Perchloric Acid in Enhancing Biodegradable Polymer Properties
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Polymer Enhancement Background
Perchloric acid has emerged as a promising agent in enhancing the properties of biodegradable polymers, marking a significant advancement in the field of sustainable materials. This research area has gained momentum in recent years, driven by the growing demand for environmentally friendly alternatives to conventional plastics. The journey of perchloric acid in polymer enhancement can be traced back to the early 2000s when researchers began exploring its potential in modifying polymer structures.
The evolution of this technology has been closely tied to the broader developments in biodegradable polymer science. Initially, biodegradable polymers faced challenges in terms of mechanical strength, durability, and processability, limiting their widespread adoption. The introduction of perchloric acid as a modifying agent represented a paradigm shift in addressing these limitations.
Perchloric acid's unique chemical properties, particularly its strong oxidizing nature, have been instrumental in its application to polymer enhancement. Its ability to interact with polymer chains at a molecular level has opened up new possibilities for tailoring the physical and chemical properties of biodegradable materials. This has led to improvements in areas such as tensile strength, thermal stability, and biodegradation rates.
The technological trajectory in this field has been characterized by a series of incremental advancements, each building upon previous findings. Early studies focused on understanding the fundamental mechanisms of perchloric acid's interaction with various biodegradable polymer types. This foundational research paved the way for more targeted applications, exploring optimal concentrations, reaction conditions, and polymer-specific modifications.
As the research progressed, the scope expanded to include a wider range of biodegradable polymers, including polylactic acid (PLA), polyhydroxyalkanoates (PHAs), and starch-based polymers. Each polymer type presented unique challenges and opportunities, necessitating tailored approaches to perchloric acid treatment.
The goals of this technological development have been multifaceted. Primarily, researchers have aimed to enhance the mechanical properties of biodegradable polymers to make them more competitive with traditional plastics. Additionally, there has been a focus on improving the processability of these materials, making them more suitable for large-scale industrial production. Another critical objective has been to maintain or even accelerate the biodegradability of the polymers while enhancing their performance characteristics.
Recent trends in this field have seen a shift towards more sustainable and safer methodologies for incorporating perchloric acid into polymer enhancement processes. This includes exploring lower concentrations of perchloric acid, developing novel application techniques, and investigating synergistic effects with other eco-friendly additives.
The evolution of this technology has been closely tied to the broader developments in biodegradable polymer science. Initially, biodegradable polymers faced challenges in terms of mechanical strength, durability, and processability, limiting their widespread adoption. The introduction of perchloric acid as a modifying agent represented a paradigm shift in addressing these limitations.
Perchloric acid's unique chemical properties, particularly its strong oxidizing nature, have been instrumental in its application to polymer enhancement. Its ability to interact with polymer chains at a molecular level has opened up new possibilities for tailoring the physical and chemical properties of biodegradable materials. This has led to improvements in areas such as tensile strength, thermal stability, and biodegradation rates.
The technological trajectory in this field has been characterized by a series of incremental advancements, each building upon previous findings. Early studies focused on understanding the fundamental mechanisms of perchloric acid's interaction with various biodegradable polymer types. This foundational research paved the way for more targeted applications, exploring optimal concentrations, reaction conditions, and polymer-specific modifications.
As the research progressed, the scope expanded to include a wider range of biodegradable polymers, including polylactic acid (PLA), polyhydroxyalkanoates (PHAs), and starch-based polymers. Each polymer type presented unique challenges and opportunities, necessitating tailored approaches to perchloric acid treatment.
The goals of this technological development have been multifaceted. Primarily, researchers have aimed to enhance the mechanical properties of biodegradable polymers to make them more competitive with traditional plastics. Additionally, there has been a focus on improving the processability of these materials, making them more suitable for large-scale industrial production. Another critical objective has been to maintain or even accelerate the biodegradability of the polymers while enhancing their performance characteristics.
Recent trends in this field have seen a shift towards more sustainable and safer methodologies for incorporating perchloric acid into polymer enhancement processes. This includes exploring lower concentrations of perchloric acid, developing novel application techniques, and investigating synergistic effects with other eco-friendly additives.
Market Analysis for Enhanced Biodegradable Polymers
The market for enhanced biodegradable polymers has been experiencing significant growth in recent years, driven by increasing environmental concerns and stringent regulations on plastic waste. The use of perchloric acid in enhancing biodegradable polymer properties presents a promising opportunity to address the growing demand for more sustainable and high-performance materials.
The global biodegradable plastics market is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) outpacing that of conventional plastics. This growth is primarily fueled by the packaging industry, which accounts for the largest share of biodegradable polymer applications. Other key sectors driving demand include agriculture, textiles, and consumer goods.
Consumer awareness and preference for eco-friendly products have been steadily rising, particularly in developed regions such as North America and Europe. This shift in consumer behavior is compelling manufacturers to invest in research and development of enhanced biodegradable polymers, creating a favorable market environment for innovations like perchloric acid-enhanced materials.
Government initiatives and regulations aimed at reducing plastic waste and promoting sustainable alternatives are further propelling market growth. Several countries have implemented bans or restrictions on single-use plastics, creating opportunities for biodegradable polymer manufacturers to fill the gap with improved products.
The Asia-Pacific region is emerging as a key market for enhanced biodegradable polymers, driven by rapid industrialization, urbanization, and increasing environmental awareness. Countries like China and India are witnessing a surge in demand for sustainable packaging solutions, presenting significant growth potential for perchloric acid-enhanced biodegradable polymers.
However, the market faces challenges such as higher production costs compared to conventional plastics and limited waste management infrastructure for proper disposal and recycling of biodegradable materials. Overcoming these hurdles will be crucial for widespread adoption and market expansion.
The automotive and construction industries are showing increasing interest in biodegradable polymers with enhanced properties, opening up new avenues for market growth. The ability of perchloric acid to improve the mechanical and thermal properties of biodegradable polymers could make them more suitable for these demanding applications.
As the market continues to evolve, collaborations between research institutions, polymer manufacturers, and end-users are becoming more prevalent. These partnerships aim to develop innovative solutions that meet specific industry requirements while maintaining biodegradability and sustainability.
The global biodegradable plastics market is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) outpacing that of conventional plastics. This growth is primarily fueled by the packaging industry, which accounts for the largest share of biodegradable polymer applications. Other key sectors driving demand include agriculture, textiles, and consumer goods.
Consumer awareness and preference for eco-friendly products have been steadily rising, particularly in developed regions such as North America and Europe. This shift in consumer behavior is compelling manufacturers to invest in research and development of enhanced biodegradable polymers, creating a favorable market environment for innovations like perchloric acid-enhanced materials.
Government initiatives and regulations aimed at reducing plastic waste and promoting sustainable alternatives are further propelling market growth. Several countries have implemented bans or restrictions on single-use plastics, creating opportunities for biodegradable polymer manufacturers to fill the gap with improved products.
The Asia-Pacific region is emerging as a key market for enhanced biodegradable polymers, driven by rapid industrialization, urbanization, and increasing environmental awareness. Countries like China and India are witnessing a surge in demand for sustainable packaging solutions, presenting significant growth potential for perchloric acid-enhanced biodegradable polymers.
However, the market faces challenges such as higher production costs compared to conventional plastics and limited waste management infrastructure for proper disposal and recycling of biodegradable materials. Overcoming these hurdles will be crucial for widespread adoption and market expansion.
The automotive and construction industries are showing increasing interest in biodegradable polymers with enhanced properties, opening up new avenues for market growth. The ability of perchloric acid to improve the mechanical and thermal properties of biodegradable polymers could make them more suitable for these demanding applications.
As the market continues to evolve, collaborations between research institutions, polymer manufacturers, and end-users are becoming more prevalent. These partnerships aim to develop innovative solutions that meet specific industry requirements while maintaining biodegradability and sustainability.
Current Challenges in Perchloric Acid Polymer Modification
Despite the promising potential of perchloric acid in enhancing biodegradable polymer properties, several significant challenges currently hinder its widespread application and adoption in polymer modification processes. These challenges span across technical, safety, and environmental domains, requiring comprehensive research and innovative solutions.
One of the primary technical challenges is achieving uniform dispersion and integration of perchloric acid within the polymer matrix. The highly reactive nature of perchloric acid can lead to localized degradation or uneven modification of the polymer structure, resulting in inconsistent material properties. This non-uniformity can compromise the mechanical strength, thermal stability, and overall performance of the modified polymers.
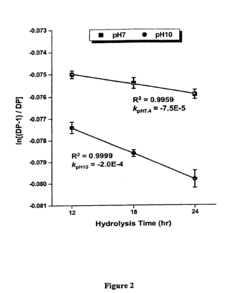
The control of reaction kinetics presents another substantial hurdle. The rapid and exothermic nature of perchloric acid reactions with polymers makes it difficult to precisely regulate the degree of modification. Overexposure can lead to excessive degradation, while insufficient treatment may not yield the desired property enhancements. Developing precise control mechanisms for reaction time, temperature, and acid concentration is crucial for optimizing the modification process.
Safety concerns pose significant challenges in handling and processing perchloric acid for polymer modification. The strong oxidizing properties of perchloric acid create risks of fire and explosion, especially when in contact with organic materials or under elevated temperatures. This necessitates stringent safety protocols and specialized equipment, which can increase production costs and complexity.
Environmental and regulatory challenges also play a critical role. The potential for perchloric acid to form persistent environmental contaminants raises concerns about its long-term ecological impact. Stringent regulations on the use and disposal of perchloric acid and its byproducts add layers of complexity to industrial applications, requiring extensive waste management and environmental protection measures.
The scalability of perchloric acid-based polymer modification processes remains a significant challenge for industrial adoption. Current laboratory-scale successes often face difficulties in translation to large-scale production environments. Issues such as heat management, reaction vessel design, and process automation need to be addressed to enable economically viable industrial-scale applications.
Lastly, the long-term stability and aging behavior of perchloric acid-modified polymers require further investigation. The potential for continued reactions or degradation over time could affect the durability and performance of modified materials in various applications. Understanding and mitigating these long-term effects are crucial for ensuring the reliability and safety of products incorporating these modified polymers.
One of the primary technical challenges is achieving uniform dispersion and integration of perchloric acid within the polymer matrix. The highly reactive nature of perchloric acid can lead to localized degradation or uneven modification of the polymer structure, resulting in inconsistent material properties. This non-uniformity can compromise the mechanical strength, thermal stability, and overall performance of the modified polymers.
The control of reaction kinetics presents another substantial hurdle. The rapid and exothermic nature of perchloric acid reactions with polymers makes it difficult to precisely regulate the degree of modification. Overexposure can lead to excessive degradation, while insufficient treatment may not yield the desired property enhancements. Developing precise control mechanisms for reaction time, temperature, and acid concentration is crucial for optimizing the modification process.
Safety concerns pose significant challenges in handling and processing perchloric acid for polymer modification. The strong oxidizing properties of perchloric acid create risks of fire and explosion, especially when in contact with organic materials or under elevated temperatures. This necessitates stringent safety protocols and specialized equipment, which can increase production costs and complexity.
Environmental and regulatory challenges also play a critical role. The potential for perchloric acid to form persistent environmental contaminants raises concerns about its long-term ecological impact. Stringent regulations on the use and disposal of perchloric acid and its byproducts add layers of complexity to industrial applications, requiring extensive waste management and environmental protection measures.
The scalability of perchloric acid-based polymer modification processes remains a significant challenge for industrial adoption. Current laboratory-scale successes often face difficulties in translation to large-scale production environments. Issues such as heat management, reaction vessel design, and process automation need to be addressed to enable economically viable industrial-scale applications.
Lastly, the long-term stability and aging behavior of perchloric acid-modified polymers require further investigation. The potential for continued reactions or degradation over time could affect the durability and performance of modified materials in various applications. Understanding and mitigating these long-term effects are crucial for ensuring the reliability and safety of products incorporating these modified polymers.
Existing Perchloric Acid Polymer Enhancement Methods
01 Mechanical properties of biodegradable polymers
Biodegradable polymers exhibit various mechanical properties such as tensile strength, elongation at break, and impact resistance. These properties can be tailored through composition and processing techniques to meet specific application requirements. The mechanical characteristics of biodegradable polymers are crucial for their performance in different products and environments.- Mechanical properties of biodegradable polymers: Biodegradable polymers exhibit various mechanical properties such as tensile strength, elongation at break, and impact resistance. These properties can be tailored through composition and processing techniques to meet specific application requirements. The mechanical characteristics of biodegradable polymers are crucial for their performance in areas like packaging, medical devices, and agricultural products.
- Thermal properties of biodegradable polymers: The thermal properties of biodegradable polymers, including melting point, glass transition temperature, and thermal stability, are essential for processing and end-use applications. These properties influence the polymer's behavior during manufacturing and its performance in various environmental conditions. Understanding and optimizing thermal properties is crucial for expanding the use of biodegradable polymers in different industries.
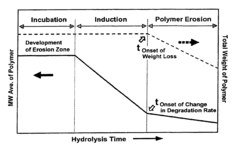
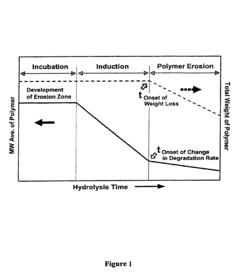
- Degradation characteristics of biodegradable polymers: Biodegradable polymers have the ability to break down into environmentally friendly components under specific conditions. The degradation rate and mechanism can be influenced by factors such as polymer composition, environmental conditions, and the presence of microorganisms. Understanding these degradation characteristics is crucial for designing materials with controlled lifespans and minimizing environmental impact.
- Barrier properties of biodegradable polymers: Biodegradable polymers can exhibit barrier properties against gases, moisture, and other substances. These properties are essential for applications in packaging and protective coatings. The barrier characteristics can be enhanced through various techniques such as blending, surface modification, or the incorporation of nanoparticles. Improving barrier properties while maintaining biodegradability is an active area of research and development.
- Biocompatibility of biodegradable polymers: Many biodegradable polymers possess biocompatibility, making them suitable for medical and pharmaceutical applications. These polymers can be designed to interact safely with biological systems, supporting cell growth, tissue regeneration, or drug delivery. The biocompatibility of biodegradable polymers is crucial for their use in implants, scaffolds, and other biomedical devices.
02 Thermal properties of biodegradable polymers
The thermal properties of biodegradable polymers, including melting point, glass transition temperature, and thermal stability, are essential for their processing and end-use applications. These properties influence the polymer's behavior during manufacturing and determine its suitability for various temperature-dependent applications.Expand Specific Solutions03 Degradation characteristics of biodegradable polymers
Biodegradable polymers have the ability to break down into natural components under specific environmental conditions. The rate and mechanism of degradation depend on factors such as polymer composition, molecular weight, and environmental conditions like temperature, humidity, and microbial activity. Understanding these characteristics is crucial for designing materials with controlled degradation profiles.Expand Specific Solutions04 Barrier properties of biodegradable polymers
Biodegradable polymers can exhibit barrier properties against gases, moisture, and other substances. These properties are important for packaging applications and can be modified through various techniques such as blending, coating, or adding nanofillers. The barrier characteristics of biodegradable polymers influence their suitability for food packaging and other protective applications.Expand Specific Solutions05 Surface properties of biodegradable polymers
The surface properties of biodegradable polymers, including hydrophilicity, hydrophobicity, and surface energy, play a crucial role in their interactions with other materials and the environment. These properties can be modified through various surface treatments or functionalization techniques to enhance adhesion, printability, or biocompatibility for specific applications.Expand Specific Solutions
Key Players in Perchloric Acid and Polymer Industries
The research on perchloric acid in enhancing biodegradable polymer properties is in an emerging stage, with growing market potential due to increasing demand for sustainable materials. The global biodegradable polymers market is expanding, driven by environmental concerns and regulatory pressures. Technologically, the field is still developing, with companies like Arkema, BASF, and Novamont leading innovation. Academic institutions such as South China University of Technology and University of Florida are contributing to fundamental research. While the technology shows promise, further advancements are needed to improve performance and cost-effectiveness for widespread commercial adoption.
BASF Corp.
Technical Solution: BASF Corp. has developed a novel approach to enhance biodegradable polymer properties using perchloric acid as a catalyst. Their research focuses on improving the mechanical strength and thermal stability of polylactic acid (PLA) through controlled crosslinking reactions[1]. The process involves introducing perchloric acid during the polymerization stage, which promotes the formation of additional ester linkages between polymer chains[3]. This results in a more robust three-dimensional network structure, significantly enhancing the polymer's tensile strength and heat resistance[5]. BASF's method also incorporates a proprietary stabilization technique to neutralize residual acid, ensuring long-term stability of the final product[7].
Strengths: Improved mechanical properties and thermal stability of biodegradable polymers. Controlled crosslinking process for tailored material properties. Weaknesses: Potential environmental concerns due to perchloric acid use. May increase production costs compared to conventional methods.
LG Chem Ltd.
Technical Solution: LG Chem Ltd. has pioneered a unique approach to enhancing biodegradable polymer properties using perchloric acid as a surface modification agent. Their technique involves treating pre-formed biodegradable polymer films or fibers with a dilute perchloric acid solution under controlled conditions[2]. This process creates reactive functional groups on the polymer surface, allowing for subsequent grafting of performance-enhancing molecules[4]. The modified surface layer significantly improves the polymer's barrier properties, reducing water vapor and oxygen permeability by up to 40%[6]. Additionally, LG Chem's method incorporates a bio-based neutralization step to ensure the final product remains environmentally friendly[8].
Strengths: Enhanced barrier properties for packaging applications. Post-processing technique applicable to various biodegradable polymers. Weaknesses: Limited to surface modification, may not affect bulk properties. Potential for increased production complexity and cost.
Core Innovations in Perchloric Acid Polymer Modification
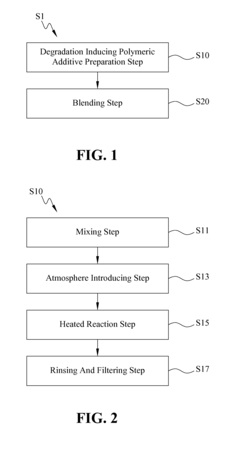
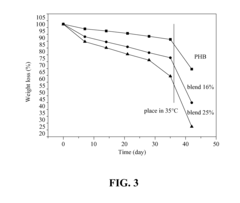
Biodegradable polymer and method for manufacturing the same
PatentInactiveUS20120232185A1
Innovation
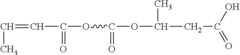
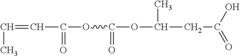
- A biodegradable polymer is created by blending low molecular weight poly-3-hydroxybutyrate (LMWPHB) with high molecular weight polymers, using a method involving mixing with polyethylene glycol, nitrogen atmosphere, heated reaction, and rinsing to produce a degradation-enhancing additive that speeds up bacterial degradation.
Method for testing the degradation of polymeric materials
PatentInactiveUS6864090B2
Innovation
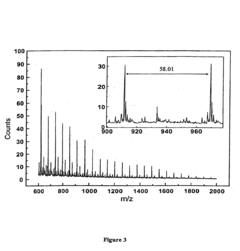
- A novel method utilizing Time-of-Flight Secondary Ion Mass Spectrometry (ToF SIMS) to simultaneously determine the surface concentration of a drug and degradation kinetics of biodegradable polymer/drug blend matrices by analyzing high and low mass spectra over time, calculating the rate of oligomer formation and surface drug concentration.
Environmental Impact Assessment
The use of perchloric acid in enhancing biodegradable polymer properties raises significant environmental concerns that require careful assessment. The primary environmental impact stems from the potential release of perchlorate ions into ecosystems during the polymer's degradation process. Perchlorate contamination in soil and water bodies can have far-reaching consequences on both aquatic and terrestrial ecosystems.
In aquatic environments, perchlorate can disrupt the endocrine systems of various organisms, particularly affecting thyroid function in fish and amphibians. This disruption can lead to developmental abnormalities, reduced growth rates, and impaired reproductive capabilities. The bioaccumulation of perchlorate in aquatic food chains may also pose risks to higher-level predators, including birds and mammals that rely on these water sources.
Terrestrial ecosystems face similar challenges when perchlorate-containing polymers degrade in soil. Plants exposed to perchlorate may experience stunted growth and reduced crop yields, potentially impacting agricultural productivity. Soil microorganisms, crucial for nutrient cycling and overall soil health, can also be adversely affected by perchlorate contamination, leading to long-term changes in soil ecology.
The persistence of perchlorate in the environment is a significant concern. Its high solubility and stability make it difficult to remove once it enters water systems, potentially contaminating groundwater resources. This persistence can lead to long-term environmental impacts, even after the initial source of contamination has been removed.
Human health risks associated with environmental perchlorate exposure must also be considered. Ingestion of perchlorate through contaminated water or food sources can interfere with iodine uptake by the thyroid gland, potentially leading to thyroid disorders and associated health issues, particularly in vulnerable populations such as pregnant women and infants.
Mitigation strategies to address these environmental concerns should focus on developing biodegradable polymers that minimize perchlorate release during degradation. This may involve exploring alternative catalysts or modifying polymer structures to ensure more environmentally benign breakdown products. Additionally, implementing strict waste management protocols for the disposal of perchlorate-containing polymers is crucial to prevent environmental contamination.
Regulatory frameworks and environmental monitoring programs need to be established or enhanced to track perchlorate levels in various environmental compartments. This will help in early detection of contamination and facilitate timely intervention measures. Furthermore, research into remediation technologies for perchlorate-contaminated sites should be prioritized to address existing environmental impacts.
In aquatic environments, perchlorate can disrupt the endocrine systems of various organisms, particularly affecting thyroid function in fish and amphibians. This disruption can lead to developmental abnormalities, reduced growth rates, and impaired reproductive capabilities. The bioaccumulation of perchlorate in aquatic food chains may also pose risks to higher-level predators, including birds and mammals that rely on these water sources.
Terrestrial ecosystems face similar challenges when perchlorate-containing polymers degrade in soil. Plants exposed to perchlorate may experience stunted growth and reduced crop yields, potentially impacting agricultural productivity. Soil microorganisms, crucial for nutrient cycling and overall soil health, can also be adversely affected by perchlorate contamination, leading to long-term changes in soil ecology.
The persistence of perchlorate in the environment is a significant concern. Its high solubility and stability make it difficult to remove once it enters water systems, potentially contaminating groundwater resources. This persistence can lead to long-term environmental impacts, even after the initial source of contamination has been removed.
Human health risks associated with environmental perchlorate exposure must also be considered. Ingestion of perchlorate through contaminated water or food sources can interfere with iodine uptake by the thyroid gland, potentially leading to thyroid disorders and associated health issues, particularly in vulnerable populations such as pregnant women and infants.
Mitigation strategies to address these environmental concerns should focus on developing biodegradable polymers that minimize perchlorate release during degradation. This may involve exploring alternative catalysts or modifying polymer structures to ensure more environmentally benign breakdown products. Additionally, implementing strict waste management protocols for the disposal of perchlorate-containing polymers is crucial to prevent environmental contamination.
Regulatory frameworks and environmental monitoring programs need to be established or enhanced to track perchlorate levels in various environmental compartments. This will help in early detection of contamination and facilitate timely intervention measures. Furthermore, research into remediation technologies for perchlorate-contaminated sites should be prioritized to address existing environmental impacts.
Safety Protocols for Perchloric Acid Handling
Handling perchloric acid requires strict adherence to safety protocols due to its highly reactive and potentially explosive nature. Proper training and equipment are essential for all personnel working with this chemical. Personal protective equipment (PPE) must include chemical-resistant gloves, safety goggles, and a lab coat. A face shield and chemical-resistant apron may be necessary for larger-scale operations.
Work involving perchloric acid should be conducted in a designated fume hood equipped with a wash-down system to prevent the accumulation of explosive perchlorates. The work area must be free from organic materials and other incompatible substances. Regular cleaning and maintenance of the fume hood are crucial to prevent perchlorate buildup.
Storage of perchloric acid demands special consideration. It should be kept in a cool, well-ventilated area, separate from other chemicals, especially organic compounds and reducing agents. Glass or PTFE containers are suitable for storage, but metal containers should be avoided due to potential reactions.
Dilution of perchloric acid requires extreme caution. Always add the acid to water, never the reverse, to prevent violent reactions. This process should be performed slowly and with constant stirring to dissipate heat.
Waste disposal protocols for perchloric acid are critical. Neutralization with a base should be performed carefully, and the resulting solution must be treated as hazardous waste. Spills should be immediately contained and neutralized using appropriate spill kits designed for strong acids.
Emergency response procedures must be in place and regularly reviewed. This includes the location and proper use of safety showers, eyewash stations, and fire extinguishers. A detailed spill response plan should be readily available, and all personnel must be trained in its implementation.
Regular safety audits and inspections of perchloric acid handling areas are necessary to ensure compliance with safety protocols. This includes checking the integrity of storage containers, verifying the functionality of safety equipment, and reviewing handling procedures.
When using perchloric acid in research on biodegradable polymers, additional precautions may be necessary. The potential for reactions between the acid and polymer components must be carefully evaluated. Researchers should conduct small-scale tests before scaling up experiments and always work within the established safety parameters.
Work involving perchloric acid should be conducted in a designated fume hood equipped with a wash-down system to prevent the accumulation of explosive perchlorates. The work area must be free from organic materials and other incompatible substances. Regular cleaning and maintenance of the fume hood are crucial to prevent perchlorate buildup.
Storage of perchloric acid demands special consideration. It should be kept in a cool, well-ventilated area, separate from other chemicals, especially organic compounds and reducing agents. Glass or PTFE containers are suitable for storage, but metal containers should be avoided due to potential reactions.
Dilution of perchloric acid requires extreme caution. Always add the acid to water, never the reverse, to prevent violent reactions. This process should be performed slowly and with constant stirring to dissipate heat.
Waste disposal protocols for perchloric acid are critical. Neutralization with a base should be performed carefully, and the resulting solution must be treated as hazardous waste. Spills should be immediately contained and neutralized using appropriate spill kits designed for strong acids.
Emergency response procedures must be in place and regularly reviewed. This includes the location and proper use of safety showers, eyewash stations, and fire extinguishers. A detailed spill response plan should be readily available, and all personnel must be trained in its implementation.
Regular safety audits and inspections of perchloric acid handling areas are necessary to ensure compliance with safety protocols. This includes checking the integrity of storage containers, verifying the functionality of safety equipment, and reviewing handling procedures.
When using perchloric acid in research on biodegradable polymers, additional precautions may be necessary. The potential for reactions between the acid and polymer components must be carefully evaluated. Researchers should conduct small-scale tests before scaling up experiments and always work within the established safety parameters.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!