How Perchloric Acid Acts as a Dehydrating Agent in Chemical Synthesis
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Dehydration Background
Perchloric acid, a powerful oxidizing agent and strong acid, has been utilized in chemical synthesis as an effective dehydrating agent for decades. Its unique properties and reactivity make it a valuable tool in various organic and inorganic reactions where water removal is crucial. The background of perchloric acid's use as a dehydrating agent dates back to the early 20th century when chemists began exploring its potential in synthetic applications.
The dehydrating capabilities of perchloric acid stem from its strong acidic nature and its ability to form stable perchlorate salts. As a strong acid, it readily donates protons, facilitating the removal of water molecules from reactants or reaction intermediates. This property is particularly useful in reactions where water is a byproduct or where the presence of water can hinder the desired reaction pathway.
In organic synthesis, perchloric acid has found applications in various dehydration reactions, including the formation of esters, ethers, and alkenes. Its ability to remove water molecules from alcohols and carboxylic acids has made it a valuable reagent in the preparation of anhydrous compounds. The acid's strong dehydrating power allows for efficient conversion of alcohols to alkenes, a process known as dehydration, which is fundamental in many organic transformations.
Inorganic chemistry has also benefited from perchloric acid's dehydrating properties. It has been used in the preparation of anhydrous metal salts and in the synthesis of complex coordination compounds. The acid's ability to remove water from hydrated metal ions has made it an essential tool in the isolation of water-sensitive compounds and in the study of anhydrous inorganic systems.
The use of perchloric acid as a dehydrating agent has evolved over time, with researchers developing safer handling protocols and exploring alternative reagents for specific applications. Despite its effectiveness, the inherent dangers associated with perchloric acid, such as its explosive nature when in contact with organic materials, have led to the development of safer alternatives in some cases.
Understanding the background of perchloric acid's role as a dehydrating agent provides crucial context for its current applications and ongoing research in chemical synthesis. This knowledge forms the foundation for exploring new methodologies and improving existing processes in both academic and industrial settings.
The dehydrating capabilities of perchloric acid stem from its strong acidic nature and its ability to form stable perchlorate salts. As a strong acid, it readily donates protons, facilitating the removal of water molecules from reactants or reaction intermediates. This property is particularly useful in reactions where water is a byproduct or where the presence of water can hinder the desired reaction pathway.
In organic synthesis, perchloric acid has found applications in various dehydration reactions, including the formation of esters, ethers, and alkenes. Its ability to remove water molecules from alcohols and carboxylic acids has made it a valuable reagent in the preparation of anhydrous compounds. The acid's strong dehydrating power allows for efficient conversion of alcohols to alkenes, a process known as dehydration, which is fundamental in many organic transformations.
Inorganic chemistry has also benefited from perchloric acid's dehydrating properties. It has been used in the preparation of anhydrous metal salts and in the synthesis of complex coordination compounds. The acid's ability to remove water from hydrated metal ions has made it an essential tool in the isolation of water-sensitive compounds and in the study of anhydrous inorganic systems.
The use of perchloric acid as a dehydrating agent has evolved over time, with researchers developing safer handling protocols and exploring alternative reagents for specific applications. Despite its effectiveness, the inherent dangers associated with perchloric acid, such as its explosive nature when in contact with organic materials, have led to the development of safer alternatives in some cases.
Understanding the background of perchloric acid's role as a dehydrating agent provides crucial context for its current applications and ongoing research in chemical synthesis. This knowledge forms the foundation for exploring new methodologies and improving existing processes in both academic and industrial settings.
Market Demand Analysis
The market demand for perchloric acid as a dehydrating agent in chemical synthesis has been steadily growing, driven by its unique properties and versatile applications across various industries. In the pharmaceutical sector, perchloric acid plays a crucial role in the synthesis of complex organic compounds, particularly in the production of active pharmaceutical ingredients (APIs). The increasing demand for novel drug formulations and the expansion of the pharmaceutical industry in emerging markets have contributed to the rising need for efficient dehydrating agents like perchloric acid.
In the field of materials science and polymer chemistry, perchloric acid's dehydrating capabilities are highly valued for the production of high-performance polymers and advanced materials. The growing demand for lightweight, durable, and heat-resistant materials in industries such as aerospace, automotive, and electronics has further fueled the market for perchloric acid. Its ability to facilitate precise control over reaction conditions and yield high-purity products makes it an attractive choice for researchers and manufacturers alike.
The petrochemical industry also represents a significant market for perchloric acid as a dehydrating agent. In the production of various petrochemical derivatives, perchloric acid is utilized to remove water from reaction mixtures, enhancing the efficiency of processes and improving product quality. As the global demand for petrochemicals continues to rise, driven by population growth and increasing industrialization, the market for perchloric acid is expected to expand correspondingly.
Environmental concerns and stringent regulations have led to an increased focus on green chemistry and sustainable production methods. This trend has created opportunities for the development of novel applications of perchloric acid as a dehydrating agent in environmentally friendly synthesis routes. Research into more efficient and selective dehydration processes using perchloric acid is ongoing, potentially opening up new market segments and applications.
The electronics industry, particularly in the production of printed circuit boards and semiconductor devices, has also contributed to the growing demand for perchloric acid. Its use in etching and cleaning processes, as well as in the synthesis of specialized electronic materials, has made it an essential component in the manufacturing of high-tech products. As the global electronics market continues to expand, driven by advancements in technologies such as 5G, IoT, and AI, the demand for perchloric acid is expected to increase accordingly.
While the market for perchloric acid as a dehydrating agent shows promising growth potential, it is important to note that safety concerns and regulatory restrictions may impact its widespread adoption in certain applications. The development of safer handling protocols and more efficient recovery and recycling methods for perchloric acid could help address these challenges and further expand its market potential in chemical synthesis applications.
In the field of materials science and polymer chemistry, perchloric acid's dehydrating capabilities are highly valued for the production of high-performance polymers and advanced materials. The growing demand for lightweight, durable, and heat-resistant materials in industries such as aerospace, automotive, and electronics has further fueled the market for perchloric acid. Its ability to facilitate precise control over reaction conditions and yield high-purity products makes it an attractive choice for researchers and manufacturers alike.
The petrochemical industry also represents a significant market for perchloric acid as a dehydrating agent. In the production of various petrochemical derivatives, perchloric acid is utilized to remove water from reaction mixtures, enhancing the efficiency of processes and improving product quality. As the global demand for petrochemicals continues to rise, driven by population growth and increasing industrialization, the market for perchloric acid is expected to expand correspondingly.
Environmental concerns and stringent regulations have led to an increased focus on green chemistry and sustainable production methods. This trend has created opportunities for the development of novel applications of perchloric acid as a dehydrating agent in environmentally friendly synthesis routes. Research into more efficient and selective dehydration processes using perchloric acid is ongoing, potentially opening up new market segments and applications.
The electronics industry, particularly in the production of printed circuit boards and semiconductor devices, has also contributed to the growing demand for perchloric acid. Its use in etching and cleaning processes, as well as in the synthesis of specialized electronic materials, has made it an essential component in the manufacturing of high-tech products. As the global electronics market continues to expand, driven by advancements in technologies such as 5G, IoT, and AI, the demand for perchloric acid is expected to increase accordingly.
While the market for perchloric acid as a dehydrating agent shows promising growth potential, it is important to note that safety concerns and regulatory restrictions may impact its widespread adoption in certain applications. The development of safer handling protocols and more efficient recovery and recycling methods for perchloric acid could help address these challenges and further expand its market potential in chemical synthesis applications.
Current Challenges in Dehydration Processes
Dehydration processes play a crucial role in chemical synthesis, particularly in the production of various organic compounds. However, these processes face several significant challenges that hinder their efficiency and widespread application. One of the primary issues is the selectivity of dehydration reactions, especially when dealing with complex molecules containing multiple functional groups.
The presence of multiple hydroxyl groups or other water-sensitive moieties can lead to undesired side reactions, resulting in lower yields and reduced product purity. This challenge is particularly pronounced in the synthesis of pharmaceuticals and fine chemicals, where precise control over reaction outcomes is essential.
Another major hurdle in dehydration processes is the energy intensity of traditional methods. Many conventional dehydrating agents require high temperatures or prolonged reaction times, leading to increased energy consumption and potential degradation of sensitive compounds. This not only impacts the economic viability of the process but also raises environmental concerns due to the associated carbon footprint.
The use of strong acids as dehydrating agents, including perchloric acid, presents safety and handling challenges. These corrosive substances pose significant risks to personnel and equipment, necessitating stringent safety protocols and specialized infrastructure. Furthermore, the disposal of spent acid and byproducts from these reactions often requires additional treatment steps, adding complexity and cost to the overall process.
Scale-up of dehydration reactions from laboratory to industrial scale presents its own set of challenges. Heat transfer limitations, mixing inefficiencies, and the need for large quantities of dehydrating agents can significantly impact process economics and practicality. Additionally, the potential for runaway reactions or unexpected byproduct formation increases with scale, necessitating careful process design and control measures.
The development of sustainable and green chemistry alternatives to traditional dehydration methods remains an ongoing challenge. While progress has been made in utilizing catalytic systems and alternative solvents, many of these approaches still struggle with issues of efficiency, cost-effectiveness, or broad applicability across different substrate classes.
Lastly, the removal and recovery of water produced during dehydration reactions pose significant challenges, particularly in equilibrium-controlled processes. Efficient water removal is crucial for driving reactions to completion and maximizing yields. However, conventional methods such as azeotropic distillation or the use of desiccants often have limitations in terms of energy efficiency or regeneration capabilities.
The presence of multiple hydroxyl groups or other water-sensitive moieties can lead to undesired side reactions, resulting in lower yields and reduced product purity. This challenge is particularly pronounced in the synthesis of pharmaceuticals and fine chemicals, where precise control over reaction outcomes is essential.
Another major hurdle in dehydration processes is the energy intensity of traditional methods. Many conventional dehydrating agents require high temperatures or prolonged reaction times, leading to increased energy consumption and potential degradation of sensitive compounds. This not only impacts the economic viability of the process but also raises environmental concerns due to the associated carbon footprint.
The use of strong acids as dehydrating agents, including perchloric acid, presents safety and handling challenges. These corrosive substances pose significant risks to personnel and equipment, necessitating stringent safety protocols and specialized infrastructure. Furthermore, the disposal of spent acid and byproducts from these reactions often requires additional treatment steps, adding complexity and cost to the overall process.
Scale-up of dehydration reactions from laboratory to industrial scale presents its own set of challenges. Heat transfer limitations, mixing inefficiencies, and the need for large quantities of dehydrating agents can significantly impact process economics and practicality. Additionally, the potential for runaway reactions or unexpected byproduct formation increases with scale, necessitating careful process design and control measures.
The development of sustainable and green chemistry alternatives to traditional dehydration methods remains an ongoing challenge. While progress has been made in utilizing catalytic systems and alternative solvents, many of these approaches still struggle with issues of efficiency, cost-effectiveness, or broad applicability across different substrate classes.
Lastly, the removal and recovery of water produced during dehydration reactions pose significant challenges, particularly in equilibrium-controlled processes. Efficient water removal is crucial for driving reactions to completion and maximizing yields. However, conventional methods such as azeotropic distillation or the use of desiccants often have limitations in terms of energy efficiency or regeneration capabilities.
Existing Perchloric Acid Dehydration Methods
01 Perchloric acid as a dehydrating agent
Perchloric acid is used as a powerful dehydrating agent in various chemical processes. Its strong oxidizing properties and ability to remove water molecules make it effective for dehydration reactions in organic synthesis and material processing.- Perchloric acid as a dehydrating agent: Perchloric acid is used as a powerful dehydrating agent in various chemical processes. Its strong oxidizing properties and ability to remove water molecules make it effective for dehydration reactions in organic synthesis and material processing.
- Dehydration of organic compounds: Perchloric acid is employed in the dehydration of organic compounds, particularly in the synthesis of alkenes from alcohols. This process involves the elimination of water molecules from the substrate, resulting in the formation of carbon-carbon double bonds.
- Dehydration in material processing: Perchloric acid dehydration is utilized in material processing, such as the preparation of desiccants, dehydration of polymers, and treatment of certain minerals. This application enhances the properties and performance of various materials by removing moisture content.
- Safety considerations in perchloric acid dehydration: Due to the highly reactive nature of perchloric acid, special safety measures are required when using it for dehydration processes. This includes the use of specialized equipment, proper handling techniques, and appropriate personal protective equipment to mitigate risks associated with its corrosive and oxidizing properties.
- Alternative dehydration methods: While perchloric acid is effective for dehydration, alternative methods and reagents are being explored to address safety concerns and environmental impact. These include the use of other strong acids, desiccants, and novel catalytic systems that can achieve similar dehydration results with reduced risks.
02 Dehydration of organic compounds
Perchloric acid is employed in the dehydration of organic compounds, particularly in the synthesis of complex molecules. It can remove water from alcohols to form alkenes, or assist in the formation of cyclic compounds through intramolecular dehydration reactions.Expand Specific Solutions03 Perchloric acid in material processing
In material science and engineering, perchloric acid is used for dehydration processes in the preparation of certain materials. This includes the treatment of polymers, ceramics, and other advanced materials to remove residual water and improve their properties.Expand Specific Solutions04 Safety considerations in perchloric acid dehydration
Due to the strong oxidizing nature of perchloric acid, special safety measures are required when using it for dehydration processes. This includes the use of specialized equipment, proper handling procedures, and appropriate personal protective equipment to mitigate risks associated with its reactivity.Expand Specific Solutions05 Alternative dehydration methods
While perchloric acid is effective for dehydration, alternative methods and reagents are also explored due to safety concerns. These may include other strong acids, desiccants, or physical dehydration techniques that can achieve similar results with potentially lower risks.Expand Specific Solutions
Key Players in Chemical Synthesis Industry
The competitive landscape for perchloric acid as a dehydrating agent in chemical synthesis is characterized by a mature market with established players. The industry is in a stable growth phase, with a moderate market size driven by demand in various chemical processes. Technological maturity is high, with companies like Henkel AG & Co. KGaA, China Petroleum & Chemical Corp., and IHI Corp. leading in research and development. These firms, along with specialized chemical manufacturers such as Adeka Corp. and TaiGen Biotechnology, are continuously improving the efficiency and safety of perchloric acid applications. The market also sees participation from academic institutions like Xi'an Jiaotong University and Tongji University, contributing to ongoing advancements in the field.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced techniques for using perchloric acid as a dehydrating agent in chemical synthesis. Their approach involves utilizing perchloric acid's strong oxidizing properties to remove water molecules from organic compounds efficiently. Sinopec has optimized the process by controlling reaction temperatures and concentrations to maximize dehydration while minimizing side reactions. They have also implemented safety protocols to handle the potentially explosive nature of perchloric acid[1]. Sinopec's method has been particularly effective in the synthesis of complex organic molecules and petrochemical products, improving yields and purity levels[2].
Strengths: Extensive experience in large-scale chemical processes, advanced safety protocols, and high efficiency in petrochemical synthesis. Weaknesses: Potential environmental concerns and the need for specialized equipment to handle perchloric acid safely.
Sinopec Research Institute of Petroleum Processing
Technical Solution: The Sinopec Research Institute of Petroleum Processing has developed innovative applications of perchloric acid as a dehydrating agent in petroleum refining processes. Their approach focuses on using perchloric acid to remove water from crude oil and intermediate products, enhancing the efficiency of catalytic cracking and hydroprocessing operations. The institute has engineered a controlled-release system for perchloric acid application, which allows for precise dosing and minimizes the risks associated with handling this strong acid[3]. They have also developed novel catalysts that work synergistically with perchloric acid, further improving dehydration efficiency and selectivity in complex hydrocarbon mixtures[4].
Strengths: Specialized expertise in petroleum processing, integration with existing refining technologies, and improved product quality. Weaknesses: Limited applicability outside the petroleum industry and potential corrosion issues in processing equipment.
Core Innovations in Dehydration Techniques
Method and apparatus for producing perchlorate
PatentInactiveEP2412847A1
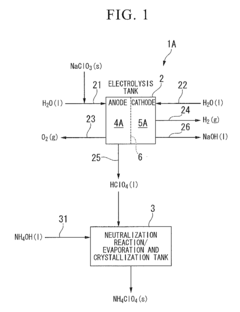
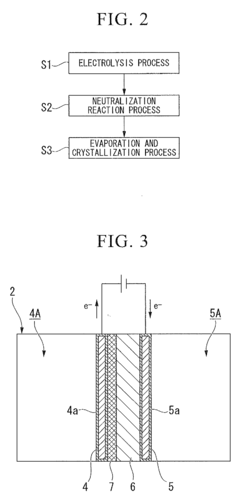
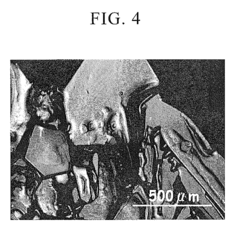
Innovation
- A method involving an electrolysis process using a cation exchange membrane to separate sodium ions from perchlorate ions, followed by a neutralization reaction with alkaline substances like ammonia, and subsequent evaporation and crystallization to produce high-purity perchlorates, reducing the need for toxic additives and simplifying the process.
Perchlorate Destruction
PatentInactiveUS20090008333A1
Innovation
- The use of methanesulfonic acid to increase the solubility of Ti(III) ions, allowing for electrochemical reduction of Ti(IV) to Ti(III) in an acidic environment, which accelerates perchlorate destruction to chloride without the need for high temperatures or organic accelerants, and can be applied to both selective and non-selective anion exchange resins.
Safety Considerations for Perchloric Acid Use
Perchloric acid is a powerful oxidizing agent and dehydrating agent widely used in chemical synthesis. However, its highly reactive nature necessitates strict safety protocols to prevent accidents and ensure safe handling. When working with perchloric acid, proper personal protective equipment (PPE) is essential. This includes chemical-resistant gloves, goggles, face shield, and a lab coat or chemical-resistant apron. A fume hood specifically designed for perchloric acid use is crucial to contain and neutralize any vapors or fumes.
Storage of perchloric acid requires special considerations. It should be kept in a cool, dry place away from organic materials, reducing agents, and other incompatible substances. Glass or PTFE containers are recommended, as perchloric acid can react with some metals. Regular inspections of storage areas and containers are necessary to detect any signs of degradation or leakage.
Handling procedures for perchloric acid must be carefully followed. It should never be allowed to come into contact with organic materials or dehydrating agents, as this can lead to the formation of explosive perchlorates. When diluting perchloric acid, it should always be added to water, never the reverse, to avoid violent reactions.
Emergency response plans must be in place for spills or accidents involving perchloric acid. This includes having appropriate spill kits, neutralizing agents, and evacuation procedures readily available. All personnel working with or around perchloric acid should be thoroughly trained in these safety protocols and emergency procedures.
Waste disposal of perchloric acid and its byproducts requires special attention. It should never be disposed of down the drain or mixed with other chemical waste. Instead, it must be properly neutralized and disposed of according to local regulations and institutional guidelines.
Regular maintenance and cleaning of equipment and work areas used with perchloric acid is crucial. This includes periodic cleaning of fume hoods to prevent the buildup of potentially explosive perchlorate salts. Any equipment or surfaces that have come into contact with perchloric acid should be thoroughly decontaminated before being used for other purposes.
Lastly, it's important to maintain accurate records of perchloric acid use, storage, and disposal. This helps in tracking inventory, ensuring proper handling procedures are followed, and facilitating quick response in case of emergencies. Regular safety audits and reviews of perchloric acid handling procedures can help identify potential risks and improve safety measures over time.
Storage of perchloric acid requires special considerations. It should be kept in a cool, dry place away from organic materials, reducing agents, and other incompatible substances. Glass or PTFE containers are recommended, as perchloric acid can react with some metals. Regular inspections of storage areas and containers are necessary to detect any signs of degradation or leakage.
Handling procedures for perchloric acid must be carefully followed. It should never be allowed to come into contact with organic materials or dehydrating agents, as this can lead to the formation of explosive perchlorates. When diluting perchloric acid, it should always be added to water, never the reverse, to avoid violent reactions.
Emergency response plans must be in place for spills or accidents involving perchloric acid. This includes having appropriate spill kits, neutralizing agents, and evacuation procedures readily available. All personnel working with or around perchloric acid should be thoroughly trained in these safety protocols and emergency procedures.
Waste disposal of perchloric acid and its byproducts requires special attention. It should never be disposed of down the drain or mixed with other chemical waste. Instead, it must be properly neutralized and disposed of according to local regulations and institutional guidelines.
Regular maintenance and cleaning of equipment and work areas used with perchloric acid is crucial. This includes periodic cleaning of fume hoods to prevent the buildup of potentially explosive perchlorate salts. Any equipment or surfaces that have come into contact with perchloric acid should be thoroughly decontaminated before being used for other purposes.
Lastly, it's important to maintain accurate records of perchloric acid use, storage, and disposal. This helps in tracking inventory, ensuring proper handling procedures are followed, and facilitating quick response in case of emergencies. Regular safety audits and reviews of perchloric acid handling procedures can help identify potential risks and improve safety measures over time.
Environmental Impact Assessment
The use of perchloric acid as a dehydrating agent in chemical synthesis raises significant environmental concerns that require careful assessment and mitigation strategies. Perchloric acid is a strong oxidizing agent and can pose serious risks to ecosystems if released into the environment. Its high reactivity and corrosive nature can lead to soil and water contamination, potentially causing long-term damage to flora and fauna.
One of the primary environmental risks associated with perchloric acid is its potential to form explosive perchlorates when it comes into contact with organic materials. This can result in hazardous situations during storage, handling, and disposal processes. Additionally, perchloric acid can contribute to the formation of acid rain if released into the atmosphere, leading to acidification of water bodies and soil, which can have detrimental effects on aquatic life and vegetation.
The production and use of perchloric acid in chemical synthesis also contribute to air pollution. Volatile organic compounds (VOCs) and other hazardous air pollutants may be released during the manufacturing process and subsequent chemical reactions. These emissions can lead to smog formation and have adverse effects on air quality, potentially impacting human health and the environment.
Water pollution is another significant concern. Improper disposal or accidental spills of perchloric acid can contaminate groundwater and surface water sources. This contamination can persist for extended periods due to the stability of perchlorate ions in aqueous environments. The presence of perchlorates in drinking water sources poses health risks to humans and wildlife, particularly affecting thyroid function.
To mitigate these environmental impacts, stringent control measures and best practices must be implemented. This includes proper containment systems, advanced air filtration technologies, and wastewater treatment processes specifically designed to handle perchloric acid and its byproducts. Recycling and recovery systems should be employed to minimize waste generation and reduce the overall environmental footprint of perchloric acid use in chemical synthesis.
Furthermore, research into alternative dehydrating agents or greener synthesis methods should be prioritized to reduce reliance on perchloric acid. This could involve exploring bio-based alternatives or developing novel catalytic processes that achieve similar dehydration effects with lower environmental impact. Implementing life cycle assessments for perchloric acid-based processes can help identify areas for improvement and guide the development of more sustainable chemical synthesis practices.
One of the primary environmental risks associated with perchloric acid is its potential to form explosive perchlorates when it comes into contact with organic materials. This can result in hazardous situations during storage, handling, and disposal processes. Additionally, perchloric acid can contribute to the formation of acid rain if released into the atmosphere, leading to acidification of water bodies and soil, which can have detrimental effects on aquatic life and vegetation.
The production and use of perchloric acid in chemical synthesis also contribute to air pollution. Volatile organic compounds (VOCs) and other hazardous air pollutants may be released during the manufacturing process and subsequent chemical reactions. These emissions can lead to smog formation and have adverse effects on air quality, potentially impacting human health and the environment.
Water pollution is another significant concern. Improper disposal or accidental spills of perchloric acid can contaminate groundwater and surface water sources. This contamination can persist for extended periods due to the stability of perchlorate ions in aqueous environments. The presence of perchlorates in drinking water sources poses health risks to humans and wildlife, particularly affecting thyroid function.
To mitigate these environmental impacts, stringent control measures and best practices must be implemented. This includes proper containment systems, advanced air filtration technologies, and wastewater treatment processes specifically designed to handle perchloric acid and its byproducts. Recycling and recovery systems should be employed to minimize waste generation and reduce the overall environmental footprint of perchloric acid use in chemical synthesis.
Furthermore, research into alternative dehydrating agents or greener synthesis methods should be prioritized to reduce reliance on perchloric acid. This could involve exploring bio-based alternatives or developing novel catalytic processes that achieve similar dehydration effects with lower environmental impact. Implementing life cycle assessments for perchloric acid-based processes can help identify areas for improvement and guide the development of more sustainable chemical synthesis practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!