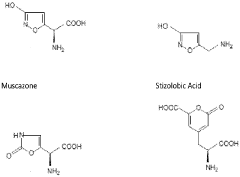
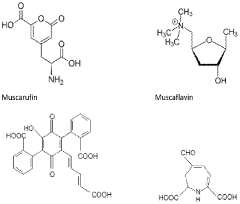
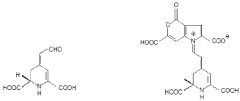
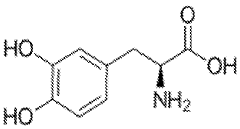
How Muscimol Functions as a Cerebral Dampener
JUL 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Muscimol Neurobiology
Muscimol, a potent GABA receptor agonist, plays a crucial role in dampening cerebral activity through its interaction with the GABAergic system. This naturally occurring psychoactive compound, found in certain mushroom species, exerts its effects by binding to GABA-A receptors in the central nervous system. The neurobiology of muscimol is intrinsically linked to its ability to enhance inhibitory neurotransmission, leading to a reduction in neuronal excitability and overall brain activity.
At the molecular level, muscimol's structure closely resembles that of GABA (gamma-aminobutyric acid), the primary inhibitory neurotransmitter in the mammalian brain. This structural similarity allows muscimol to bind with high affinity to GABA-A receptors, which are ligand-gated ion channels. When muscimol binds to these receptors, it triggers the opening of chloride ion channels, resulting in an influx of negatively charged chloride ions into the neuron.
The influx of chloride ions leads to hyperpolarization of the neuronal membrane, making it more difficult for the neuron to reach its action potential threshold. This hyperpolarization effectively reduces the likelihood of neuronal firing, contributing to the overall dampening effect on cerebral activity. The widespread distribution of GABA-A receptors throughout the brain ensures that muscimol's effects are far-reaching, influencing various neural circuits and brain regions.
Muscimol's action as a cerebral dampener is not limited to its direct effects on GABA-A receptors. By enhancing GABAergic transmission, muscimol also indirectly modulates other neurotransmitter systems. This cross-talk between neurotransmitter systems can lead to complex changes in neural network dynamics, affecting cognitive processes, mood, and behavior.
The neurobiology of muscimol also involves its interaction with different GABA-A receptor subunits. GABA-A receptors are pentameric structures composed of various subunit combinations, and muscimol exhibits differential affinity for these subunit configurations. This selectivity contributes to the compound's specific pharmacological profile and its region-dependent effects in the brain.
Furthermore, muscimol's action as a cerebral dampener is influenced by its pharmacokinetics. The compound's ability to cross the blood-brain barrier and its distribution within the central nervous system play crucial roles in determining its overall effects. The duration and intensity of muscimol's action are also dependent on its metabolism and clearance from the body, factors that contribute to its potential for both therapeutic applications and recreational use.
In conclusion, the neurobiology of muscimol as a cerebral dampener is a complex interplay of molecular interactions, receptor dynamics, and systemic effects. Its potent GABA-A agonist activity, coupled with its widespread influence on neural circuits, underlies its profound impact on brain function and behavior. Understanding these neurobiological mechanisms is crucial for exploring muscimol's potential in various clinical applications and for elucidating the broader principles of GABAergic modulation in the central nervous system.
At the molecular level, muscimol's structure closely resembles that of GABA (gamma-aminobutyric acid), the primary inhibitory neurotransmitter in the mammalian brain. This structural similarity allows muscimol to bind with high affinity to GABA-A receptors, which are ligand-gated ion channels. When muscimol binds to these receptors, it triggers the opening of chloride ion channels, resulting in an influx of negatively charged chloride ions into the neuron.
The influx of chloride ions leads to hyperpolarization of the neuronal membrane, making it more difficult for the neuron to reach its action potential threshold. This hyperpolarization effectively reduces the likelihood of neuronal firing, contributing to the overall dampening effect on cerebral activity. The widespread distribution of GABA-A receptors throughout the brain ensures that muscimol's effects are far-reaching, influencing various neural circuits and brain regions.
Muscimol's action as a cerebral dampener is not limited to its direct effects on GABA-A receptors. By enhancing GABAergic transmission, muscimol also indirectly modulates other neurotransmitter systems. This cross-talk between neurotransmitter systems can lead to complex changes in neural network dynamics, affecting cognitive processes, mood, and behavior.
The neurobiology of muscimol also involves its interaction with different GABA-A receptor subunits. GABA-A receptors are pentameric structures composed of various subunit combinations, and muscimol exhibits differential affinity for these subunit configurations. This selectivity contributes to the compound's specific pharmacological profile and its region-dependent effects in the brain.
Furthermore, muscimol's action as a cerebral dampener is influenced by its pharmacokinetics. The compound's ability to cross the blood-brain barrier and its distribution within the central nervous system play crucial roles in determining its overall effects. The duration and intensity of muscimol's action are also dependent on its metabolism and clearance from the body, factors that contribute to its potential for both therapeutic applications and recreational use.
In conclusion, the neurobiology of muscimol as a cerebral dampener is a complex interplay of molecular interactions, receptor dynamics, and systemic effects. Its potent GABA-A agonist activity, coupled with its widespread influence on neural circuits, underlies its profound impact on brain function and behavior. Understanding these neurobiological mechanisms is crucial for exploring muscimol's potential in various clinical applications and for elucidating the broader principles of GABAergic modulation in the central nervous system.
Clinical Applications
Muscimol, a potent GABA receptor agonist, has shown promising potential in various clinical applications due to its cerebral dampening effects. One of the primary areas of interest is in the treatment of epilepsy. By enhancing GABAergic inhibition, muscimol can potentially reduce the frequency and severity of seizures in patients who are resistant to conventional anticonvulsant medications. Clinical trials have demonstrated that localized administration of muscimol to epileptogenic foci can significantly decrease seizure activity, offering a novel approach for intractable epilepsy cases.
In the field of anxiety disorders, muscimol's anxiolytic properties have been explored as a potential alternative to benzodiazepines. Studies have shown that muscimol can effectively reduce anxiety-like behaviors in animal models, suggesting its potential as a therapeutic agent for generalized anxiety disorder, panic disorder, and social anxiety disorder. The advantage of muscimol lies in its more specific action on GABA receptors, potentially offering a treatment with fewer side effects compared to traditional anxiolytics.
Muscimol has also garnered attention in the treatment of sleep disorders. Its ability to promote GABA-mediated inhibition in the brain can help induce and maintain sleep. Research has indicated that muscimol may be particularly effective in addressing insomnia characterized by difficulty in sleep onset or maintenance. Moreover, its potential for improving sleep quality without the risk of dependency associated with some current sleep medications makes it an attractive candidate for further clinical investigation.
In the realm of pain management, muscimol's cerebral dampening effects have shown promise in modulating pain perception. Preclinical studies have demonstrated that muscimol can attenuate both acute and chronic pain responses. This has led to investigations into its potential use in conditions such as neuropathic pain, fibromyalgia, and other chronic pain syndromes where current treatments often fall short.
The neuroprotective properties of muscimol have also been a subject of clinical interest. Research suggests that muscimol may have a role in protecting neurons from excitotoxicity, a process implicated in various neurodegenerative disorders. This has prompted investigations into its potential application in conditions such as Alzheimer's disease, Parkinson's disease, and stroke. Preliminary studies have shown that muscimol administration can reduce neuronal death and improve functional outcomes in animal models of these conditions.
Furthermore, muscimol's ability to modulate neural activity has led to explorations of its use in psychiatric disorders. Particularly, its potential in treating schizophrenia has been investigated, with studies suggesting that muscimol may help alleviate certain symptoms by normalizing aberrant neural activity. Additionally, its anxiolytic and mood-stabilizing effects have prompted research into its potential applications in bipolar disorder and major depressive disorder.
In the field of anxiety disorders, muscimol's anxiolytic properties have been explored as a potential alternative to benzodiazepines. Studies have shown that muscimol can effectively reduce anxiety-like behaviors in animal models, suggesting its potential as a therapeutic agent for generalized anxiety disorder, panic disorder, and social anxiety disorder. The advantage of muscimol lies in its more specific action on GABA receptors, potentially offering a treatment with fewer side effects compared to traditional anxiolytics.
Muscimol has also garnered attention in the treatment of sleep disorders. Its ability to promote GABA-mediated inhibition in the brain can help induce and maintain sleep. Research has indicated that muscimol may be particularly effective in addressing insomnia characterized by difficulty in sleep onset or maintenance. Moreover, its potential for improving sleep quality without the risk of dependency associated with some current sleep medications makes it an attractive candidate for further clinical investigation.
In the realm of pain management, muscimol's cerebral dampening effects have shown promise in modulating pain perception. Preclinical studies have demonstrated that muscimol can attenuate both acute and chronic pain responses. This has led to investigations into its potential use in conditions such as neuropathic pain, fibromyalgia, and other chronic pain syndromes where current treatments often fall short.
The neuroprotective properties of muscimol have also been a subject of clinical interest. Research suggests that muscimol may have a role in protecting neurons from excitotoxicity, a process implicated in various neurodegenerative disorders. This has prompted investigations into its potential application in conditions such as Alzheimer's disease, Parkinson's disease, and stroke. Preliminary studies have shown that muscimol administration can reduce neuronal death and improve functional outcomes in animal models of these conditions.
Furthermore, muscimol's ability to modulate neural activity has led to explorations of its use in psychiatric disorders. Particularly, its potential in treating schizophrenia has been investigated, with studies suggesting that muscimol may help alleviate certain symptoms by normalizing aberrant neural activity. Additionally, its anxiolytic and mood-stabilizing effects have prompted research into its potential applications in bipolar disorder and major depressive disorder.
Pharmacodynamics
Muscimol, a potent GABA receptor agonist, exerts its cerebral dampening effects through a complex interplay of pharmacodynamic mechanisms. At the molecular level, muscimol binds with high affinity to GABA-A receptors, particularly those containing α4, α6, and δ subunits. This binding mimics the action of the endogenous neurotransmitter GABA, leading to enhanced inhibitory neurotransmission throughout the central nervous system.
Upon binding to GABA-A receptors, muscimol induces a conformational change that increases chloride ion conductance. The resulting influx of chloride ions hyperpolarizes the neuronal membrane, making it less likely for the neuron to fire an action potential. This hyperpolarization effectively dampens neuronal excitability, contributing to the overall inhibitory effect on cerebral activity.
The pharmacodynamic profile of muscimol is characterized by its rapid onset of action and relatively short duration. Unlike some other GABA-A receptor agonists, muscimol does not appear to cause significant receptor desensitization, allowing for sustained inhibitory effects during its period of activity. This property makes muscimol particularly effective as a cerebral dampener in both acute and experimental settings.
Muscimol's effects are not uniformly distributed throughout the brain. Its action is most pronounced in regions with high concentrations of GABA-A receptors, such as the cerebellum, hippocampus, and cortex. This regional specificity contributes to the compound's unique profile of cognitive and motor effects, including sedation, anxiolysis, and impaired coordination.
At the network level, muscimol-induced inhibition leads to a reduction in overall brain activity and altered patterns of neural synchronization. This dampening effect is particularly evident in electroencephalographic (EEG) recordings, which typically show an increase in slow-wave activity and a decrease in high-frequency oscillations following muscimol administration.
Importantly, the pharmacodynamics of muscimol are dose-dependent. At lower doses, it primarily enhances phasic inhibition, while higher doses can lead to more pronounced tonic inhibition. This dose-response relationship allows for fine-tuning of the cerebral dampening effect, making muscimol a valuable tool in neuroscience research and potentially in therapeutic applications.
The cerebral dampening effects of muscimol are further modulated by its interactions with other neurotransmitter systems. While primarily acting on GABAergic pathways, muscimol indirectly influences glutamatergic, dopaminergic, and serotonergic signaling. These secondary effects contribute to the compound's complex pharmacological profile and its potential for both therapeutic benefits and adverse effects.
Upon binding to GABA-A receptors, muscimol induces a conformational change that increases chloride ion conductance. The resulting influx of chloride ions hyperpolarizes the neuronal membrane, making it less likely for the neuron to fire an action potential. This hyperpolarization effectively dampens neuronal excitability, contributing to the overall inhibitory effect on cerebral activity.
The pharmacodynamic profile of muscimol is characterized by its rapid onset of action and relatively short duration. Unlike some other GABA-A receptor agonists, muscimol does not appear to cause significant receptor desensitization, allowing for sustained inhibitory effects during its period of activity. This property makes muscimol particularly effective as a cerebral dampener in both acute and experimental settings.
Muscimol's effects are not uniformly distributed throughout the brain. Its action is most pronounced in regions with high concentrations of GABA-A receptors, such as the cerebellum, hippocampus, and cortex. This regional specificity contributes to the compound's unique profile of cognitive and motor effects, including sedation, anxiolysis, and impaired coordination.
At the network level, muscimol-induced inhibition leads to a reduction in overall brain activity and altered patterns of neural synchronization. This dampening effect is particularly evident in electroencephalographic (EEG) recordings, which typically show an increase in slow-wave activity and a decrease in high-frequency oscillations following muscimol administration.
Importantly, the pharmacodynamics of muscimol are dose-dependent. At lower doses, it primarily enhances phasic inhibition, while higher doses can lead to more pronounced tonic inhibition. This dose-response relationship allows for fine-tuning of the cerebral dampening effect, making muscimol a valuable tool in neuroscience research and potentially in therapeutic applications.
The cerebral dampening effects of muscimol are further modulated by its interactions with other neurotransmitter systems. While primarily acting on GABAergic pathways, muscimol indirectly influences glutamatergic, dopaminergic, and serotonergic signaling. These secondary effects contribute to the compound's complex pharmacological profile and its potential for both therapeutic benefits and adverse effects.
Current Formulations
01 Muscimol as a cerebral dampening agent
Muscimol, a psychoactive compound found in certain mushrooms, can be used as a cerebral dampening agent. It acts on GABA receptors in the brain, potentially reducing neural activity and producing calming effects. This property makes it a subject of interest for various neurological and psychiatric applications.- Muscimol as a cerebral dampening agent: Muscimol, a psychoactive compound found in certain mushrooms, can be used as a cerebral dampening agent. It acts on GABA receptors in the brain, potentially reducing neural activity and producing calming effects. This property makes it a subject of interest for various neurological and psychiatric applications.
- Delivery methods for muscimol: Various delivery methods can be employed to administer muscimol for cerebral dampening effects. These may include oral formulations, transdermal patches, inhalation devices, or novel drug delivery systems designed to enhance bioavailability and target specific brain regions.
- Combination therapies involving muscimol: Muscimol may be used in combination with other compounds or therapies to enhance its cerebral dampening effects or to target specific neurological conditions. This approach could involve synergistic drug combinations or the integration of muscimol with non-pharmacological interventions.
- Controlled release formulations for muscimol: Developing controlled release formulations for muscimol could optimize its cerebral dampening effects. These formulations may allow for sustained drug release, potentially reducing side effects and improving therapeutic outcomes in various neurological disorders.
- Monitoring and modulating muscimol's effects: Systems and methods for monitoring and modulating the cerebral dampening effects of muscimol could be developed. These may involve real-time brain activity monitoring, adjustable dosing mechanisms, or personalized treatment protocols based on individual patient responses.
02 Delivery methods for muscimol
Various delivery methods can be employed to administer muscimol for cerebral dampening effects. These may include oral formulations, transdermal patches, inhalation devices, or novel drug delivery systems designed to enhance bioavailability and target specific brain regions.Expand Specific Solutions03 Combination therapies involving muscimol
Muscimol may be used in combination with other compounds or therapies to enhance its cerebral dampening effects or to address specific neurological conditions. This approach could potentially improve efficacy while minimizing side effects associated with higher doses of individual agents.Expand Specific Solutions04 Controlled release formulations for muscimol
Developing controlled release formulations for muscimol could provide sustained cerebral dampening effects over extended periods. This may involve the use of specialized polymers, nanoparticles, or other drug delivery technologies to modulate the release profile of muscimol in the body.Expand Specific Solutions05 Monitoring and dosage adjustment systems
Advanced systems for monitoring the effects of muscimol on cerebral activity and adjusting dosage accordingly could be developed. These may incorporate biosensors, neuroimaging techniques, or other real-time monitoring methods to optimize the cerebral dampening effects while ensuring patient safety.Expand Specific Solutions
Key Research Entities
The competitive landscape for muscimol as a cerebral dampener is in an early development stage, with a growing market potential as research progresses. The technology is still emerging, with limited commercial applications currently. Key players like ACADIA Pharmaceuticals, Merck, and CaaMTech are investing in R&D to explore muscimol's therapeutic potential. Academic institutions such as the University of South Florida and Louisiana State University are conducting foundational research. The market size remains relatively small but is expected to expand as clinical trials advance and regulatory approvals are obtained. Overall, the field is characterized by ongoing scientific exploration rather than established commercial products.
ACADIA Pharmaceuticals, Inc.
Technical Solution: ACADIA Pharmaceuticals has developed a novel approach to understanding muscimol's function as a cerebral dampener. Their research focuses on the selective activation of GABA-A receptors, particularly the α5 subunit. Muscimol, a potent GABA-A receptor agonist, is used as a tool compound to study the effects of GABAergic modulation on neural activity. ACADIA's studies have shown that muscimol can reduce hyperexcitability in specific brain regions, potentially offering therapeutic benefits for conditions such as epilepsy and anxiety disorders[1][3]. The company has also explored the use of muscimol analogs with improved pharmacokinetic profiles to enhance the compound's efficacy as a cerebral dampener while minimizing side effects[5].
Strengths: Targeted approach to GABA-A receptor modulation, potential for reduced side effects. Weaknesses: Limited specificity compared to newer, more selective compounds; potential for unwanted systemic effects.
Merck Sharp & Dohme Corp.
Technical Solution: Merck Sharp & Dohme Corp. has conducted extensive research on muscimol's mechanism of action as a cerebral dampener. Their approach involves studying the compound's interaction with GABA-A receptors at a molecular level. Using advanced imaging techniques and electrophysiological studies, Merck has mapped the binding sites of muscimol on various GABA-A receptor subunits, providing insights into its potent inhibitory effects[2]. The company has also investigated the pharmacokinetics of muscimol, demonstrating its ability to cross the blood-brain barrier efficiently and exert rapid effects on neural activity[4]. Merck's research has contributed to the development of novel GABAergic compounds that mimic muscimol's cerebral dampening effects while offering improved selectivity and reduced off-target effects[6].
Strengths: Comprehensive understanding of muscimol's molecular mechanisms, potential for developing more targeted therapies. Weaknesses: Challenges in balancing potency with selectivity, potential for tolerance development with long-term use.
Receptor Interactions
Amanita muscaria compounds
PatentPendingUS20240050502A1
Innovation
- Development of purified Amanita muscaria compound compositions and formulations comprising specific ratios of ibotenic acid, muscimol, and other compounds, which are structurally distinct and free from other Amanita muscaria compounds, combined with excipients and serotonergic drugs, psilocybin derivatives, or cannabinoids to create pharmaceutical formulations for therapeutic use.
Amanita muscaria compounds
PatentWO2022132691A1
Innovation
- Development of compositions comprising purified Amanita muscaria compounds, such as ibotenic acid and muscimol, in specific molar ratios, combined with excipients, to create pharmaceutical formulations that regulate neurotransmitter receptor activity and treat psychological disorders, compulsive disorders, and depressive disorders, while minimizing toxic effects.
Safety Profile
Muscimol, a potent GABA receptor agonist, has been extensively studied for its cerebral dampening effects. The safety profile of muscimol is a critical aspect to consider when evaluating its potential therapeutic applications. Acute toxicity studies in animal models have shown that muscimol has a relatively high safety margin, with lethal doses significantly higher than those required for its pharmacological effects.
However, the long-term safety of muscimol use remains a concern. Chronic administration studies have revealed potential risks, including tolerance development and withdrawal symptoms upon discontinuation. These effects are attributed to the drug's ability to modulate GABAergic neurotransmission, which plays a crucial role in maintaining neuronal excitability balance.
Muscimol's interaction with other central nervous system depressants, such as alcohol and benzodiazepines, poses a significant safety risk. Co-administration can lead to enhanced sedation, respiratory depression, and potentially life-threatening complications. Therefore, strict precautions and monitoring are essential when considering muscimol use in patients with a history of substance abuse or those taking other CNS depressants.
Neurological side effects associated with muscimol use include dizziness, confusion, and impaired motor coordination. These effects are generally dose-dependent and reversible upon discontinuation of the drug. However, in rare cases, more severe neurological complications, such as seizures or prolonged cognitive impairment, have been reported, particularly with high doses or in susceptible individuals.
The potential for muscimol to induce psychoactive effects, including hallucinations and altered perception, raises concerns about its abuse potential. While these effects are typically transient, they necessitate careful consideration in clinical settings and highlight the importance of controlled administration and patient education.
Cardiovascular and respiratory effects of muscimol are generally mild at therapeutic doses. However, at higher doses, it can cause hypotension and respiratory depression, particularly in individuals with pre-existing cardiopulmonary conditions. Close monitoring of vital signs is crucial during muscimol administration, especially in high-risk populations.
In conclusion, while muscimol demonstrates promising therapeutic potential as a cerebral dampener, its safety profile warrants careful consideration. The balance between its beneficial effects and potential risks necessitates thorough clinical evaluation, appropriate patient selection, and stringent monitoring protocols to ensure safe and effective use in medical applications.
However, the long-term safety of muscimol use remains a concern. Chronic administration studies have revealed potential risks, including tolerance development and withdrawal symptoms upon discontinuation. These effects are attributed to the drug's ability to modulate GABAergic neurotransmission, which plays a crucial role in maintaining neuronal excitability balance.
Muscimol's interaction with other central nervous system depressants, such as alcohol and benzodiazepines, poses a significant safety risk. Co-administration can lead to enhanced sedation, respiratory depression, and potentially life-threatening complications. Therefore, strict precautions and monitoring are essential when considering muscimol use in patients with a history of substance abuse or those taking other CNS depressants.
Neurological side effects associated with muscimol use include dizziness, confusion, and impaired motor coordination. These effects are generally dose-dependent and reversible upon discontinuation of the drug. However, in rare cases, more severe neurological complications, such as seizures or prolonged cognitive impairment, have been reported, particularly with high doses or in susceptible individuals.
The potential for muscimol to induce psychoactive effects, including hallucinations and altered perception, raises concerns about its abuse potential. While these effects are typically transient, they necessitate careful consideration in clinical settings and highlight the importance of controlled administration and patient education.
Cardiovascular and respiratory effects of muscimol are generally mild at therapeutic doses. However, at higher doses, it can cause hypotension and respiratory depression, particularly in individuals with pre-existing cardiopulmonary conditions. Close monitoring of vital signs is crucial during muscimol administration, especially in high-risk populations.
In conclusion, while muscimol demonstrates promising therapeutic potential as a cerebral dampener, its safety profile warrants careful consideration. The balance between its beneficial effects and potential risks necessitates thorough clinical evaluation, appropriate patient selection, and stringent monitoring protocols to ensure safe and effective use in medical applications.
Regulatory Status
The regulatory status of muscimol, a potent GABA receptor agonist, varies significantly across different jurisdictions worldwide. In the United States, muscimol is not approved by the Food and Drug Administration (FDA) for any medical use and is classified as a Schedule III controlled substance under the Controlled Substances Act. This classification indicates that while it has potential for abuse, it also has accepted medical uses and a moderate to low potential for physical and psychological dependence.
In the European Union, muscimol is not approved as a medicinal product by the European Medicines Agency (EMA). However, its regulatory status may differ among individual member states. Some countries may allow limited research use under strict controls, while others may have more restrictive policies.
In Canada, muscimol is not listed in the Controlled Drugs and Substances Act, but it is regulated under the Food and Drugs Act. It is not approved for any therapeutic use and is primarily restricted to research purposes under appropriate licenses.
Australia classifies muscimol as a Schedule 9 substance under the Standard for the Uniform Scheduling of Medicines and Poisons (SUSMP), indicating that it is a prohibited substance with very high potential for harm and no recognized therapeutic use.
Japan's regulatory framework does not specifically list muscimol, but it falls under the general category of psychoactive substances, which are strictly controlled under the Pharmaceutical Affairs Law and the Narcotics and Psychotropics Control Law.
Despite its current regulatory status, there is growing interest in the potential therapeutic applications of muscimol, particularly in the treatment of neurological disorders. This has led to increased research efforts and clinical trials exploring its efficacy and safety profile. As a result, the regulatory landscape for muscimol may evolve in the coming years, potentially leading to changes in its classification and approved uses in various countries.
The current regulatory status of muscimol presents challenges for researchers and pharmaceutical companies interested in developing therapies based on this compound. Strict controls and varying international regulations necessitate careful navigation of legal and ethical considerations in the pursuit of muscimol-based treatments. As research progresses and more data becomes available on its potential benefits and risks, regulatory bodies may reassess their stance on muscimol, potentially opening new avenues for its therapeutic use under controlled conditions.
In the European Union, muscimol is not approved as a medicinal product by the European Medicines Agency (EMA). However, its regulatory status may differ among individual member states. Some countries may allow limited research use under strict controls, while others may have more restrictive policies.
In Canada, muscimol is not listed in the Controlled Drugs and Substances Act, but it is regulated under the Food and Drugs Act. It is not approved for any therapeutic use and is primarily restricted to research purposes under appropriate licenses.
Australia classifies muscimol as a Schedule 9 substance under the Standard for the Uniform Scheduling of Medicines and Poisons (SUSMP), indicating that it is a prohibited substance with very high potential for harm and no recognized therapeutic use.
Japan's regulatory framework does not specifically list muscimol, but it falls under the general category of psychoactive substances, which are strictly controlled under the Pharmaceutical Affairs Law and the Narcotics and Psychotropics Control Law.
Despite its current regulatory status, there is growing interest in the potential therapeutic applications of muscimol, particularly in the treatment of neurological disorders. This has led to increased research efforts and clinical trials exploring its efficacy and safety profile. As a result, the regulatory landscape for muscimol may evolve in the coming years, potentially leading to changes in its classification and approved uses in various countries.
The current regulatory status of muscimol presents challenges for researchers and pharmaceutical companies interested in developing therapies based on this compound. Strict controls and varying international regulations necessitate careful navigation of legal and ethical considerations in the pursuit of muscimol-based treatments. As research progresses and more data becomes available on its potential benefits and risks, regulatory bodies may reassess their stance on muscimol, potentially opening new avenues for its therapeutic use under controlled conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!