The Use of Perchloric Acid in the Synthesis of Polyoxometalates
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polyoxometalate Synthesis Background and Objectives
Polyoxometalates (POMs) represent a diverse class of metal-oxygen cluster compounds with a rich history dating back to the early 19th century. These molecular metal oxides have garnered significant attention due to their unique structural properties and wide-ranging applications in catalysis, materials science, and medicine. The synthesis of POMs has evolved considerably over the years, with perchloric acid emerging as a crucial reagent in modern synthetic methodologies.
The use of perchloric acid in POM synthesis can be traced back to the mid-20th century when researchers began exploring more efficient and controlled methods for producing these complex structures. Perchloric acid's strong oxidizing properties and ability to stabilize high oxidation states of transition metals made it an ideal candidate for POM synthesis. Its introduction marked a significant milestone in the field, enabling the creation of novel POM architectures and expanding the range of accessible structures.
As research in POM chemistry progressed, the role of perchloric acid became increasingly prominent. Its unique properties allowed for the formation of previously unattainable POM structures, particularly those containing high-valent metal centers. This breakthrough opened up new avenues for exploring the fundamental chemistry of POMs and their potential applications in various fields.
The primary objective of utilizing perchloric acid in POM synthesis is to achieve greater control over the assembly process and to access a broader range of POM structures. By manipulating reaction conditions and precursor compositions, researchers aim to develop more efficient and selective synthetic routes. This approach not only enhances the yield and purity of desired POM products but also facilitates the discovery of new POM families with tailored properties.
Another critical goal in this field is to understand the mechanistic aspects of POM formation in the presence of perchloric acid. Elucidating the reaction pathways and intermediate species involved in these syntheses can provide valuable insights into the fundamental principles governing POM assembly. This knowledge is essential for developing predictive models and rational design strategies for creating POMs with specific structures and properties.
Furthermore, researchers are exploring the potential of perchloric acid-based syntheses to produce POMs with enhanced stability, reactivity, and functionality. By fine-tuning the synthetic conditions and incorporating various metal centers and heteroatoms, scientists aim to create POMs with improved catalytic activity, redox properties, and biocompatibility. These advancements are crucial for expanding the practical applications of POMs in areas such as energy conversion, environmental remediation, and drug delivery.
As the field of POM chemistry continues to evolve, the use of perchloric acid in synthesis remains a key area of investigation. Researchers are constantly seeking to optimize reaction conditions, develop new synthetic protocols, and explore alternative reagents that can complement or potentially replace perchloric acid in certain applications. The ongoing efforts in this domain are driven by the desire to unlock the full potential of POMs and harness their unique properties for addressing global challenges in energy, environment, and healthcare.
The use of perchloric acid in POM synthesis can be traced back to the mid-20th century when researchers began exploring more efficient and controlled methods for producing these complex structures. Perchloric acid's strong oxidizing properties and ability to stabilize high oxidation states of transition metals made it an ideal candidate for POM synthesis. Its introduction marked a significant milestone in the field, enabling the creation of novel POM architectures and expanding the range of accessible structures.
As research in POM chemistry progressed, the role of perchloric acid became increasingly prominent. Its unique properties allowed for the formation of previously unattainable POM structures, particularly those containing high-valent metal centers. This breakthrough opened up new avenues for exploring the fundamental chemistry of POMs and their potential applications in various fields.
The primary objective of utilizing perchloric acid in POM synthesis is to achieve greater control over the assembly process and to access a broader range of POM structures. By manipulating reaction conditions and precursor compositions, researchers aim to develop more efficient and selective synthetic routes. This approach not only enhances the yield and purity of desired POM products but also facilitates the discovery of new POM families with tailored properties.
Another critical goal in this field is to understand the mechanistic aspects of POM formation in the presence of perchloric acid. Elucidating the reaction pathways and intermediate species involved in these syntheses can provide valuable insights into the fundamental principles governing POM assembly. This knowledge is essential for developing predictive models and rational design strategies for creating POMs with specific structures and properties.
Furthermore, researchers are exploring the potential of perchloric acid-based syntheses to produce POMs with enhanced stability, reactivity, and functionality. By fine-tuning the synthetic conditions and incorporating various metal centers and heteroatoms, scientists aim to create POMs with improved catalytic activity, redox properties, and biocompatibility. These advancements are crucial for expanding the practical applications of POMs in areas such as energy conversion, environmental remediation, and drug delivery.
As the field of POM chemistry continues to evolve, the use of perchloric acid in synthesis remains a key area of investigation. Researchers are constantly seeking to optimize reaction conditions, develop new synthetic protocols, and explore alternative reagents that can complement or potentially replace perchloric acid in certain applications. The ongoing efforts in this domain are driven by the desire to unlock the full potential of POMs and harness their unique properties for addressing global challenges in energy, environment, and healthcare.
Market Analysis for Polyoxometalate Applications
The market for polyoxometalate (POM) applications has been experiencing significant growth in recent years, driven by the unique properties and versatile applications of these inorganic metal-oxygen cluster compounds. The global POM market is projected to expand at a steady rate, with increasing demand across various industries such as catalysis, materials science, and energy storage.
In the catalysis sector, POMs have gained traction due to their high catalytic activity, selectivity, and stability. They are being increasingly utilized in petrochemical processes, fine chemical synthesis, and environmental remediation. The automotive industry, in particular, has shown growing interest in POM-based catalysts for emission control systems, as they offer improved performance and durability compared to traditional catalysts.
The materials science field has also witnessed a surge in POM applications. These compounds are being incorporated into functional materials such as self-healing polymers, anti-corrosion coatings, and smart materials with stimuli-responsive properties. The electronics industry is exploring POMs for their potential in developing next-generation memory devices and molecular switches, leveraging their redox properties and structural diversity.
Energy storage represents another promising market for POMs. Research into POM-based electrolytes and electrode materials for batteries and supercapacitors has intensified, driven by the need for more efficient and sustainable energy storage solutions. The renewable energy sector is particularly interested in POMs for their potential to enhance the performance and longevity of solar cells and fuel cells.
The healthcare and biomedical industries are emerging as new frontiers for POM applications. These compounds show promise in drug delivery systems, biosensors, and as anti-tumor and anti-viral agents. The unique ability of POMs to interact with biological systems while maintaining their structural integrity has opened up novel therapeutic possibilities.
Geographically, Asia-Pacific is expected to be the fastest-growing market for POM applications, fueled by rapid industrialization and increasing investments in research and development. North America and Europe continue to be significant markets, particularly in advanced applications such as nanotechnology and green chemistry.
Despite the promising outlook, challenges remain in scaling up POM production and reducing costs to make them more commercially viable for widespread adoption. Additionally, concerns about the environmental impact of perchloric acid used in POM synthesis are driving research into greener synthesis methods. As these challenges are addressed, the market for POM applications is poised for further expansion, with innovations in synthesis techniques and new application discoveries expected to drive growth in the coming years.
In the catalysis sector, POMs have gained traction due to their high catalytic activity, selectivity, and stability. They are being increasingly utilized in petrochemical processes, fine chemical synthesis, and environmental remediation. The automotive industry, in particular, has shown growing interest in POM-based catalysts for emission control systems, as they offer improved performance and durability compared to traditional catalysts.
The materials science field has also witnessed a surge in POM applications. These compounds are being incorporated into functional materials such as self-healing polymers, anti-corrosion coatings, and smart materials with stimuli-responsive properties. The electronics industry is exploring POMs for their potential in developing next-generation memory devices and molecular switches, leveraging their redox properties and structural diversity.
Energy storage represents another promising market for POMs. Research into POM-based electrolytes and electrode materials for batteries and supercapacitors has intensified, driven by the need for more efficient and sustainable energy storage solutions. The renewable energy sector is particularly interested in POMs for their potential to enhance the performance and longevity of solar cells and fuel cells.
The healthcare and biomedical industries are emerging as new frontiers for POM applications. These compounds show promise in drug delivery systems, biosensors, and as anti-tumor and anti-viral agents. The unique ability of POMs to interact with biological systems while maintaining their structural integrity has opened up novel therapeutic possibilities.
Geographically, Asia-Pacific is expected to be the fastest-growing market for POM applications, fueled by rapid industrialization and increasing investments in research and development. North America and Europe continue to be significant markets, particularly in advanced applications such as nanotechnology and green chemistry.
Despite the promising outlook, challenges remain in scaling up POM production and reducing costs to make them more commercially viable for widespread adoption. Additionally, concerns about the environmental impact of perchloric acid used in POM synthesis are driving research into greener synthesis methods. As these challenges are addressed, the market for POM applications is poised for further expansion, with innovations in synthesis techniques and new application discoveries expected to drive growth in the coming years.
Current Challenges in Perchloric Acid-Based Synthesis
The synthesis of polyoxometalates using perchloric acid presents several significant challenges that researchers and industry professionals must address. One of the primary concerns is the inherent safety risks associated with handling perchloric acid. This highly corrosive and strong oxidizing agent requires specialized equipment and rigorous safety protocols, which can limit its widespread use in both research and industrial settings.
Another challenge lies in controlling the reaction conditions precisely. The synthesis of polyoxometalates often requires careful pH control and specific temperature ranges. Perchloric acid's high reactivity can make it difficult to maintain these conditions consistently, potentially leading to unwanted side reactions or incomplete formation of the desired polyoxometalate structures.
The purity of the final product is also a critical issue. Perchloric acid can introduce perchlorate ions as impurities in the synthesized polyoxometalates, which may affect their properties and applications. Removing these impurities often requires additional purification steps, increasing the complexity and cost of the synthesis process.
Scalability presents another significant hurdle. While perchloric acid-based synthesis may be effective on a laboratory scale, translating these processes to industrial production levels can be problematic. The increased volumes of perchloric acid required for large-scale synthesis exacerbate safety concerns and may necessitate substantial investments in specialized equipment and facilities.
Environmental considerations also pose challenges. The use of perchloric acid can generate hazardous waste that requires careful disposal. Developing more environmentally friendly synthesis routes or finding effective ways to recycle and reuse perchloric acid are important areas of ongoing research.
Furthermore, the high cost of perchloric acid compared to other acids used in polyoxometalate synthesis can make it economically unfavorable for certain applications. This economic factor often drives researchers to seek alternative synthetic routes that use less expensive or more readily available reagents.
Lastly, the limited understanding of the precise reaction mechanisms involved in perchloric acid-based polyoxometalate synthesis hinders the development of more efficient and controlled processes. Elucidating these mechanisms through advanced characterization techniques and computational studies remains a significant challenge in the field.
Another challenge lies in controlling the reaction conditions precisely. The synthesis of polyoxometalates often requires careful pH control and specific temperature ranges. Perchloric acid's high reactivity can make it difficult to maintain these conditions consistently, potentially leading to unwanted side reactions or incomplete formation of the desired polyoxometalate structures.
The purity of the final product is also a critical issue. Perchloric acid can introduce perchlorate ions as impurities in the synthesized polyoxometalates, which may affect their properties and applications. Removing these impurities often requires additional purification steps, increasing the complexity and cost of the synthesis process.
Scalability presents another significant hurdle. While perchloric acid-based synthesis may be effective on a laboratory scale, translating these processes to industrial production levels can be problematic. The increased volumes of perchloric acid required for large-scale synthesis exacerbate safety concerns and may necessitate substantial investments in specialized equipment and facilities.
Environmental considerations also pose challenges. The use of perchloric acid can generate hazardous waste that requires careful disposal. Developing more environmentally friendly synthesis routes or finding effective ways to recycle and reuse perchloric acid are important areas of ongoing research.
Furthermore, the high cost of perchloric acid compared to other acids used in polyoxometalate synthesis can make it economically unfavorable for certain applications. This economic factor often drives researchers to seek alternative synthetic routes that use less expensive or more readily available reagents.
Lastly, the limited understanding of the precise reaction mechanisms involved in perchloric acid-based polyoxometalate synthesis hinders the development of more efficient and controlled processes. Elucidating these mechanisms through advanced characterization techniques and computational studies remains a significant challenge in the field.
Existing Perchloric Acid Synthesis Protocols
01 Synthesis and structure of polyoxometalates
Polyoxometalates are synthesized through various methods, resulting in diverse structures. These compounds are typically composed of early transition metals in their highest oxidation states, forming complex anionic clusters. The synthesis often involves controlled hydrolysis and condensation reactions, leading to the formation of metal-oxygen frameworks with unique properties.- Synthesis and structure of polyoxometalates: Polyoxometalates are synthesized through various methods, resulting in diverse structures. These compounds are typically composed of early transition metals in their highest oxidation states, often forming clusters with oxygen atoms. The synthesis processes and resulting structures can be tailored for specific applications in catalysis, materials science, and medicine.
- Catalytic applications of polyoxometalates: Polyoxometalates exhibit excellent catalytic properties due to their unique electronic structure and redox capabilities. They are used in various catalytic processes, including oxidation reactions, acid-catalyzed reactions, and photocatalysis. Their high stability and tunable properties make them valuable in industrial and environmental applications.
- Polyoxometalates in energy storage and conversion: These compounds show promise in energy-related applications, particularly in the development of advanced batteries and fuel cells. Their ability to undergo reversible redox reactions and store multiple electrons makes them attractive for use in electrochemical energy storage systems and as electrocatalysts for water splitting and fuel cell reactions.
- Biomedical applications of polyoxometalates: Polyoxometalates have shown potential in various biomedical applications due to their unique properties. They exhibit antiviral, antibacterial, and anticancer activities, making them promising candidates for drug development. Additionally, their use in biosensors and diagnostic imaging has been explored, leveraging their redox properties and interactions with biomolecules.
- Polyoxometalates in materials science: These compounds are utilized in the development of advanced materials with unique properties. They are incorporated into polymers, nanocomposites, and thin films to create materials with enhanced electrical, optical, and mechanical characteristics. Applications include smart windows, sensors, and functional coatings with self-healing or stimuli-responsive properties.
02 Applications in catalysis
Polyoxometalates exhibit excellent catalytic properties, making them valuable in various chemical processes. They are used as homogeneous and heterogeneous catalysts in oxidation reactions, acid-catalyzed transformations, and selective organic syntheses. Their high acidity, redox properties, and structural versatility contribute to their effectiveness as catalysts in both industrial and laboratory settings.Expand Specific Solutions03 Use in energy storage and conversion
Polyoxometalates show promise in energy-related applications, particularly in the development of advanced energy storage and conversion devices. They are investigated for use in batteries, fuel cells, and supercapacitors due to their unique redox properties and ability to store and transfer electrons. These compounds can enhance the performance and efficiency of various electrochemical systems.Expand Specific Solutions04 Environmental applications
Polyoxometalates are utilized in environmental remediation and water treatment processes. Their ability to catalyze the degradation of pollutants, remove heavy metals, and act as photocatalysts for water splitting makes them valuable in addressing environmental challenges. They are also explored for their potential in developing more sustainable and eco-friendly industrial processes.Expand Specific Solutions05 Biomedical and pharmaceutical applications
The diverse properties of polyoxometalates are being explored for potential biomedical and pharmaceutical applications. These compounds show promise in drug delivery systems, as antiviral and antibacterial agents, and in diagnostic imaging. Their ability to interact with biological systems while maintaining stability makes them interesting candidates for various medical applications.Expand Specific Solutions
Key Players in Polyoxometalate Research and Production
The synthesis of polyoxometalates using perchloric acid is a niche field within inorganic chemistry, currently in its growth phase. The market size is relatively small but expanding, driven by applications in catalysis, materials science, and energy storage. Technologically, the process is moderately mature, with ongoing research to optimize synthesis methods and explore new applications. Key players in this area include academic institutions like Hubei University of Technology, Fuzhou University, and the University of Delaware, which are actively publishing research. Industrial involvement is limited but growing, with companies like ExxonMobil Chemical Patents and Merck Patent GmbH showing interest through patent activities, indicating potential for commercial applications in the near future.
University of Delaware
Technical Solution: The University of Delaware has made significant advancements in the use of perchloric acid for polyoxometalate synthesis. Their approach focuses on the development of green and sustainable synthesis methods. By optimizing the reaction conditions and using perchloric acid in combination with environmentally friendly solvents, they have achieved high-yield synthesis of polyoxometalates with reduced environmental impact[1]. The university has also explored the use of microwave-assisted synthesis techniques in conjunction with perchloric acid, significantly reducing reaction times and energy consumption[2]. Furthermore, they have developed novel polyoxometalate structures with enhanced stability and catalytic activity, particularly for applications in water treatment and environmental remediation[3].
Strengths: Green and sustainable synthesis methods, reduced reaction times, enhanced stability and catalytic activity of products. Weaknesses: Potential limitations in the diversity of achievable structures, challenges in scaling up microwave-assisted synthesis.
Jilin University
Technical Solution: Jilin University has developed an innovative approach for the synthesis of polyoxometalates using perchloric acid. Their method involves a controlled acidification process, where perchloric acid is used as a proton source to initiate the formation of polyoxometalate clusters. The researchers have optimized the reaction conditions, including temperature, concentration, and pH, to achieve high yields and structural diversity[1]. They have successfully synthesized novel polyoxometalate structures with enhanced catalytic properties and potential applications in materials science and energy storage[2]. The university has also explored the use of perchloric acid in combination with other acids to fine-tune the assembly process of polyoxometalates, leading to the creation of hierarchical nanostructures with unique properties[3].
Strengths: High yield and structural diversity of polyoxometalates, enhanced catalytic properties, potential for hierarchical nanostructures. Weaknesses: Safety concerns associated with handling perchloric acid, potential scalability issues for industrial production.
Innovative Approaches in Perchloric Acid Utilization
A process for site-specific selective oxidation of aromatic amines on patterned micro catalytic trail
PatentActiveIN202031035481A
Innovation
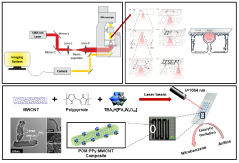
- A simple and cost-effective process using polyoxometalate-based catalytic composites with vanadium or tungsten oxometalates, multiwalled carbon nanotubes, and polypyrrole, patterned using laser-induced microbubble self-assembly and thermo-optical tweezers to create micro-catalytic reactors for site-specific oxidation of aromatic amines on a 'lab-on-a-chip' platform.
Process for obtaining a formate from a reaction mixture
PatentWO2016078698A1
Innovation
- A process involving a polyoxometalate ion catalyst at temperatures below 120°C, where formic acid is separated from the reaction mixture by reverse osmosis or vaporization and then reacted with a hydroxide solution to form a formate, allowing continuous removal and concentration without interrupting the reaction.
Safety Considerations for Perchloric Acid Handling
Perchloric acid is a powerful oxidizing agent widely used in the synthesis of polyoxometalates. However, its highly reactive nature necessitates stringent safety measures during handling and storage. Proper risk assessment and implementation of safety protocols are crucial to prevent accidents and ensure the well-being of laboratory personnel.
When working with perchloric acid, it is essential to use appropriate personal protective equipment (PPE). This includes chemical-resistant gloves, safety goggles, and a lab coat. A face shield may also be necessary when handling large quantities. Respiratory protection should be considered if there is a risk of vapor inhalation.
The storage of perchloric acid requires special attention. It should be kept in a cool, dry, and well-ventilated area, away from combustible materials and other chemicals. Glass or PTFE containers are recommended, as perchloric acid can react with many metals. Regular inspections of storage areas and containers are necessary to detect any signs of degradation or leakage.
Dedicated fume hoods equipped with wash-down systems are essential for work involving perchloric acid. These specialized hoods prevent the accumulation of potentially explosive perchlorates on surfaces. Regular cleaning and maintenance of these hoods are critical to ensure their effectiveness and prevent hazardous buildup.
Proper waste disposal is another crucial aspect of perchloric acid safety. Neutralization and dilution should be performed before disposal, following local regulations and institutional guidelines. It is important to note that perchloric acid should never be allowed to dry on organic materials, as this can lead to the formation of explosive compounds.
Emergency response procedures must be in place and well-communicated to all laboratory personnel. This includes the location and proper use of safety showers, eyewash stations, and spill kits specifically designed for perchloric acid. Regular safety training and drills should be conducted to ensure preparedness in case of accidents.
When using perchloric acid in the synthesis of polyoxometalates, additional precautions may be necessary. The combination with certain metal ions or organic compounds can lead to the formation of unstable intermediates. Therefore, a thorough understanding of the reaction chemistry and potential side reactions is essential for safe experimental design.
Lastly, it is crucial to maintain accurate records of perchloric acid usage, storage, and disposal. This documentation aids in inventory management and ensures compliance with safety regulations. Regular safety audits should be conducted to identify and address any potential hazards or areas for improvement in handling procedures.
When working with perchloric acid, it is essential to use appropriate personal protective equipment (PPE). This includes chemical-resistant gloves, safety goggles, and a lab coat. A face shield may also be necessary when handling large quantities. Respiratory protection should be considered if there is a risk of vapor inhalation.
The storage of perchloric acid requires special attention. It should be kept in a cool, dry, and well-ventilated area, away from combustible materials and other chemicals. Glass or PTFE containers are recommended, as perchloric acid can react with many metals. Regular inspections of storage areas and containers are necessary to detect any signs of degradation or leakage.
Dedicated fume hoods equipped with wash-down systems are essential for work involving perchloric acid. These specialized hoods prevent the accumulation of potentially explosive perchlorates on surfaces. Regular cleaning and maintenance of these hoods are critical to ensure their effectiveness and prevent hazardous buildup.
Proper waste disposal is another crucial aspect of perchloric acid safety. Neutralization and dilution should be performed before disposal, following local regulations and institutional guidelines. It is important to note that perchloric acid should never be allowed to dry on organic materials, as this can lead to the formation of explosive compounds.
Emergency response procedures must be in place and well-communicated to all laboratory personnel. This includes the location and proper use of safety showers, eyewash stations, and spill kits specifically designed for perchloric acid. Regular safety training and drills should be conducted to ensure preparedness in case of accidents.
When using perchloric acid in the synthesis of polyoxometalates, additional precautions may be necessary. The combination with certain metal ions or organic compounds can lead to the formation of unstable intermediates. Therefore, a thorough understanding of the reaction chemistry and potential side reactions is essential for safe experimental design.
Lastly, it is crucial to maintain accurate records of perchloric acid usage, storage, and disposal. This documentation aids in inventory management and ensures compliance with safety regulations. Regular safety audits should be conducted to identify and address any potential hazards or areas for improvement in handling procedures.
Environmental Impact of Perchloric Acid Use
The use of perchloric acid in the synthesis of polyoxometalates raises significant environmental concerns due to its highly reactive and potentially hazardous nature. When released into the environment, perchloric acid can have severe impacts on ecosystems and human health.
In aquatic environments, perchloric acid can disrupt the natural pH balance, leading to acidification of water bodies. This pH change can have detrimental effects on aquatic life, including fish, amphibians, and microorganisms. The altered pH can interfere with the respiratory functions of aquatic organisms and affect their ability to maintain proper ion balance.
Soil contamination is another major concern associated with perchloric acid use. When perchloric acid or its salts enter the soil, they can persist for extended periods due to their high stability. This persistence can lead to long-term contamination of groundwater and surface water sources. Additionally, perchloric acid in soil can alter soil chemistry, affecting plant growth and microbial communities essential for soil health.
The atmospheric release of perchloric acid vapors during synthesis processes poses risks to air quality. These vapors can contribute to the formation of acidic aerosols, which may lead to acid rain and its associated environmental impacts. Acid rain can damage vegetation, acidify water bodies, and corrode infrastructure.
Human exposure to perchloric acid through contaminated air, water, or soil can result in various health issues. Inhalation of perchloric acid vapors can cause respiratory irritation and, in severe cases, lung damage. Skin contact can lead to burns and dermatitis, while ingestion can cause severe gastrointestinal problems.
To mitigate these environmental risks, strict handling and disposal protocols must be implemented in laboratories and industrial settings where perchloric acid is used for polyoxometalate synthesis. Proper containment systems, including fume hoods and specialized waste treatment facilities, are essential to prevent environmental release.
Recycling and neutralization of perchloric acid waste are crucial steps in minimizing environmental impact. Advanced treatment technologies, such as ion exchange and electrochemical reduction, can be employed to convert perchlorate ions into less harmful compounds before disposal.
Research into alternative synthesis methods that reduce or eliminate the use of perchloric acid is ongoing. Green chemistry approaches, including the use of less hazardous oxidizing agents or novel catalytic systems, are being explored to develop more environmentally friendly polyoxometalate synthesis processes.
Regulatory bodies worldwide have established guidelines for the use and disposal of perchloric acid. Compliance with these regulations is essential for minimizing environmental risks and ensuring sustainable practices in polyoxometalate synthesis.
In aquatic environments, perchloric acid can disrupt the natural pH balance, leading to acidification of water bodies. This pH change can have detrimental effects on aquatic life, including fish, amphibians, and microorganisms. The altered pH can interfere with the respiratory functions of aquatic organisms and affect their ability to maintain proper ion balance.
Soil contamination is another major concern associated with perchloric acid use. When perchloric acid or its salts enter the soil, they can persist for extended periods due to their high stability. This persistence can lead to long-term contamination of groundwater and surface water sources. Additionally, perchloric acid in soil can alter soil chemistry, affecting plant growth and microbial communities essential for soil health.
The atmospheric release of perchloric acid vapors during synthesis processes poses risks to air quality. These vapors can contribute to the formation of acidic aerosols, which may lead to acid rain and its associated environmental impacts. Acid rain can damage vegetation, acidify water bodies, and corrode infrastructure.
Human exposure to perchloric acid through contaminated air, water, or soil can result in various health issues. Inhalation of perchloric acid vapors can cause respiratory irritation and, in severe cases, lung damage. Skin contact can lead to burns and dermatitis, while ingestion can cause severe gastrointestinal problems.
To mitigate these environmental risks, strict handling and disposal protocols must be implemented in laboratories and industrial settings where perchloric acid is used for polyoxometalate synthesis. Proper containment systems, including fume hoods and specialized waste treatment facilities, are essential to prevent environmental release.
Recycling and neutralization of perchloric acid waste are crucial steps in minimizing environmental impact. Advanced treatment technologies, such as ion exchange and electrochemical reduction, can be employed to convert perchlorate ions into less harmful compounds before disposal.
Research into alternative synthesis methods that reduce or eliminate the use of perchloric acid is ongoing. Green chemistry approaches, including the use of less hazardous oxidizing agents or novel catalytic systems, are being explored to develop more environmentally friendly polyoxometalate synthesis processes.
Regulatory bodies worldwide have established guidelines for the use and disposal of perchloric acid. Compliance with these regulations is essential for minimizing environmental risks and ensuring sustainable practices in polyoxometalate synthesis.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!