, and managing fluoride concentrations to prevent equipment corrosion, while maintaining high selectivity and yield of chlorohydrins.
Process of recovery of base metals from oxide ores
PatentActiveEP2454389A1
Innovation
- A process involving the in-situ generation of hydrochloric acid by agglomerating ferric or ferrous chloride with the ore, followed by selective hydrolysis to form metal chlorides, which are then solubilized in water, eliminating the need for iron and aluminum removal stages and reducing acid consumption.
Environmental Impact and Safety Considerations
The reaction between muriatic acid (hydrochloric acid) and various metals has significant environmental and safety implications that must be carefully considered. When these reactions occur, they can release harmful gases, create hazardous waste, and pose risks to human health and the environment.
One of the primary environmental concerns is the potential for acid runoff. If not properly contained, the acidic solution resulting from metal-acid reactions can contaminate soil and water sources. This contamination can disrupt ecosystems, harm aquatic life, and potentially enter the food chain. The pH changes caused by acid runoff can have far-reaching effects on local flora and fauna, altering habitats and biodiversity.
The release of hydrogen gas during these reactions presents both safety and environmental hazards. Hydrogen is highly flammable and can form explosive mixtures with air, posing a significant risk in enclosed spaces. From an environmental perspective, while hydrogen itself is not a greenhouse gas, its production and potential combustion can indirectly contribute to climate change through energy consumption and the release of other pollutants.
Metal chlorides, formed as byproducts of these reactions, can also have environmental impacts. Some metal chlorides are water-soluble and can leach into groundwater, potentially affecting drinking water quality and aquatic ecosystems. Certain metal chlorides may also be toxic to plants and animals, causing long-term ecological damage if released into the environment.
Safety considerations for handling muriatic acid and its reactions with metals are paramount. Personal protective equipment (PPE) such as acid-resistant gloves, goggles, and respiratory protection is essential to prevent skin contact, eye damage, and inhalation of harmful fumes. Proper ventilation is crucial to disperse potentially dangerous gases and vapors.
Waste management is another critical aspect of environmental and safety considerations. The proper disposal of spent acid solutions and metal byproducts is necessary to prevent environmental contamination. This often involves neutralization processes and adherence to local regulations regarding hazardous waste disposal.
In industrial settings, engineering controls such as closed systems, fume hoods, and scrubbers are important for containing and treating emissions from these reactions. Regular maintenance and inspection of equipment used in these processes are essential to prevent leaks and ensure safe operation.
Education and training for those working with muriatic acid and metals are crucial for maintaining a safe environment. This includes understanding the specific hazards associated with different metal-acid combinations, proper handling and storage procedures, and emergency response protocols in case of spills or accidents.
One of the primary environmental concerns is the potential for acid runoff. If not properly contained, the acidic solution resulting from metal-acid reactions can contaminate soil and water sources. This contamination can disrupt ecosystems, harm aquatic life, and potentially enter the food chain. The pH changes caused by acid runoff can have far-reaching effects on local flora and fauna, altering habitats and biodiversity.
The release of hydrogen gas during these reactions presents both safety and environmental hazards. Hydrogen is highly flammable and can form explosive mixtures with air, posing a significant risk in enclosed spaces. From an environmental perspective, while hydrogen itself is not a greenhouse gas, its production and potential combustion can indirectly contribute to climate change through energy consumption and the release of other pollutants.
Metal chlorides, formed as byproducts of these reactions, can also have environmental impacts. Some metal chlorides are water-soluble and can leach into groundwater, potentially affecting drinking water quality and aquatic ecosystems. Certain metal chlorides may also be toxic to plants and animals, causing long-term ecological damage if released into the environment.
Safety considerations for handling muriatic acid and its reactions with metals are paramount. Personal protective equipment (PPE) such as acid-resistant gloves, goggles, and respiratory protection is essential to prevent skin contact, eye damage, and inhalation of harmful fumes. Proper ventilation is crucial to disperse potentially dangerous gases and vapors.
Waste management is another critical aspect of environmental and safety considerations. The proper disposal of spent acid solutions and metal byproducts is necessary to prevent environmental contamination. This often involves neutralization processes and adherence to local regulations regarding hazardous waste disposal.
In industrial settings, engineering controls such as closed systems, fume hoods, and scrubbers are important for containing and treating emissions from these reactions. Regular maintenance and inspection of equipment used in these processes are essential to prevent leaks and ensure safe operation.
Education and training for those working with muriatic acid and metals are crucial for maintaining a safe environment. This includes understanding the specific hazards associated with different metal-acid combinations, proper handling and storage procedures, and emergency response protocols in case of spills or accidents.
Corrosion Prevention Strategies
Corrosion prevention strategies are crucial when dealing with the reaction between muriatic acid and different types of metals. The primary goal is to mitigate or eliminate the corrosive effects of the acid on metal surfaces. One of the most effective approaches is the application of protective coatings. These coatings create a barrier between the metal surface and the corrosive environment, significantly reducing the rate of corrosion.
Cathodic protection is another widely used method, particularly for large metal structures. This technique involves connecting the metal to be protected to a more easily corroded "sacrificial" metal, which preferentially corrodes and protects the primary metal. In the context of muriatic acid reactions, this method can be adapted to protect specific metal components in industrial settings where acid exposure is unavoidable.
Chemical inhibitors offer another line of defense against corrosion. These substances can be added to the muriatic acid solution to reduce its corrosive properties without compromising its intended function. Inhibitors work by forming a protective film on the metal surface or by altering the chemical properties of the acid itself.
Material selection plays a crucial role in corrosion prevention. Choosing metals or alloys that are inherently resistant to muriatic acid can significantly reduce corrosion risks. For instance, certain stainless steel grades and titanium alloys exhibit excellent resistance to hydrochloric acid (muriatic acid) and can be used in applications where acid exposure is expected.
Surface modification techniques, such as nitriding or carburizing, can enhance the corrosion resistance of metals. These processes alter the surface composition of the metal, creating a more resistant outer layer while maintaining the bulk properties of the material.
Regular inspection and maintenance are essential components of any corrosion prevention strategy. This includes routine checks for signs of corrosion, prompt repair of damaged protective coatings, and periodic replacement of sacrificial anodes in cathodic protection systems.
Environmental control measures can also be implemented to reduce corrosion risks. This may involve controlling humidity levels, temperature, or air quality in areas where metals are exposed to muriatic acid. In some cases, complete isolation of acid-containing systems from metal components may be necessary.
Lastly, proper handling and storage of muriatic acid are critical in preventing unintended corrosion. This includes using appropriate containment materials, implementing spill prevention measures, and ensuring proper ventilation in areas where the acid is used or stored. By combining these strategies, the corrosive effects of muriatic acid on different types of metals can be effectively managed and minimized.
Cathodic protection is another widely used method, particularly for large metal structures. This technique involves connecting the metal to be protected to a more easily corroded "sacrificial" metal, which preferentially corrodes and protects the primary metal. In the context of muriatic acid reactions, this method can be adapted to protect specific metal components in industrial settings where acid exposure is unavoidable.
Chemical inhibitors offer another line of defense against corrosion. These substances can be added to the muriatic acid solution to reduce its corrosive properties without compromising its intended function. Inhibitors work by forming a protective film on the metal surface or by altering the chemical properties of the acid itself.
Material selection plays a crucial role in corrosion prevention. Choosing metals or alloys that are inherently resistant to muriatic acid can significantly reduce corrosion risks. For instance, certain stainless steel grades and titanium alloys exhibit excellent resistance to hydrochloric acid (muriatic acid) and can be used in applications where acid exposure is expected.
Surface modification techniques, such as nitriding or carburizing, can enhance the corrosion resistance of metals. These processes alter the surface composition of the metal, creating a more resistant outer layer while maintaining the bulk properties of the material.
Regular inspection and maintenance are essential components of any corrosion prevention strategy. This includes routine checks for signs of corrosion, prompt repair of damaged protective coatings, and periodic replacement of sacrificial anodes in cathodic protection systems.
Environmental control measures can also be implemented to reduce corrosion risks. This may involve controlling humidity levels, temperature, or air quality in areas where metals are exposed to muriatic acid. In some cases, complete isolation of acid-containing systems from metal components may be necessary.
Lastly, proper handling and storage of muriatic acid are critical in preventing unintended corrosion. This includes using appropriate containment materials, implementing spill prevention measures, and ensuring proper ventilation in areas where the acid is used or stored. By combining these strategies, the corrosive effects of muriatic acid on different types of metals can be effectively managed and minimized.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!