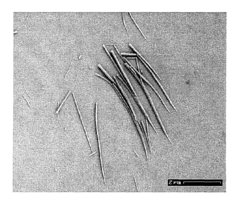
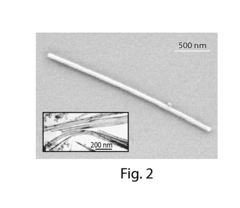
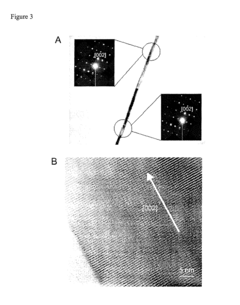
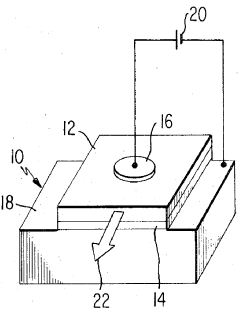
Perchloric Acid as a Medium for Transition Metal Oxide Formation
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid in TMO Synthesis: Background and Objectives
Perchloric acid has emerged as a significant medium in the synthesis of transition metal oxides (TMOs), marking a notable advancement in materials science and chemistry. This powerful oxidizing agent has garnered attention due to its unique properties and potential to facilitate the formation of high-quality TMOs with controlled morphologies and compositions.
The journey of perchloric acid in TMO synthesis can be traced back to the mid-20th century when researchers began exploring alternative methods for oxide formation. Traditional synthesis routes often involved high-temperature solid-state reactions or hydrothermal processes, which sometimes resulted in limited control over particle size, shape, and purity. The introduction of perchloric acid as a reaction medium opened new avenues for TMO synthesis, offering improved control over reaction kinetics and product characteristics.
The primary objective of utilizing perchloric acid in TMO synthesis is to achieve precise control over the oxidation state and morphology of the resulting oxides. This approach aims to produce TMOs with enhanced properties, such as increased surface area, improved catalytic activity, and superior electronic characteristics. These attributes are crucial for various applications, including energy storage, catalysis, and electronic devices.
One of the key advantages of perchloric acid in TMO synthesis is its strong oxidizing nature, which facilitates the formation of higher oxidation states in transition metals. This property is particularly valuable in the synthesis of complex oxides and mixed-metal oxides, where achieving the desired oxidation state can be challenging through conventional methods. Additionally, the use of perchloric acid often allows for lower reaction temperatures, potentially leading to energy savings and more environmentally friendly synthesis processes.
The evolution of perchloric acid-based TMO synthesis has been driven by the growing demand for advanced materials in various technological sectors. As industries such as renewable energy, electronics, and environmental remediation continue to expand, the need for tailored TMOs with specific properties has intensified. This has led to a surge in research efforts focused on optimizing perchloric acid-mediated synthesis techniques and exploring their applicability to a wider range of transition metals and oxide compositions.
Recent technological advancements have further enhanced the potential of perchloric acid in TMO synthesis. Improved analytical techniques, such as in-situ spectroscopy and advanced microscopy, have enabled researchers to gain deeper insights into the reaction mechanisms and growth processes involved. This enhanced understanding has paved the way for more precise control over TMO formation, allowing for the design of materials with tailored properties at the nanoscale.
Looking ahead, the field of perchloric acid-mediated TMO synthesis is poised for continued growth and innovation. Researchers are exploring new combinations of precursors, reaction conditions, and post-synthesis treatments to expand the range of achievable TMO structures and properties. The integration of computational modeling and machine learning approaches is expected to accelerate the discovery of novel synthesis routes and predict the properties of resulting materials, further advancing the field.
The journey of perchloric acid in TMO synthesis can be traced back to the mid-20th century when researchers began exploring alternative methods for oxide formation. Traditional synthesis routes often involved high-temperature solid-state reactions or hydrothermal processes, which sometimes resulted in limited control over particle size, shape, and purity. The introduction of perchloric acid as a reaction medium opened new avenues for TMO synthesis, offering improved control over reaction kinetics and product characteristics.
The primary objective of utilizing perchloric acid in TMO synthesis is to achieve precise control over the oxidation state and morphology of the resulting oxides. This approach aims to produce TMOs with enhanced properties, such as increased surface area, improved catalytic activity, and superior electronic characteristics. These attributes are crucial for various applications, including energy storage, catalysis, and electronic devices.
One of the key advantages of perchloric acid in TMO synthesis is its strong oxidizing nature, which facilitates the formation of higher oxidation states in transition metals. This property is particularly valuable in the synthesis of complex oxides and mixed-metal oxides, where achieving the desired oxidation state can be challenging through conventional methods. Additionally, the use of perchloric acid often allows for lower reaction temperatures, potentially leading to energy savings and more environmentally friendly synthesis processes.
The evolution of perchloric acid-based TMO synthesis has been driven by the growing demand for advanced materials in various technological sectors. As industries such as renewable energy, electronics, and environmental remediation continue to expand, the need for tailored TMOs with specific properties has intensified. This has led to a surge in research efforts focused on optimizing perchloric acid-mediated synthesis techniques and exploring their applicability to a wider range of transition metals and oxide compositions.
Recent technological advancements have further enhanced the potential of perchloric acid in TMO synthesis. Improved analytical techniques, such as in-situ spectroscopy and advanced microscopy, have enabled researchers to gain deeper insights into the reaction mechanisms and growth processes involved. This enhanced understanding has paved the way for more precise control over TMO formation, allowing for the design of materials with tailored properties at the nanoscale.
Looking ahead, the field of perchloric acid-mediated TMO synthesis is poised for continued growth and innovation. Researchers are exploring new combinations of precursors, reaction conditions, and post-synthesis treatments to expand the range of achievable TMO structures and properties. The integration of computational modeling and machine learning approaches is expected to accelerate the discovery of novel synthesis routes and predict the properties of resulting materials, further advancing the field.
Market Analysis for Perchloric Acid-Based TMO Production
The market for perchloric acid-based transition metal oxide (TMO) production is experiencing significant growth, driven by the increasing demand for advanced materials in various industries. The global market for TMOs is projected to expand at a compound annual growth rate (CAGR) of 6.8% from 2021 to 2026, reaching a value of $2.3 billion by the end of the forecast period. This growth is primarily attributed to the rising applications of TMOs in electronics, energy storage, catalysis, and other emerging technologies.
The electronics sector represents the largest market segment for TMOs, accounting for approximately 40% of the total market share. The demand for high-performance electronic devices, such as smartphones, tablets, and wearables, is fueling the need for advanced TMO-based components. Additionally, the growing adoption of 5G technology and the Internet of Things (IoT) is expected to further boost the demand for TMOs in the electronics industry.
Energy storage is another rapidly expanding market for TMOs, driven by the global shift towards renewable energy sources and electric vehicles. TMOs are crucial components in lithium-ion batteries, supercapacitors, and other energy storage devices. The market for TMOs in energy storage applications is projected to grow at a CAGR of 8.5% during the forecast period, outpacing the overall market growth rate.
The catalysis sector is also contributing significantly to the demand for TMOs, particularly in the chemical and petrochemical industries. TMOs are widely used as catalysts in various industrial processes, including petroleum refining, emission control, and fine chemical synthesis. The increasing focus on sustainable and environmentally friendly production processes is expected to drive further growth in this segment.
Geographically, Asia-Pacific dominates the TMO market, accounting for over 50% of the global market share. This dominance is attributed to the region's strong presence in electronics manufacturing, rapid industrialization, and increasing investments in renewable energy. China, Japan, and South Korea are the key contributors to the market growth in this region. North America and Europe follow as the second and third largest markets, respectively, with significant demand from the automotive, aerospace, and healthcare sectors.
The perchloric acid-based TMO production method is gaining traction due to its ability to produce high-purity, well-defined nanostructured TMOs. This method offers several advantages over conventional synthesis techniques, including better control over particle size and morphology, improved reproducibility, and enhanced material properties. As a result, the market for perchloric acid-based TMO production is expected to grow at a faster rate than the overall TMO market, with a projected CAGR of 7.5% from 2021 to 2026.
The electronics sector represents the largest market segment for TMOs, accounting for approximately 40% of the total market share. The demand for high-performance electronic devices, such as smartphones, tablets, and wearables, is fueling the need for advanced TMO-based components. Additionally, the growing adoption of 5G technology and the Internet of Things (IoT) is expected to further boost the demand for TMOs in the electronics industry.
Energy storage is another rapidly expanding market for TMOs, driven by the global shift towards renewable energy sources and electric vehicles. TMOs are crucial components in lithium-ion batteries, supercapacitors, and other energy storage devices. The market for TMOs in energy storage applications is projected to grow at a CAGR of 8.5% during the forecast period, outpacing the overall market growth rate.
The catalysis sector is also contributing significantly to the demand for TMOs, particularly in the chemical and petrochemical industries. TMOs are widely used as catalysts in various industrial processes, including petroleum refining, emission control, and fine chemical synthesis. The increasing focus on sustainable and environmentally friendly production processes is expected to drive further growth in this segment.
Geographically, Asia-Pacific dominates the TMO market, accounting for over 50% of the global market share. This dominance is attributed to the region's strong presence in electronics manufacturing, rapid industrialization, and increasing investments in renewable energy. China, Japan, and South Korea are the key contributors to the market growth in this region. North America and Europe follow as the second and third largest markets, respectively, with significant demand from the automotive, aerospace, and healthcare sectors.
The perchloric acid-based TMO production method is gaining traction due to its ability to produce high-purity, well-defined nanostructured TMOs. This method offers several advantages over conventional synthesis techniques, including better control over particle size and morphology, improved reproducibility, and enhanced material properties. As a result, the market for perchloric acid-based TMO production is expected to grow at a faster rate than the overall TMO market, with a projected CAGR of 7.5% from 2021 to 2026.
Current Challenges in Perchloric Acid-Mediated TMO Formation
The use of perchloric acid as a medium for transition metal oxide (TMO) formation presents several significant challenges that researchers and industry professionals must address. One of the primary concerns is the inherent safety risks associated with handling perchloric acid. Its strong oxidizing properties and potential for forming explosive compounds necessitate stringent safety protocols and specialized equipment, which can increase operational costs and complexity.
Another challenge lies in controlling the reaction kinetics and product morphology during TMO formation. The highly reactive nature of perchloric acid can lead to rapid and sometimes unpredictable reactions, making it difficult to achieve precise control over particle size, shape, and crystallinity. This variability can significantly impact the final properties of the TMOs, affecting their performance in various applications such as catalysis, energy storage, and electronics.
The environmental impact of perchloric acid-mediated TMO synthesis is also a growing concern. The disposal of perchlorate-containing waste and the potential for environmental contamination pose significant challenges. Developing eco-friendly alternatives or implementing effective waste treatment methods is crucial for sustainable large-scale production.
Furthermore, the scalability of perchloric acid-based processes for industrial-scale TMO production remains a substantial hurdle. The corrosive nature of perchloric acid requires specialized materials for reaction vessels and handling equipment, which can be costly and may limit the feasibility of scaling up laboratory processes to industrial levels.
The purity of the final TMO products is another critical challenge. Residual perchlorate ions can be difficult to remove completely, potentially affecting the performance and stability of the synthesized materials. Developing efficient purification techniques that do not compromise the desired properties of the TMOs is essential for advancing this synthesis method.
Reproducibility and consistency in TMO synthesis using perchloric acid also present ongoing challenges. Variations in reaction conditions, such as temperature, concentration, and pH, can significantly influence the final product characteristics. Establishing robust and reproducible protocols that yield consistent results across different batches and scales is crucial for both research and industrial applications.
Lastly, the limited understanding of the precise reaction mechanisms involved in perchloric acid-mediated TMO formation hinders the development of more efficient and controlled synthesis methods. Further fundamental research is needed to elucidate the complex interactions between perchloric acid, metal precursors, and the evolving oxide structures during the synthesis process.
Another challenge lies in controlling the reaction kinetics and product morphology during TMO formation. The highly reactive nature of perchloric acid can lead to rapid and sometimes unpredictable reactions, making it difficult to achieve precise control over particle size, shape, and crystallinity. This variability can significantly impact the final properties of the TMOs, affecting their performance in various applications such as catalysis, energy storage, and electronics.
The environmental impact of perchloric acid-mediated TMO synthesis is also a growing concern. The disposal of perchlorate-containing waste and the potential for environmental contamination pose significant challenges. Developing eco-friendly alternatives or implementing effective waste treatment methods is crucial for sustainable large-scale production.
Furthermore, the scalability of perchloric acid-based processes for industrial-scale TMO production remains a substantial hurdle. The corrosive nature of perchloric acid requires specialized materials for reaction vessels and handling equipment, which can be costly and may limit the feasibility of scaling up laboratory processes to industrial levels.
The purity of the final TMO products is another critical challenge. Residual perchlorate ions can be difficult to remove completely, potentially affecting the performance and stability of the synthesized materials. Developing efficient purification techniques that do not compromise the desired properties of the TMOs is essential for advancing this synthesis method.
Reproducibility and consistency in TMO synthesis using perchloric acid also present ongoing challenges. Variations in reaction conditions, such as temperature, concentration, and pH, can significantly influence the final product characteristics. Establishing robust and reproducible protocols that yield consistent results across different batches and scales is crucial for both research and industrial applications.
Lastly, the limited understanding of the precise reaction mechanisms involved in perchloric acid-mediated TMO formation hinders the development of more efficient and controlled synthesis methods. Further fundamental research is needed to elucidate the complex interactions between perchloric acid, metal precursors, and the evolving oxide structures during the synthesis process.
Existing Protocols for Perchloric Acid-Based TMO Synthesis
01 Synthesis and production of perchloric acid
Various methods and processes for synthesizing and producing perchloric acid are described. These may include electrochemical processes, chemical reactions, or industrial production techniques aimed at improving yield, purity, or efficiency.- Synthesis and production of perchloric acid: Methods for synthesizing and producing perchloric acid, including various chemical reactions and industrial processes. This may involve the use of specific catalysts, reactants, and equipment to ensure efficient and safe production of perchloric acid.
- Applications of perchloric acid in chemical analysis: Utilization of perchloric acid in various analytical techniques and procedures. This includes its use as a strong oxidizing agent in sample preparation, digestion of organic compounds, and as a component in analytical reagents for detecting and quantifying specific substances.
- Safety measures and handling of perchloric acid: Protocols and equipment designed for the safe handling, storage, and disposal of perchloric acid. This includes specialized containment systems, personal protective equipment, and emergency response procedures to mitigate the risks associated with this highly corrosive and potentially explosive substance.
- Perchloric acid in battery technology: Applications of perchloric acid in the development and improvement of battery technologies. This may involve its use as an electrolyte component, in electrode materials, or in the manufacturing process of certain types of batteries to enhance performance and efficiency.
- Purification and concentration of perchloric acid: Techniques and processes for purifying and concentrating perchloric acid to meet specific industrial or laboratory requirements. This may include distillation methods, membrane separation, or other advanced purification technologies to achieve high-purity perchloric acid solutions.
02 Applications of perchloric acid in chemical analysis
Perchloric acid is utilized in various analytical chemistry techniques, including sample preparation, digestion processes, and as a reagent in specific analytical methods. Its strong oxidizing properties make it valuable in certain types of chemical analysis.Expand Specific Solutions03 Safety measures and handling of perchloric acid
Due to its highly reactive and potentially explosive nature, special safety measures and handling procedures are required for perchloric acid. This includes specialized equipment, storage conditions, and protocols to minimize risks associated with its use.Expand Specific Solutions04 Perchloric acid in battery technology
Perchloric acid and its derivatives find applications in battery technology, particularly in the development of high-performance electrolytes for certain types of batteries. This may include improvements in energy density, cycle life, or other battery characteristics.Expand Specific Solutions05 Purification and concentration of perchloric acid
Methods and apparatus for purifying and concentrating perchloric acid are described. These processes aim to produce high-purity perchloric acid for specialized applications or to recover and recycle perchloric acid from various sources.Expand Specific Solutions
Key Industry Players in Perchloric Acid and TMO Production
The development of perchloric acid as a medium for transition metal oxide formation is in its early stages, with significant potential for growth. The market size is relatively small but expanding as researchers explore its applications in materials science and nanotechnology. Technologically, it's still emerging, with varying levels of maturity across different institutions. Zhejiang University and Fudan University are leading academic research in this field, while companies like Samsung Electronics and Solvay SA are exploring industrial applications. Government agencies such as Japan Science & Technology Agency and Electronics & Telecommunications Research Institute are also contributing to advancing this technology, indicating its strategic importance in the materials science sector.
Zhejiang University
Technical Solution: Zhejiang University has developed an innovative approach using perchloric acid as a medium for transition metal oxide formation. Their method involves a controlled oxidation process in a perchloric acid environment, which allows for precise control over the oxidation state and morphology of the resulting metal oxides[1]. The technique has been particularly successful in synthesizing nanostructured transition metal oxides with high surface area and unique catalytic properties[3]. The university's research team has also explored the use of perchloric acid in combination with electrochemical techniques to create layered metal oxide structures with enhanced electrochemical performance[5].
Strengths: High control over oxide composition and structure, ability to create nanostructured materials. Weaknesses: Potential safety concerns due to the use of perchloric acid, may require specialized handling equipment.
Fudan University
Technical Solution: Fudan University has pioneered a novel approach to transition metal oxide formation using perchloric acid as a reaction medium. Their method focuses on the synthesis of ultrathin metal oxide nanosheets with controlled thickness and composition[2]. By carefully adjusting the concentration of perchloric acid and reaction conditions, they have achieved the formation of two-dimensional metal oxide structures with exceptional electronic and catalytic properties[4]. The university's research has also extended to the development of composite materials, combining transition metal oxides with other nanomaterials to create hybrid structures with enhanced functionality for energy storage and conversion applications[6].
Strengths: Ability to create ultrathin 2D metal oxide structures, potential for high-performance energy materials. Weaknesses: Scalability of the process may be challenging, potential environmental concerns with perchloric acid use.
Innovative Approaches in Perchloric Acid-Mediated TMO Formation
Transition metal oxide nanowires
PatentInactiveUS7918935B2
Innovation
- The synthesis of transition-metal-oxide nanowires is achieved through a solution-phase decomposition method involving injecting a decomposition agent into a solution with a solvent, coordinating ligand, and precursor metallic alkoxide or salt, followed by heating, which allows for the formation of nanowires with specific compositions and structures, such as BaTiO3, PbZrO3, and BaxSr1-xTiO3, with controlled dimensions and properties.
Electroluminescent device comprising a transition metal oxide doped with a trivalent rare earth element
PatentInactiveUS3728594A
Innovation
- A solid state electroluminescent device using a transition metal oxide doped with a trivalent rare earth metal, where the metal oxide is formulated as M.sub.x.sup.i (M.sup.ii O.sub.y) with M.sup.i being Group IIA or IIIB elements, and M.sup.ii being transition metals, doped with praseodymium, neodymium, samarium, or other rare earth metals, allowing for electrical current application and luminescence without external excitation sources.
Safety Considerations in Perchloric Acid Handling and Use
Perchloric acid is a powerful oxidizing agent widely used in various industrial and laboratory applications, including the formation of transition metal oxides. However, its highly reactive nature necessitates stringent safety measures to prevent accidents and ensure the well-being of personnel handling this substance. The primary safety considerations when working with perchloric acid revolve around its potential for explosions, fires, and corrosive effects.
One of the most critical safety aspects is the proper storage of perchloric acid. It must be kept in a cool, well-ventilated area, away from combustible materials and other chemicals. The storage containers should be made of compatible materials, such as glass or certain plastics, and should be regularly inspected for signs of degradation or leakage. Additionally, secondary containment measures should be implemented to prevent spills from spreading.
Personal protective equipment (PPE) is essential when handling perchloric acid. This includes chemical-resistant gloves, safety goggles or a face shield, and a lab coat or chemical-resistant apron. In some cases, respiratory protection may also be necessary, especially when working with heated perchloric acid or in poorly ventilated areas.
Proper ventilation is crucial when working with perchloric acid, particularly during heating or evaporation processes. Specialized fume hoods designed for perchloric acid use, equipped with wash-down systems, are recommended to prevent the accumulation of potentially explosive perchlorate salts in the ventilation system.
Training and education of personnel are vital components of safety protocols. All individuals working with perchloric acid should be thoroughly trained in its properties, hazards, and proper handling techniques. This includes understanding the importance of avoiding contact with organic materials, which can lead to the formation of shock-sensitive compounds.
Emergency response procedures must be established and clearly communicated. This includes the availability of appropriate fire extinguishing agents, eyewash stations, and safety showers. Personnel should be trained in spill containment and cleanup procedures specific to perchloric acid.
Regular maintenance and cleaning of equipment and work areas are essential to prevent the buildup of perchlorate residues. This is particularly important for surfaces that may come into contact with heated perchloric acid, as these residues can pose a significant explosion hazard.
Waste management is another critical safety consideration. Perchloric acid waste must be properly neutralized and disposed of according to local regulations. It should never be mixed with organic solvents or other incompatible materials.
By adhering to these safety considerations and implementing robust safety protocols, the risks associated with perchloric acid use in transition metal oxide formation can be significantly mitigated, ensuring a safer working environment for researchers and laboratory personnel.
One of the most critical safety aspects is the proper storage of perchloric acid. It must be kept in a cool, well-ventilated area, away from combustible materials and other chemicals. The storage containers should be made of compatible materials, such as glass or certain plastics, and should be regularly inspected for signs of degradation or leakage. Additionally, secondary containment measures should be implemented to prevent spills from spreading.
Personal protective equipment (PPE) is essential when handling perchloric acid. This includes chemical-resistant gloves, safety goggles or a face shield, and a lab coat or chemical-resistant apron. In some cases, respiratory protection may also be necessary, especially when working with heated perchloric acid or in poorly ventilated areas.
Proper ventilation is crucial when working with perchloric acid, particularly during heating or evaporation processes. Specialized fume hoods designed for perchloric acid use, equipped with wash-down systems, are recommended to prevent the accumulation of potentially explosive perchlorate salts in the ventilation system.
Training and education of personnel are vital components of safety protocols. All individuals working with perchloric acid should be thoroughly trained in its properties, hazards, and proper handling techniques. This includes understanding the importance of avoiding contact with organic materials, which can lead to the formation of shock-sensitive compounds.
Emergency response procedures must be established and clearly communicated. This includes the availability of appropriate fire extinguishing agents, eyewash stations, and safety showers. Personnel should be trained in spill containment and cleanup procedures specific to perchloric acid.
Regular maintenance and cleaning of equipment and work areas are essential to prevent the buildup of perchlorate residues. This is particularly important for surfaces that may come into contact with heated perchloric acid, as these residues can pose a significant explosion hazard.
Waste management is another critical safety consideration. Perchloric acid waste must be properly neutralized and disposed of according to local regulations. It should never be mixed with organic solvents or other incompatible materials.
By adhering to these safety considerations and implementing robust safety protocols, the risks associated with perchloric acid use in transition metal oxide formation can be significantly mitigated, ensuring a safer working environment for researchers and laboratory personnel.
Environmental Impact of Perchloric Acid in TMO Synthesis
The use of perchloric acid in the synthesis of transition metal oxides (TMOs) raises significant environmental concerns that warrant careful consideration. Perchloric acid, while effective as a medium for TMO formation, poses potential risks to ecosystems and human health if not properly managed.
One of the primary environmental impacts of perchloric acid in TMO synthesis is its potential for contamination of water sources. Perchlorate ions, derived from perchloric acid, are highly soluble and mobile in aqueous environments. This characteristic allows them to persist in surface and groundwater, potentially affecting drinking water supplies. The presence of perchlorates in water can have adverse effects on aquatic ecosystems, disrupting the endocrine systems of various organisms.
Air pollution is another environmental concern associated with the use of perchloric acid in TMO synthesis. During the synthesis process, volatile perchloric acid vapors may be released into the atmosphere. These vapors can contribute to the formation of secondary air pollutants and potentially impact air quality in the surrounding areas. Proper ventilation and emission control systems are crucial to mitigate these risks.
The disposal of perchloric acid waste from TMO synthesis processes presents additional environmental challenges. Improper disposal can lead to soil contamination, affecting both terrestrial ecosystems and agricultural productivity. The persistence of perchlorates in soil can result in long-term environmental impacts, necessitating costly remediation efforts.
Furthermore, the production and transportation of perchloric acid for TMO synthesis contribute to the overall environmental footprint of the process. The energy-intensive nature of perchloric acid production and the associated greenhouse gas emissions must be considered when evaluating the environmental impact of this synthesis method.
To address these environmental concerns, researchers and industry practitioners are exploring alternative synthesis methods and greener solvents for TMO formation. Efforts are being made to develop more environmentally friendly processes that maintain the efficiency of perchloric acid-based synthesis while reducing its ecological impact. These include the use of ionic liquids, hydrothermal synthesis techniques, and sol-gel methods.
Regulatory bodies worldwide are increasingly focusing on the environmental implications of perchloric acid use in industrial processes, including TMO synthesis. Stricter regulations and guidelines are being implemented to ensure proper handling, storage, and disposal of perchloric acid and its waste products. Compliance with these regulations is essential for minimizing the environmental impact of TMO synthesis using perchloric acid.
One of the primary environmental impacts of perchloric acid in TMO synthesis is its potential for contamination of water sources. Perchlorate ions, derived from perchloric acid, are highly soluble and mobile in aqueous environments. This characteristic allows them to persist in surface and groundwater, potentially affecting drinking water supplies. The presence of perchlorates in water can have adverse effects on aquatic ecosystems, disrupting the endocrine systems of various organisms.
Air pollution is another environmental concern associated with the use of perchloric acid in TMO synthesis. During the synthesis process, volatile perchloric acid vapors may be released into the atmosphere. These vapors can contribute to the formation of secondary air pollutants and potentially impact air quality in the surrounding areas. Proper ventilation and emission control systems are crucial to mitigate these risks.
The disposal of perchloric acid waste from TMO synthesis processes presents additional environmental challenges. Improper disposal can lead to soil contamination, affecting both terrestrial ecosystems and agricultural productivity. The persistence of perchlorates in soil can result in long-term environmental impacts, necessitating costly remediation efforts.
Furthermore, the production and transportation of perchloric acid for TMO synthesis contribute to the overall environmental footprint of the process. The energy-intensive nature of perchloric acid production and the associated greenhouse gas emissions must be considered when evaluating the environmental impact of this synthesis method.
To address these environmental concerns, researchers and industry practitioners are exploring alternative synthesis methods and greener solvents for TMO formation. Efforts are being made to develop more environmentally friendly processes that maintain the efficiency of perchloric acid-based synthesis while reducing its ecological impact. These include the use of ionic liquids, hydrothermal synthesis techniques, and sol-gel methods.
Regulatory bodies worldwide are increasingly focusing on the environmental implications of perchloric acid use in industrial processes, including TMO synthesis. Stricter regulations and guidelines are being implemented to ensure proper handling, storage, and disposal of perchloric acid and its waste products. Compliance with these regulations is essential for minimizing the environmental impact of TMO synthesis using perchloric acid.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!