Reaction Mechanisms of Perchloric Acid in Protein Denaturation
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Protein Denaturation Background
Perchloric acid (HClO4) has long been recognized as a potent protein denaturant, playing a crucial role in various biochemical and analytical applications. The interaction between perchloric acid and proteins has been a subject of extensive research due to its significant impact on protein structure and function. This powerful oxidizing agent is known for its ability to disrupt the intricate three-dimensional structure of proteins, leading to their denaturation.
The process of protein denaturation by perchloric acid involves complex mechanisms that affect both the primary and secondary structures of proteins. At the molecular level, perchloric acid interacts with the peptide bonds and side chains of amino acids, causing the unfolding of protein molecules. This interaction results in the loss of the protein's native conformation, which is essential for its biological activity.
One of the primary mechanisms by which perchloric acid denatures proteins is through the disruption of hydrogen bonds. These bonds are crucial for maintaining the secondary and tertiary structures of proteins. Perchloric acid, being a strong acid, can protonate the carbonyl oxygen atoms in the peptide backbone, weakening the hydrogen bonds that stabilize the protein's structure. This protonation leads to the unraveling of alpha-helices and beta-sheets, two fundamental secondary structure elements in proteins.
Additionally, perchloric acid affects the electrostatic interactions within proteins. It can neutralize charged amino acid side chains, altering the delicate balance of attractive and repulsive forces that contribute to protein stability. This neutralization can lead to the exposure of hydrophobic regions that are typically buried within the protein's core, further contributing to the denaturation process.
The concentration of perchloric acid plays a significant role in the extent and rate of protein denaturation. At lower concentrations, the acid may cause partial unfolding of proteins, while higher concentrations can lead to complete denaturation and potential fragmentation of the protein structure. The pH of the solution, temperature, and the specific properties of the protein in question also influence the denaturation process.
Understanding the reaction mechanisms of perchloric acid in protein denaturation is crucial for various applications in biochemistry and biotechnology. This knowledge is particularly valuable in protein purification techniques, where controlled denaturation can be used to isolate and study specific protein components. Furthermore, insights into these mechanisms contribute to the development of more effective protein stabilization methods and the design of novel protein-based materials.
The process of protein denaturation by perchloric acid involves complex mechanisms that affect both the primary and secondary structures of proteins. At the molecular level, perchloric acid interacts with the peptide bonds and side chains of amino acids, causing the unfolding of protein molecules. This interaction results in the loss of the protein's native conformation, which is essential for its biological activity.
One of the primary mechanisms by which perchloric acid denatures proteins is through the disruption of hydrogen bonds. These bonds are crucial for maintaining the secondary and tertiary structures of proteins. Perchloric acid, being a strong acid, can protonate the carbonyl oxygen atoms in the peptide backbone, weakening the hydrogen bonds that stabilize the protein's structure. This protonation leads to the unraveling of alpha-helices and beta-sheets, two fundamental secondary structure elements in proteins.
Additionally, perchloric acid affects the electrostatic interactions within proteins. It can neutralize charged amino acid side chains, altering the delicate balance of attractive and repulsive forces that contribute to protein stability. This neutralization can lead to the exposure of hydrophobic regions that are typically buried within the protein's core, further contributing to the denaturation process.
The concentration of perchloric acid plays a significant role in the extent and rate of protein denaturation. At lower concentrations, the acid may cause partial unfolding of proteins, while higher concentrations can lead to complete denaturation and potential fragmentation of the protein structure. The pH of the solution, temperature, and the specific properties of the protein in question also influence the denaturation process.
Understanding the reaction mechanisms of perchloric acid in protein denaturation is crucial for various applications in biochemistry and biotechnology. This knowledge is particularly valuable in protein purification techniques, where controlled denaturation can be used to isolate and study specific protein components. Furthermore, insights into these mechanisms contribute to the development of more effective protein stabilization methods and the design of novel protein-based materials.
Market Analysis for Protein Denaturation Techniques
The protein denaturation techniques market has witnessed significant growth in recent years, driven by the increasing demand for protein analysis in various fields such as biotechnology, pharmaceuticals, and food science. The global market for protein denaturation techniques is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to be in the high single digits over the next five years.
One of the key factors contributing to this market growth is the rising adoption of proteomics research in drug discovery and development processes. As pharmaceutical companies intensify their efforts to develop novel therapeutics, the need for efficient protein denaturation techniques has become more pronounced. This trend is particularly evident in the field of personalized medicine, where understanding protein structures and functions is crucial for developing targeted therapies.
The food and beverage industry has also emerged as a significant driver of demand for protein denaturation techniques. With increasing consumer awareness about food quality and safety, manufacturers are investing in advanced protein analysis methods to ensure product integrity and compliance with regulatory standards. This has led to a surge in the adoption of protein denaturation techniques in food testing laboratories and quality control departments.
In the academic and research sector, there is a growing interest in studying protein folding and unfolding mechanisms, which has further fueled the demand for sophisticated protein denaturation techniques. Universities and research institutions are allocating substantial resources to proteomics research, contributing to the overall market expansion.
Geographically, North America currently holds the largest share of the protein denaturation techniques market, owing to its well-established biotechnology and pharmaceutical industries. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in life sciences research and the rapid expansion of the biopharmaceutical sector in countries like China and India.
The market for protein denaturation techniques is characterized by the presence of several key players, including major life sciences companies and specialized instrument manufacturers. These companies are focusing on developing innovative solutions to enhance the efficiency and accuracy of protein denaturation processes. The competitive landscape is marked by strategic collaborations, mergers and acquisitions, and continuous product innovations aimed at gaining a larger market share.
As the field of proteomics continues to evolve, there is a growing demand for more advanced and high-throughput protein denaturation techniques. This has led to the development of novel approaches, such as microfluidic-based systems and automated protein denaturation platforms, which offer improved speed and precision in protein analysis. These technological advancements are expected to further drive market growth and open up new opportunities for both established players and new entrants in the protein denaturation techniques market.
One of the key factors contributing to this market growth is the rising adoption of proteomics research in drug discovery and development processes. As pharmaceutical companies intensify their efforts to develop novel therapeutics, the need for efficient protein denaturation techniques has become more pronounced. This trend is particularly evident in the field of personalized medicine, where understanding protein structures and functions is crucial for developing targeted therapies.
The food and beverage industry has also emerged as a significant driver of demand for protein denaturation techniques. With increasing consumer awareness about food quality and safety, manufacturers are investing in advanced protein analysis methods to ensure product integrity and compliance with regulatory standards. This has led to a surge in the adoption of protein denaturation techniques in food testing laboratories and quality control departments.
In the academic and research sector, there is a growing interest in studying protein folding and unfolding mechanisms, which has further fueled the demand for sophisticated protein denaturation techniques. Universities and research institutions are allocating substantial resources to proteomics research, contributing to the overall market expansion.
Geographically, North America currently holds the largest share of the protein denaturation techniques market, owing to its well-established biotechnology and pharmaceutical industries. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in life sciences research and the rapid expansion of the biopharmaceutical sector in countries like China and India.
The market for protein denaturation techniques is characterized by the presence of several key players, including major life sciences companies and specialized instrument manufacturers. These companies are focusing on developing innovative solutions to enhance the efficiency and accuracy of protein denaturation processes. The competitive landscape is marked by strategic collaborations, mergers and acquisitions, and continuous product innovations aimed at gaining a larger market share.
As the field of proteomics continues to evolve, there is a growing demand for more advanced and high-throughput protein denaturation techniques. This has led to the development of novel approaches, such as microfluidic-based systems and automated protein denaturation platforms, which offer improved speed and precision in protein analysis. These technological advancements are expected to further drive market growth and open up new opportunities for both established players and new entrants in the protein denaturation techniques market.
Current Challenges in Perchloric Acid Protein Denaturation
Despite the widespread use of perchloric acid in protein denaturation studies, several challenges persist in fully understanding and controlling the reaction mechanisms involved. One of the primary difficulties lies in the complex nature of protein-perchlorate interactions, which can vary significantly depending on the specific protein structure and environmental conditions.
The high reactivity of perchloric acid poses a significant challenge in maintaining consistent experimental conditions. Even slight variations in concentration or temperature can lead to substantial differences in denaturation rates and outcomes, making it difficult to reproduce results across different studies or laboratories. This variability hinders the development of standardized protocols for protein denaturation using perchloric acid.
Another major challenge is the potential for side reactions and unintended modifications to protein structures. Perchloric acid's strong oxidizing properties can lead to the formation of unwanted byproducts or cause irreversible changes to amino acid residues, particularly those containing sulfur or aromatic groups. These modifications can complicate the interpretation of experimental results and may lead to inaccurate conclusions about protein structure and function.
The mechanism by which perchloric acid denatures proteins is not fully elucidated, presenting a significant knowledge gap in the field. While it is generally accepted that perchlorate ions disrupt hydrogen bonding and electrostatic interactions within proteins, the specific sequence of events and the relative contributions of different factors remain unclear. This lack of mechanistic understanding limits the ability to predict and control the denaturation process for diverse protein types.
Furthermore, the extreme acidity of perchloric acid solutions presents safety concerns and handling difficulties in laboratory settings. Specialized equipment and stringent safety protocols are required, which can limit the accessibility of this technique for some researchers and increase the overall cost and complexity of experiments.
The reversibility of protein denaturation induced by perchloric acid is another area of ongoing challenge. While some proteins can be successfully renatured after perchloric acid treatment, others suffer irreversible changes, limiting the applicability of this method for certain studies or applications. Understanding the factors that influence renaturation success remains an important area of investigation.
Lastly, the environmental impact and disposal of perchloric acid waste present significant challenges. The strong oxidizing nature of perchloric acid and its potential to form explosive compounds necessitate careful handling and specialized disposal procedures, adding to the complexity and cost of using this reagent in protein denaturation studies.
The high reactivity of perchloric acid poses a significant challenge in maintaining consistent experimental conditions. Even slight variations in concentration or temperature can lead to substantial differences in denaturation rates and outcomes, making it difficult to reproduce results across different studies or laboratories. This variability hinders the development of standardized protocols for protein denaturation using perchloric acid.
Another major challenge is the potential for side reactions and unintended modifications to protein structures. Perchloric acid's strong oxidizing properties can lead to the formation of unwanted byproducts or cause irreversible changes to amino acid residues, particularly those containing sulfur or aromatic groups. These modifications can complicate the interpretation of experimental results and may lead to inaccurate conclusions about protein structure and function.
The mechanism by which perchloric acid denatures proteins is not fully elucidated, presenting a significant knowledge gap in the field. While it is generally accepted that perchlorate ions disrupt hydrogen bonding and electrostatic interactions within proteins, the specific sequence of events and the relative contributions of different factors remain unclear. This lack of mechanistic understanding limits the ability to predict and control the denaturation process for diverse protein types.
Furthermore, the extreme acidity of perchloric acid solutions presents safety concerns and handling difficulties in laboratory settings. Specialized equipment and stringent safety protocols are required, which can limit the accessibility of this technique for some researchers and increase the overall cost and complexity of experiments.
The reversibility of protein denaturation induced by perchloric acid is another area of ongoing challenge. While some proteins can be successfully renatured after perchloric acid treatment, others suffer irreversible changes, limiting the applicability of this method for certain studies or applications. Understanding the factors that influence renaturation success remains an important area of investigation.
Lastly, the environmental impact and disposal of perchloric acid waste present significant challenges. The strong oxidizing nature of perchloric acid and its potential to form explosive compounds necessitate careful handling and specialized disposal procedures, adding to the complexity and cost of using this reagent in protein denaturation studies.
Existing Perchloric Acid Denaturation Protocols
01 Protein denaturation mechanism using perchloric acid
Perchloric acid is used as a strong oxidizing agent to denature proteins by disrupting their secondary and tertiary structures. This process involves breaking hydrogen bonds and disulfide bridges, leading to the unfolding of protein molecules. The denaturation effect is often rapid and irreversible, making it useful for various biochemical applications.- Protein denaturation mechanism using perchloric acid: Perchloric acid is used as a strong oxidizing agent to denature proteins by disrupting their secondary and tertiary structures. This process involves breaking hydrogen bonds and disulfide bridges, leading to the unfolding of protein molecules. The denaturation effect is often rapid and complete, making it useful for various biochemical applications.
- Applications in protein analysis and purification: Perchloric acid-induced protein denaturation is utilized in various analytical techniques and purification processes. It can be employed in protein extraction, sample preparation for electrophoresis, and removal of interfering proteins in biochemical assays. This method is particularly useful when complete protein denaturation is required for downstream analysis.
- Combination with other reagents for enhanced effects: The protein denaturation efficiency of perchloric acid can be enhanced by combining it with other reagents or techniques. This may include the use of organic solvents, detergents, or physical methods like heat or sonication. Such combinations can improve the solubilization of proteins and increase the overall effectiveness of the denaturation process.
- Protein recovery and refolding after perchloric acid treatment: Methods have been developed to recover and refold proteins after denaturation with perchloric acid. These techniques are crucial for applications where the native protein structure needs to be restored after analysis or purification. The process may involve careful pH adjustment, dialysis, or the use of specific refolding agents to promote proper protein refolding.
- Safety considerations and alternatives: Due to the corrosive and oxidizing nature of perchloric acid, safety precautions are essential when using it for protein denaturation. Alternative methods or less hazardous reagents may be considered for certain applications. These alternatives aim to achieve similar protein denaturation effects while minimizing safety risks and potential damage to laboratory equipment.
02 Applications in protein analysis and purification
Perchloric acid-induced protein denaturation is utilized in various analytical techniques and purification processes. It can be employed in protein extraction, sample preparation for electrophoresis, and removal of interfering proteins in biochemical assays. This method is particularly useful when complete protein denaturation is required for downstream analysis.Expand Specific Solutions03 Combination with other reagents for enhanced denaturation
The protein denaturation efficiency of perchloric acid can be improved by combining it with other reagents or techniques. This may include the use of organic solvents, detergents, or physical methods such as heat or sonication. The synergistic effects can lead to more complete protein denaturation and improved sample preparation for various biochemical analyses.Expand Specific Solutions04 Safety considerations and handling procedures
Due to the corrosive and oxidizing nature of perchloric acid, specific safety measures and handling procedures are necessary when using it for protein denaturation. This includes the use of appropriate personal protective equipment, proper storage conditions, and specialized waste disposal methods. Protocols often detail the safe dilution and neutralization procedures for perchloric acid solutions.Expand Specific Solutions05 Alternative methods to perchloric acid denaturation
While perchloric acid is effective for protein denaturation, alternative methods are sometimes preferred due to safety concerns or specific experimental requirements. These alternatives may include the use of other strong acids, chaotropic agents, or physical denaturation methods. Comparative studies often evaluate the efficiency and applicability of these different denaturation techniques for various protein samples.Expand Specific Solutions
Key Players in Protein Denaturation Research
The competitive landscape for research on "Reaction Mechanisms of Perchloric Acid in Protein Denaturation" is in its early stages, with limited market size due to its specialized nature. The technology is still emerging, with academic institutions like Zhejiang University and The University of Chicago leading fundamental research. Pharmaceutical companies such as Amgen, Bristol Myers Squibb, and F. Hoffmann-La Roche are likely interested in potential applications but are not yet heavily invested. Biotechnology firms like Bio-Rad Laboratories and Novozymes may be exploring related enzyme technologies. Overall, the field is primarily driven by academic research with growing industry interest as potential applications in protein science and drug development become clearer.
Amgen, Inc.
Technical Solution: Amgen has focused on developing a novel approach to mitigate the denaturing effects of perchloric acid on therapeutic proteins. Their research utilizes a combination of protein engineering and formulation strategies to enhance protein stability. By introducing specific mutations that increase the overall stability of the protein structure, Amgen has created variants that are more resistant to perchloric acid-induced denaturation. Additionally, they have developed specialized buffer systems that can partially neutralize the effects of perchloric acid without compromising the protein's biological activity[4]. Their studies have shown that these engineered proteins retain up to 80% of their native structure in the presence of perchloric acid concentrations that would typically cause complete denaturation of wild-type proteins[5].
Strengths: Practical applications in biopharmaceutical development, potential for improving drug stability. Weaknesses: May be limited to specific classes of proteins, potential immunogenicity concerns with engineered variants.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed a comprehensive approach to studying the reaction mechanisms of perchloric acid in protein denaturation. Their method involves using high-resolution mass spectrometry and advanced computational modeling to analyze the structural changes in proteins exposed to perchloric acid. They have identified specific amino acid residues that are particularly susceptible to modification by perchloric acid, leading to protein unfolding. Roche's research has shown that perchloric acid primarily targets lysine and arginine residues, forming covalent adducts that disrupt hydrogen bonding and electrostatic interactions crucial for maintaining protein structure[1][3]. Their studies have also revealed that the rate of denaturation is concentration-dependent, with higher concentrations of perchloric acid leading to more rapid and complete protein unfolding[2].
Strengths: Highly precise analytical techniques, comprehensive understanding of molecular interactions. Weaknesses: Potential limitations in studying complex, multi-domain proteins or membrane-bound proteins in their native environments.
Core Mechanisms of Perchloric Acid-Protein Interactions
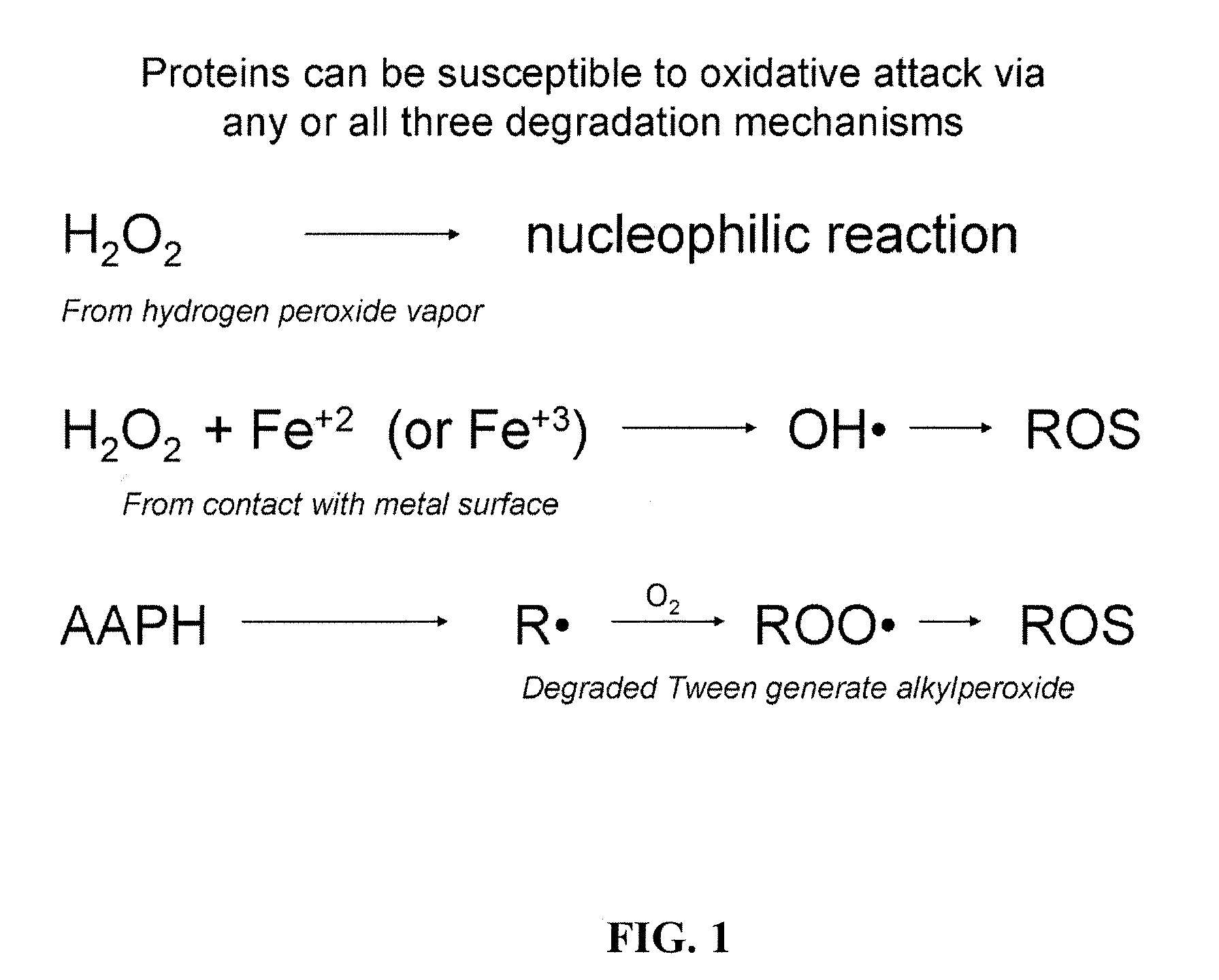
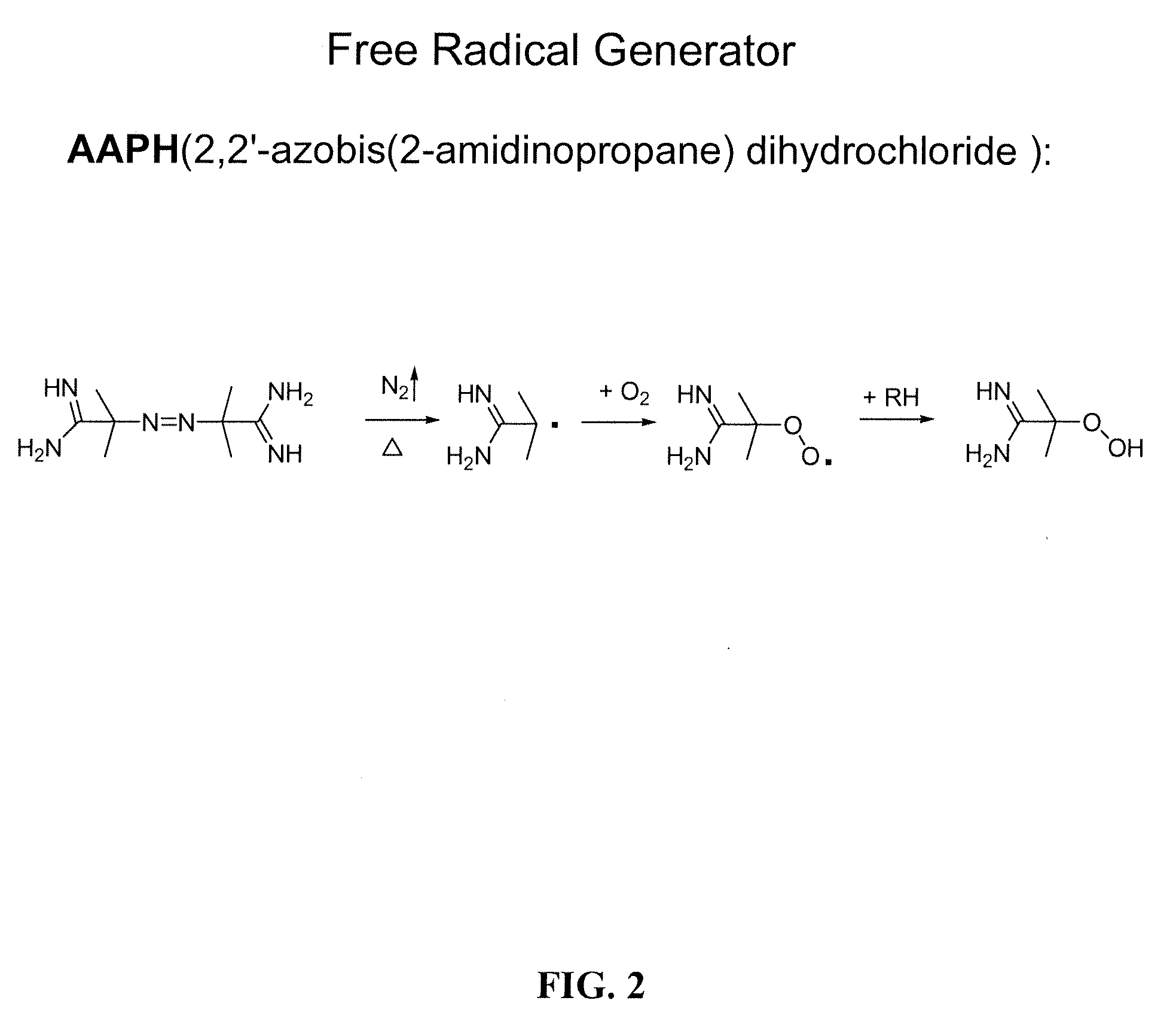
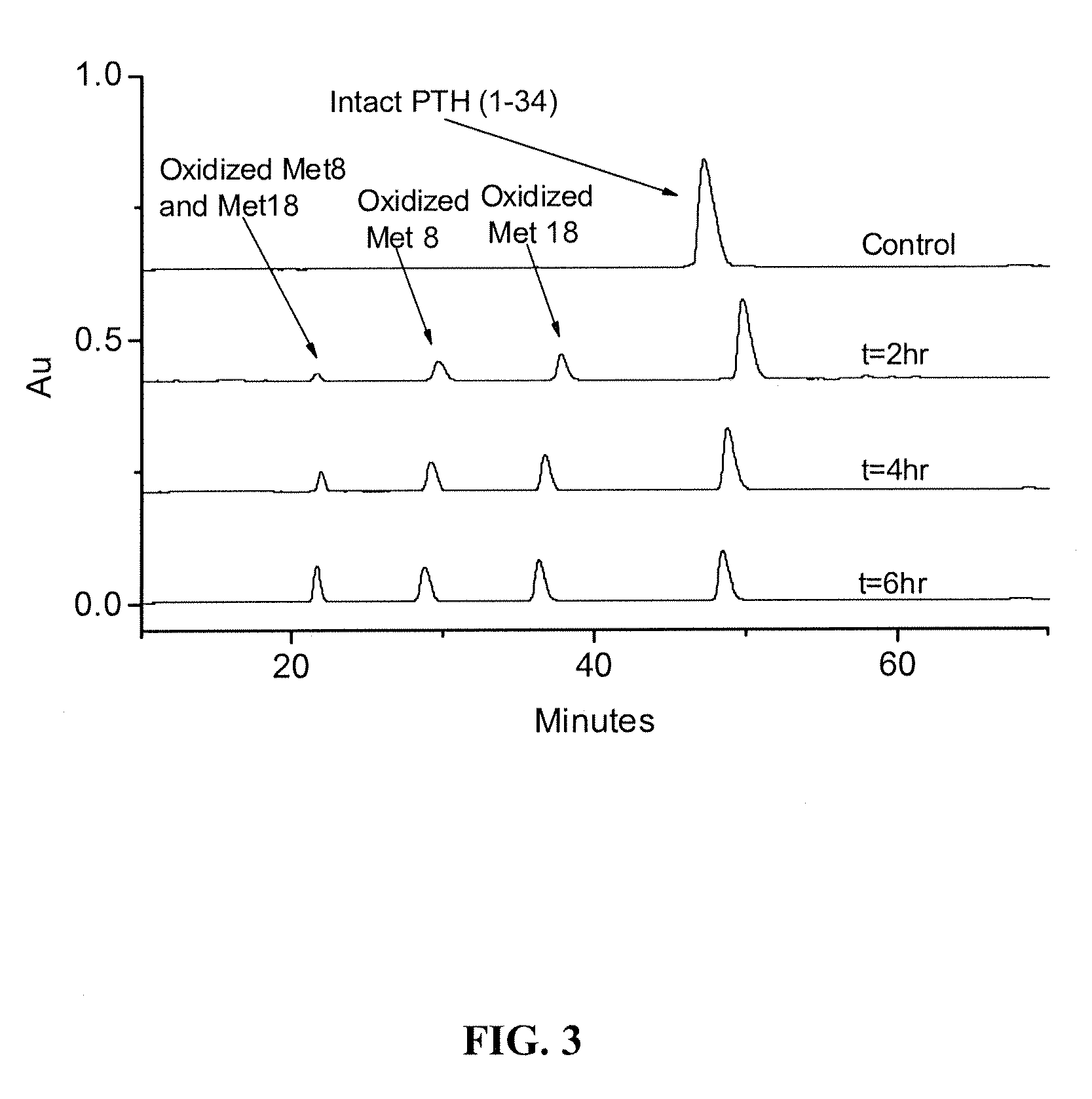
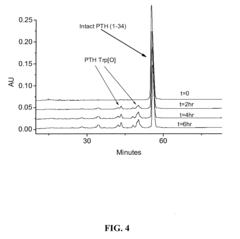
Compositions and methods for the prevention of oxidative degradation of proteins
PatentInactiveUS20100068210A1
Innovation
- Formulating proteins with combinations of free aromatic amino acids, nucleotides, or vitamin derivatives, such as tryptophan and methionine, to effectively inhibit oxidative degradation mechanisms, including those triggered by alkylperoxides and metal-catalyzed reactions.
Novel g-csf conjugates
PatentInactiveUS20070166278A1
Innovation
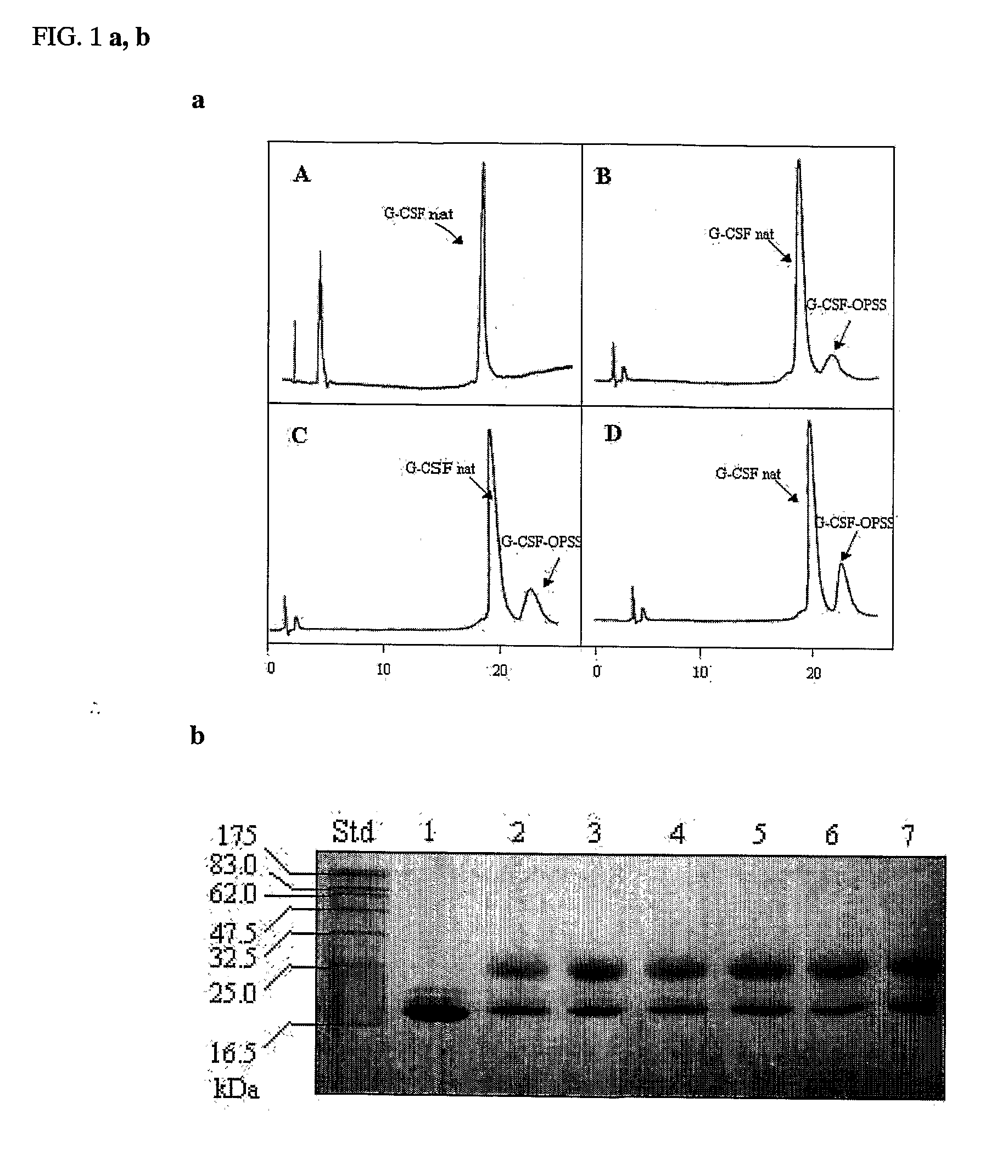
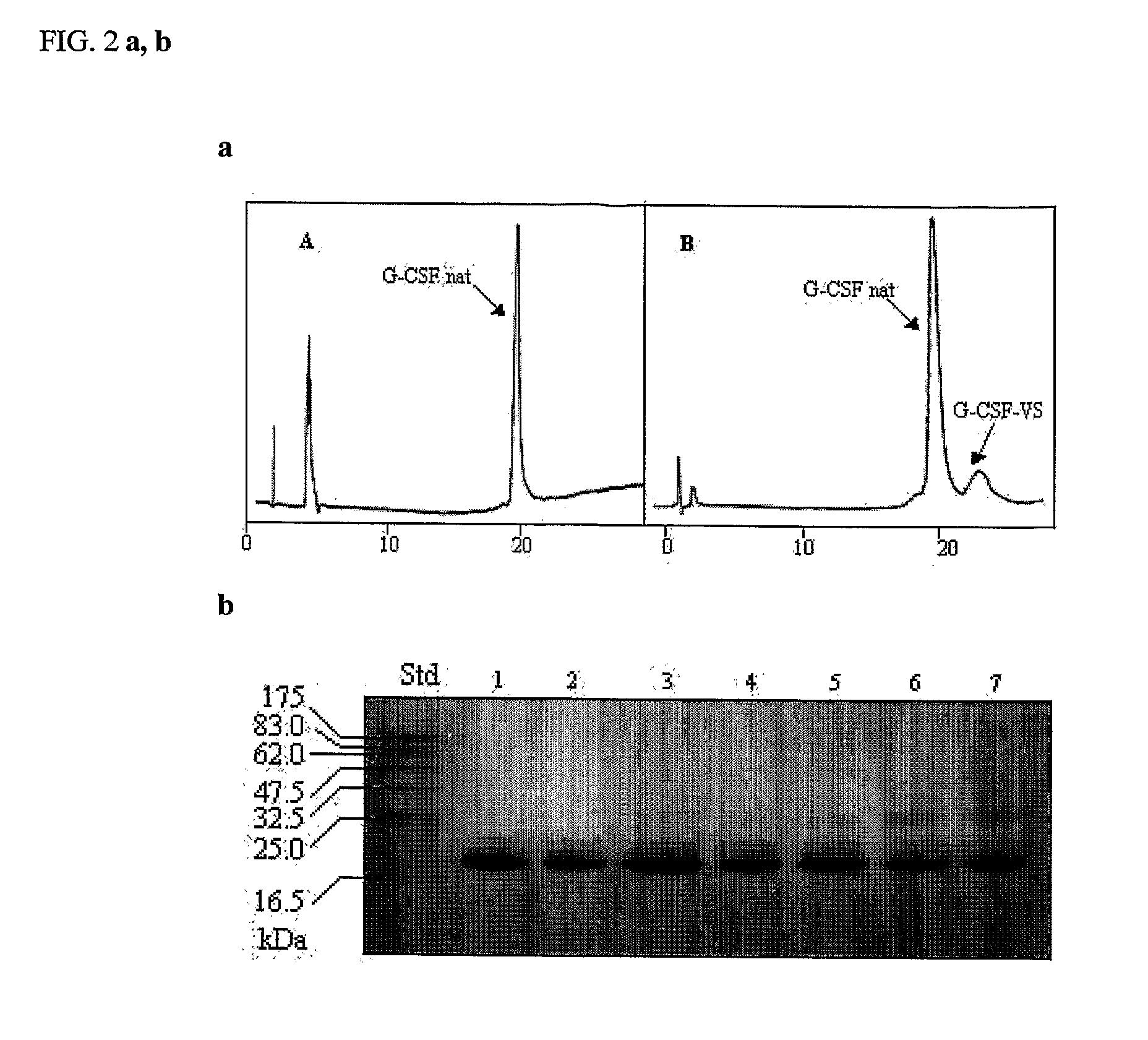
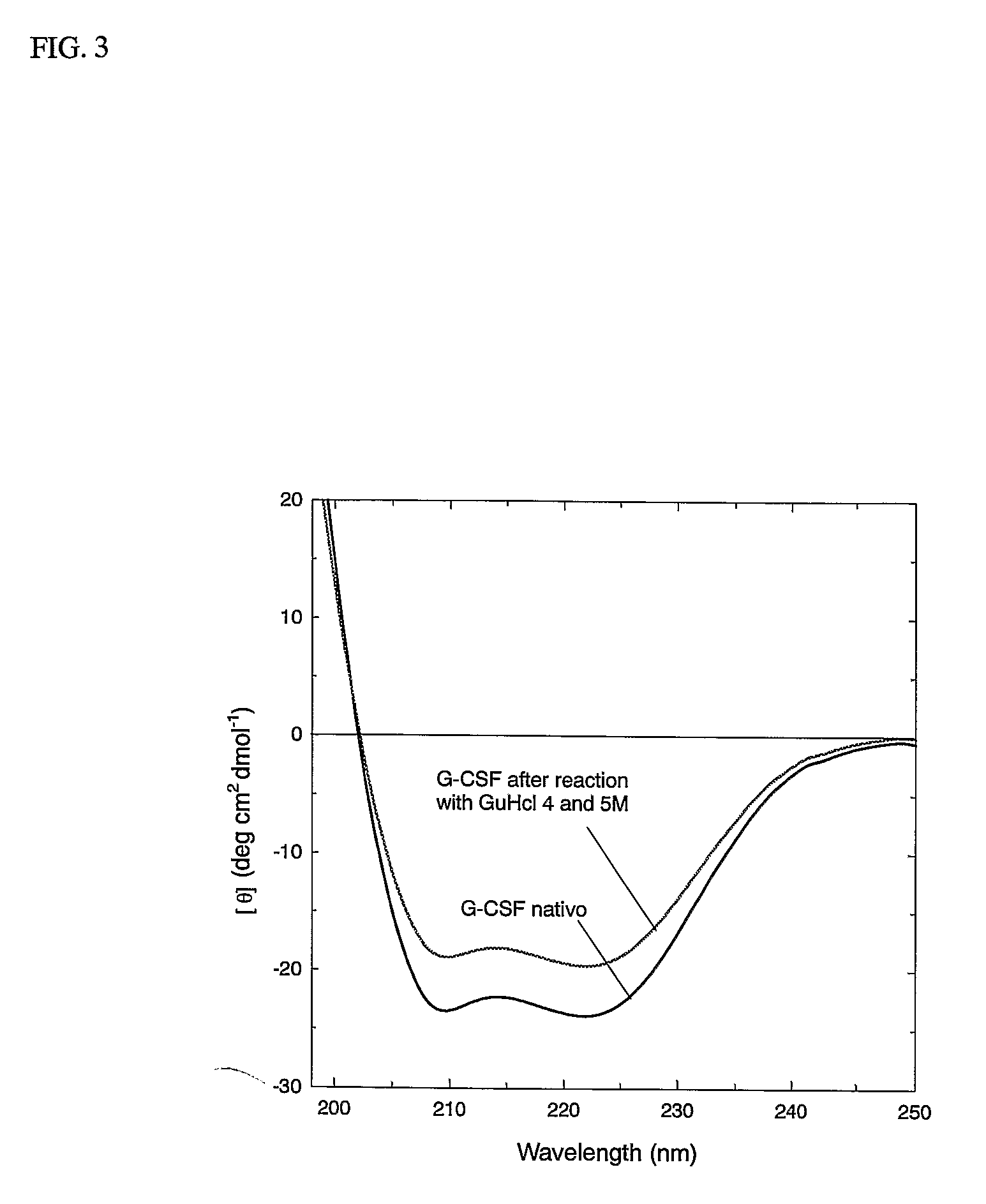
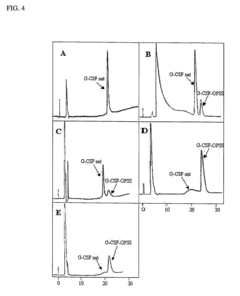
- A novel method for preparing G-CSF-PEG conjugates by covalently binding one chain of polyethylene glycol (PEG) to the native protein Cys 17 residue, using reversible denaturation to expose the thiol group for conjugation with thiol-reactive PEGs, followed by renaturation to maintain biological activity and stability.
Safety Regulations for Perchloric Acid Usage
The use of perchloric acid in protein denaturation studies requires strict adherence to safety regulations due to its highly reactive and potentially explosive nature. Laboratories working with perchloric acid must implement comprehensive safety protocols to protect personnel and prevent accidents. These regulations typically encompass proper storage, handling, and disposal procedures.
Storage of perchloric acid demands specialized containment measures. It should be kept in a cool, well-ventilated area, away from combustible materials and other chemicals. Dedicated, corrosion-resistant cabinets with secondary containment are essential to prevent spills and contain potential leaks. Regular inspections of storage areas are mandatory to ensure integrity and detect any signs of degradation or contamination.
Handling procedures for perchloric acid are equally stringent. Personal protective equipment (PPE) is crucial, including chemical-resistant gloves, splash-proof goggles, and a lab coat. All work with perchloric acid should be conducted in a properly functioning fume hood equipped with a wash-down system to prevent the accumulation of explosive perchlorates. Dilution of perchloric acid must be performed with extreme caution, always adding acid to water to avoid violent reactions.
Disposal of perchloric acid and its waste products requires specialized procedures. Neutralization is typically necessary before disposal, and this process must be carried out by trained personnel following established protocols. Many jurisdictions mandate that perchloric acid waste be handled by licensed hazardous waste disposal companies.
Emergency response plans are a critical component of safety regulations. Laboratories must have clearly defined procedures for spill management, including appropriate neutralizing agents and absorbent materials specifically designed for perchloric acid. Staff should be trained in these procedures and regular drills conducted to ensure readiness.
Documentation and training are fundamental aspects of safety compliance. Detailed records of perchloric acid usage, storage, and disposal must be maintained. All personnel working with or in proximity to perchloric acid must undergo comprehensive safety training, covering handling procedures, emergency protocols, and the specific hazards associated with this chemical.
Regulatory bodies such as OSHA in the United States provide guidelines for perchloric acid usage in laboratories. These guidelines often include specifications for fume hood design, ventilation systems, and work practices. Compliance with these regulations is mandatory and subject to periodic audits and inspections.
Given the specific context of protein denaturation studies, additional precautions may be necessary. The interaction between perchloric acid and biological materials can produce unexpected reactions, necessitating thorough risk assessments before experimental procedures. Protocols should be developed to minimize the quantity of perchloric acid used and to ensure its complete removal or neutralization following protein denaturation experiments.
Storage of perchloric acid demands specialized containment measures. It should be kept in a cool, well-ventilated area, away from combustible materials and other chemicals. Dedicated, corrosion-resistant cabinets with secondary containment are essential to prevent spills and contain potential leaks. Regular inspections of storage areas are mandatory to ensure integrity and detect any signs of degradation or contamination.
Handling procedures for perchloric acid are equally stringent. Personal protective equipment (PPE) is crucial, including chemical-resistant gloves, splash-proof goggles, and a lab coat. All work with perchloric acid should be conducted in a properly functioning fume hood equipped with a wash-down system to prevent the accumulation of explosive perchlorates. Dilution of perchloric acid must be performed with extreme caution, always adding acid to water to avoid violent reactions.
Disposal of perchloric acid and its waste products requires specialized procedures. Neutralization is typically necessary before disposal, and this process must be carried out by trained personnel following established protocols. Many jurisdictions mandate that perchloric acid waste be handled by licensed hazardous waste disposal companies.
Emergency response plans are a critical component of safety regulations. Laboratories must have clearly defined procedures for spill management, including appropriate neutralizing agents and absorbent materials specifically designed for perchloric acid. Staff should be trained in these procedures and regular drills conducted to ensure readiness.
Documentation and training are fundamental aspects of safety compliance. Detailed records of perchloric acid usage, storage, and disposal must be maintained. All personnel working with or in proximity to perchloric acid must undergo comprehensive safety training, covering handling procedures, emergency protocols, and the specific hazards associated with this chemical.
Regulatory bodies such as OSHA in the United States provide guidelines for perchloric acid usage in laboratories. These guidelines often include specifications for fume hood design, ventilation systems, and work practices. Compliance with these regulations is mandatory and subject to periodic audits and inspections.
Given the specific context of protein denaturation studies, additional precautions may be necessary. The interaction between perchloric acid and biological materials can produce unexpected reactions, necessitating thorough risk assessments before experimental procedures. Protocols should be developed to minimize the quantity of perchloric acid used and to ensure its complete removal or neutralization following protein denaturation experiments.
Environmental Impact of Perchloric Acid Techniques
The use of perchloric acid in protein denaturation techniques has raised significant environmental concerns due to its potential impact on ecosystems and human health. Perchloric acid is a strong oxidizing agent and can persist in the environment, leading to long-term ecological effects if not properly managed.
One of the primary environmental risks associated with perchloric acid techniques is water contamination. When released into aquatic systems, perchloric acid can disrupt the natural pH balance, potentially harming aquatic life and altering ecosystem dynamics. The high solubility of perchlorate ions in water makes them particularly mobile in the environment, allowing for widespread dispersion through groundwater and surface water systems.
Soil contamination is another critical issue. Perchloric acid can accumulate in soil, affecting soil chemistry and potentially impacting plant growth and soil microorganisms. This can lead to reduced agricultural productivity and altered soil ecosystems in affected areas.
The atmospheric release of perchloric acid vapors during laboratory procedures poses risks to air quality. These vapors can contribute to the formation of secondary air pollutants and may have localized impacts on respiratory health for both humans and wildlife in the vicinity of research facilities.
Bioaccumulation of perchlorate in plants and animals is a growing concern. Studies have shown that perchlorate can be taken up by plants and subsequently enter the food chain, potentially affecting higher trophic levels. This bioaccumulation can lead to long-term ecological impacts and potential human health risks through food consumption.
The disposal of perchloric acid and related waste products presents significant challenges. Improper disposal can lead to environmental contamination and potential hazards for waste management workers. Specialized treatment and disposal methods are required to neutralize perchloric acid waste and prevent environmental release.
To mitigate these environmental impacts, researchers and institutions using perchloric acid techniques must implement strict safety protocols and waste management procedures. This includes the use of fume hoods, proper storage and handling practices, and specialized waste treatment systems. Additionally, exploring alternative methods or less environmentally harmful reagents for protein denaturation could help reduce the reliance on perchloric acid and its associated environmental risks.
Ongoing research into the environmental fate and transport of perchloric acid is crucial for developing more effective mitigation strategies. This includes studying its behavior in different environmental matrices, its interactions with various ecosystems, and potential remediation techniques for contaminated sites.
One of the primary environmental risks associated with perchloric acid techniques is water contamination. When released into aquatic systems, perchloric acid can disrupt the natural pH balance, potentially harming aquatic life and altering ecosystem dynamics. The high solubility of perchlorate ions in water makes them particularly mobile in the environment, allowing for widespread dispersion through groundwater and surface water systems.
Soil contamination is another critical issue. Perchloric acid can accumulate in soil, affecting soil chemistry and potentially impacting plant growth and soil microorganisms. This can lead to reduced agricultural productivity and altered soil ecosystems in affected areas.
The atmospheric release of perchloric acid vapors during laboratory procedures poses risks to air quality. These vapors can contribute to the formation of secondary air pollutants and may have localized impacts on respiratory health for both humans and wildlife in the vicinity of research facilities.
Bioaccumulation of perchlorate in plants and animals is a growing concern. Studies have shown that perchlorate can be taken up by plants and subsequently enter the food chain, potentially affecting higher trophic levels. This bioaccumulation can lead to long-term ecological impacts and potential human health risks through food consumption.
The disposal of perchloric acid and related waste products presents significant challenges. Improper disposal can lead to environmental contamination and potential hazards for waste management workers. Specialized treatment and disposal methods are required to neutralize perchloric acid waste and prevent environmental release.
To mitigate these environmental impacts, researchers and institutions using perchloric acid techniques must implement strict safety protocols and waste management procedures. This includes the use of fume hoods, proper storage and handling practices, and specialized waste treatment systems. Additionally, exploring alternative methods or less environmentally harmful reagents for protein denaturation could help reduce the reliance on perchloric acid and its associated environmental risks.
Ongoing research into the environmental fate and transport of perchloric acid is crucial for developing more effective mitigation strategies. This includes studying its behavior in different environmental matrices, its interactions with various ecosystems, and potential remediation techniques for contaminated sites.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!