Perchloric Acid's Role in the Precipitation of Metals from Solutions
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Background and Objectives
Perchloric acid, a powerful oxidizing agent and strong acid, has played a significant role in various industrial and analytical processes, particularly in the precipitation of metals from solutions. The development of this technology can be traced back to the early 20th century when perchloric acid was first synthesized and its unique properties were discovered.
The evolution of perchloric acid's use in metal precipitation has been driven by the increasing demand for efficient and selective metal recovery methods in industries such as mining, metallurgy, and waste treatment. Over the years, researchers and engineers have explored various applications of perchloric acid, leading to advancements in metal extraction techniques and the development of novel precipitation processes.
One of the key objectives in utilizing perchloric acid for metal precipitation is to achieve high selectivity and efficiency in separating specific metals from complex solutions. This is particularly important in the recovery of valuable metals from industrial waste streams or in the purification of metal compounds for high-tech applications.
Another crucial goal is to optimize the precipitation process to minimize environmental impact and reduce operational costs. As environmental regulations become more stringent, there is a growing need for sustainable metal recovery methods that can effectively precipitate metals while minimizing the generation of hazardous waste.
The technology surrounding perchloric acid's role in metal precipitation continues to evolve, with ongoing research focused on improving reaction kinetics, enhancing selectivity, and developing novel precipitation agents. Recent trends include the exploration of green chemistry principles to make the process more environmentally friendly and the integration of advanced separation technologies to further refine the precipitated metals.
As we look towards the future, the objectives for perchloric acid in metal precipitation are likely to include the development of more efficient and sustainable processes, the expansion of its application to a wider range of metals and alloys, and the integration of this technology with other emerging fields such as nanotechnology and materials science.
Understanding the background and objectives of perchloric acid's role in metal precipitation is crucial for identifying potential areas of innovation and guiding future research efforts. This knowledge will be instrumental in addressing the growing global demand for metals while meeting the challenges of resource scarcity and environmental sustainability.
The evolution of perchloric acid's use in metal precipitation has been driven by the increasing demand for efficient and selective metal recovery methods in industries such as mining, metallurgy, and waste treatment. Over the years, researchers and engineers have explored various applications of perchloric acid, leading to advancements in metal extraction techniques and the development of novel precipitation processes.
One of the key objectives in utilizing perchloric acid for metal precipitation is to achieve high selectivity and efficiency in separating specific metals from complex solutions. This is particularly important in the recovery of valuable metals from industrial waste streams or in the purification of metal compounds for high-tech applications.
Another crucial goal is to optimize the precipitation process to minimize environmental impact and reduce operational costs. As environmental regulations become more stringent, there is a growing need for sustainable metal recovery methods that can effectively precipitate metals while minimizing the generation of hazardous waste.
The technology surrounding perchloric acid's role in metal precipitation continues to evolve, with ongoing research focused on improving reaction kinetics, enhancing selectivity, and developing novel precipitation agents. Recent trends include the exploration of green chemistry principles to make the process more environmentally friendly and the integration of advanced separation technologies to further refine the precipitated metals.
As we look towards the future, the objectives for perchloric acid in metal precipitation are likely to include the development of more efficient and sustainable processes, the expansion of its application to a wider range of metals and alloys, and the integration of this technology with other emerging fields such as nanotechnology and materials science.
Understanding the background and objectives of perchloric acid's role in metal precipitation is crucial for identifying potential areas of innovation and guiding future research efforts. This knowledge will be instrumental in addressing the growing global demand for metals while meeting the challenges of resource scarcity and environmental sustainability.
Market Analysis for Metal Precipitation Applications
The market for metal precipitation applications is experiencing significant growth, driven by increasing industrial activities and environmental regulations. Perchloric acid plays a crucial role in this market, particularly in the extraction and purification of metals from solutions. The global metal precipitation market is expected to expand due to rising demand in various sectors, including mining, wastewater treatment, and electronics manufacturing.
In the mining industry, metal precipitation is essential for recovering valuable metals from ore leachates and process solutions. Perchloric acid's ability to selectively precipitate certain metals makes it a valuable tool in this sector. The growing demand for precious and rare earth metals, driven by technological advancements and the shift towards renewable energy, is fueling market growth in this area.
The wastewater treatment sector represents another significant market for metal precipitation applications. As environmental regulations become more stringent worldwide, industries are increasingly required to remove heavy metals from their effluents before discharge. Perchloric acid-based precipitation methods offer an effective solution for treating industrial wastewater, contributing to market expansion in this segment.
Electronics manufacturing is another key market driver for metal precipitation applications. The production of high-purity metals and compounds is critical for the semiconductor industry, where perchloric acid is used in etching and cleaning processes. The ongoing miniaturization of electronic components and the growth of the Internet of Things (IoT) are expected to sustain demand in this sector.
Geographically, Asia-Pacific is anticipated to be the fastest-growing market for metal precipitation applications, driven by rapid industrialization in countries like China and India. North America and Europe are also significant markets, primarily due to stringent environmental regulations and the presence of established mining and electronics industries.
The market is characterized by a mix of large multinational corporations and specialized chemical companies. Key players are focusing on developing more efficient and environmentally friendly precipitation methods, including those utilizing perchloric acid. Innovation in this space is likely to center around improving selectivity, reducing chemical consumption, and minimizing waste generation.
Challenges in the market include the handling and disposal of perchloric acid, which requires specialized safety measures due to its strong oxidizing properties. This factor may limit adoption in some applications and drive research into alternative precipitation methods. However, the unique properties of perchloric acid in metal precipitation continue to make it a valuable tool in many industrial processes, ensuring its ongoing relevance in the market.
In the mining industry, metal precipitation is essential for recovering valuable metals from ore leachates and process solutions. Perchloric acid's ability to selectively precipitate certain metals makes it a valuable tool in this sector. The growing demand for precious and rare earth metals, driven by technological advancements and the shift towards renewable energy, is fueling market growth in this area.
The wastewater treatment sector represents another significant market for metal precipitation applications. As environmental regulations become more stringent worldwide, industries are increasingly required to remove heavy metals from their effluents before discharge. Perchloric acid-based precipitation methods offer an effective solution for treating industrial wastewater, contributing to market expansion in this segment.
Electronics manufacturing is another key market driver for metal precipitation applications. The production of high-purity metals and compounds is critical for the semiconductor industry, where perchloric acid is used in etching and cleaning processes. The ongoing miniaturization of electronic components and the growth of the Internet of Things (IoT) are expected to sustain demand in this sector.
Geographically, Asia-Pacific is anticipated to be the fastest-growing market for metal precipitation applications, driven by rapid industrialization in countries like China and India. North America and Europe are also significant markets, primarily due to stringent environmental regulations and the presence of established mining and electronics industries.
The market is characterized by a mix of large multinational corporations and specialized chemical companies. Key players are focusing on developing more efficient and environmentally friendly precipitation methods, including those utilizing perchloric acid. Innovation in this space is likely to center around improving selectivity, reducing chemical consumption, and minimizing waste generation.
Challenges in the market include the handling and disposal of perchloric acid, which requires specialized safety measures due to its strong oxidizing properties. This factor may limit adoption in some applications and drive research into alternative precipitation methods. However, the unique properties of perchloric acid in metal precipitation continue to make it a valuable tool in many industrial processes, ensuring its ongoing relevance in the market.
Current Challenges in Metal Precipitation Techniques
Metal precipitation techniques play a crucial role in various industrial processes, including wastewater treatment, metal recovery, and purification. However, several challenges persist in current metal precipitation methods, hindering their efficiency and widespread application.
One of the primary challenges is the selectivity of precipitation. Many conventional techniques struggle to selectively precipitate specific metals from complex solutions containing multiple metal ions. This lack of selectivity often results in co-precipitation of unwanted metals, reducing the purity of the final product and necessitating additional purification steps.
The formation of stable and easily filterable precipitates is another significant challenge. Some precipitation reactions produce fine, colloidal particles that are difficult to separate from the solution. This issue not only affects the recovery efficiency but also increases the operational costs associated with filtration and solid-liquid separation processes.
pH control and buffering present ongoing difficulties in metal precipitation. Many precipitation reactions are highly pH-dependent, and maintaining the optimal pH range throughout the process can be challenging, especially when dealing with large volumes or continuous operations. Fluctuations in pH can lead to incomplete precipitation or re-dissolution of precipitated metals.
The presence of complexing agents in industrial effluents poses a substantial challenge to metal precipitation. These agents, such as EDTA or ammonia, form stable complexes with metal ions, making them resistant to conventional precipitation methods. Overcoming the strong metal-ligand bonds requires specialized techniques or pre-treatment steps, adding complexity to the process.
Environmental concerns and regulatory compliance add another layer of complexity to metal precipitation techniques. Stringent discharge limits for metals in effluents necessitate highly efficient precipitation methods. Additionally, the management and disposal of metal-containing sludge generated during precipitation processes present environmental and economic challenges.
The energy intensity and chemical consumption of some precipitation techniques are areas of concern. High-temperature processes or those requiring large quantities of precipitating agents can be cost-prohibitive and environmentally unsustainable. Developing more energy-efficient and chemically economical precipitation methods remains an ongoing challenge in the field.
Scaling up laboratory-proven precipitation techniques to industrial scales presents its own set of challenges. Factors such as mixing efficiency, reaction kinetics, and heat transfer can behave differently at larger scales, affecting the overall precipitation performance and product quality.
One of the primary challenges is the selectivity of precipitation. Many conventional techniques struggle to selectively precipitate specific metals from complex solutions containing multiple metal ions. This lack of selectivity often results in co-precipitation of unwanted metals, reducing the purity of the final product and necessitating additional purification steps.
The formation of stable and easily filterable precipitates is another significant challenge. Some precipitation reactions produce fine, colloidal particles that are difficult to separate from the solution. This issue not only affects the recovery efficiency but also increases the operational costs associated with filtration and solid-liquid separation processes.
pH control and buffering present ongoing difficulties in metal precipitation. Many precipitation reactions are highly pH-dependent, and maintaining the optimal pH range throughout the process can be challenging, especially when dealing with large volumes or continuous operations. Fluctuations in pH can lead to incomplete precipitation or re-dissolution of precipitated metals.
The presence of complexing agents in industrial effluents poses a substantial challenge to metal precipitation. These agents, such as EDTA or ammonia, form stable complexes with metal ions, making them resistant to conventional precipitation methods. Overcoming the strong metal-ligand bonds requires specialized techniques or pre-treatment steps, adding complexity to the process.
Environmental concerns and regulatory compliance add another layer of complexity to metal precipitation techniques. Stringent discharge limits for metals in effluents necessitate highly efficient precipitation methods. Additionally, the management and disposal of metal-containing sludge generated during precipitation processes present environmental and economic challenges.
The energy intensity and chemical consumption of some precipitation techniques are areas of concern. High-temperature processes or those requiring large quantities of precipitating agents can be cost-prohibitive and environmentally unsustainable. Developing more energy-efficient and chemically economical precipitation methods remains an ongoing challenge in the field.
Scaling up laboratory-proven precipitation techniques to industrial scales presents its own set of challenges. Factors such as mixing efficiency, reaction kinetics, and heat transfer can behave differently at larger scales, affecting the overall precipitation performance and product quality.
Existing Perchloric Acid-based Precipitation Methods
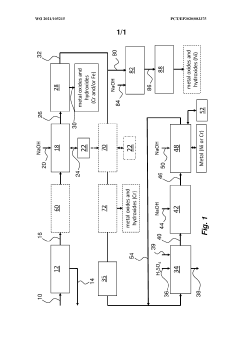
01 Perchloric acid precipitation methods
Various methods for precipitating substances using perchloric acid are described. These methods can be applied in different fields such as chemistry, materials science, and environmental engineering. The precipitation process typically involves the addition of perchloric acid to a solution containing the target substance, resulting in the formation of insoluble compounds.- Perchloric acid precipitation methods: Various methods for precipitating substances using perchloric acid are described. These methods can be applied in different fields such as chemistry, materials science, and environmental engineering. The precipitation process typically involves the addition of perchloric acid to a solution containing the target substance, resulting in the formation of insoluble compounds.
- Purification and separation techniques: Perchloric acid precipitation is utilized in purification and separation processes for various compounds and materials. This technique can be particularly effective for isolating specific elements or molecules from complex mixtures. The precipitated products can be further processed or analyzed for different applications.
- Equipment and apparatus for perchloric acid precipitation: Specialized equipment and apparatus have been developed for conducting perchloric acid precipitation reactions safely and efficiently. These may include reaction vessels, mixing systems, temperature control units, and safety features to handle the corrosive nature of perchloric acid.
- Applications in material synthesis: Perchloric acid precipitation is employed in the synthesis of various materials, including nanoparticles, catalysts, and functional compounds. This method can offer advantages such as control over particle size, morphology, and composition, leading to materials with specific properties for different applications.
- Safety measures and handling procedures: Due to the highly reactive and potentially explosive nature of perchloric acid, specific safety measures and handling procedures are crucial when performing precipitation reactions. These may include the use of specialized protective equipment, proper storage conditions, and protocols for waste disposal to minimize risks associated with perchloric acid use.
02 Purification and separation techniques
Perchloric acid precipitation is utilized in purification and separation processes for various compounds and materials. This technique can be employed to isolate specific substances from complex mixtures or to remove impurities from solutions. The selective precipitation properties of perchloric acid make it a valuable tool in analytical chemistry and industrial applications.Expand Specific Solutions03 Safety measures and handling procedures
Due to the highly reactive nature of perchloric acid, special safety measures and handling procedures are necessary when using it for precipitation processes. This includes the use of appropriate personal protective equipment, proper storage conditions, and specialized laboratory equipment designed to handle perchloric acid safely. Proper disposal methods for perchloric acid waste are also crucial.Expand Specific Solutions04 Applications in material synthesis
Perchloric acid precipitation is employed in the synthesis of various materials, including nanoparticles, catalysts, and advanced functional materials. The technique allows for precise control over particle size, morphology, and composition, making it valuable in fields such as nanotechnology, electronics, and energy storage.Expand Specific Solutions05 Analysis and characterization techniques
Various analytical and characterization techniques are used in conjunction with perchloric acid precipitation to study the properties of the precipitated materials. These methods may include spectroscopy, microscopy, and diffraction techniques, which provide valuable information about the structure, composition, and properties of the precipitated substances.Expand Specific Solutions
Key Players in Perchloric Acid and Metal Processing
The competitive landscape for perchloric acid's role in metal precipitation from solutions is characterized by a mature market with established players and ongoing research. The industry is in a stable growth phase, with a moderate market size driven by applications in analytical chemistry, electrochemistry, and materials science. Technological maturity is high, with companies like Halliburton Energy Services, China Petroleum & Chemical Corp., and BP Corporation North America leading in industrial applications. Academic institutions such as Central South University and Tongji University contribute to advancing the technology through research. Specialized chemical companies like Haldor Topsøe and Chemetics focus on process optimization and equipment development, while larger conglomerates like FUJIFILM and Mitsubishi Heavy Industries incorporate the technology into broader product lines.
Halliburton Energy Services, Inc.
Technical Solution: Halliburton has developed a novel approach for metal precipitation using perchloric acid in oil and gas well stimulation. Their method involves injecting a perchloric acid solution into the formation, which reacts with metal ions to form insoluble perchlorates. This process effectively removes problematic metals such as iron, calcium, and barium from the solution[1]. The company has optimized the concentration and injection parameters to achieve maximum precipitation efficiency while minimizing formation damage[2]. Additionally, Halliburton has implemented a proprietary post-treatment process to neutralize excess perchloric acid and safely dispose of the precipitated metals[3].
Strengths: Highly effective for removing specific metal ions in oil and gas applications. Customizable for different formation types. Weaknesses: Potential safety concerns due to perchloric acid's reactivity. May require specialized handling and disposal procedures.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative perchloric acid-based metal precipitation technique for use in refinery wastewater treatment. Their approach utilizes a controlled-release perchloric acid formulation that gradually releases the acid into the wastewater stream, allowing for more efficient metal precipitation over time[4]. Sinopec's method incorporates a multi-stage reaction process, where different metal ions are selectively precipitated at varying pH levels achieved through precise perchloric acid dosing[5]. The company has also integrated an advanced filtration system to separate the precipitated metals, resulting in high-purity recovered metals and cleaner effluent water[6].
Strengths: Efficient metal removal from complex wastewater streams. Potential for valuable metal recovery. Weaknesses: May require significant capital investment for implementation. Ongoing operational costs for perchloric acid and filtration media.
Innovative Approaches in Perchloric Acid Metal Extraction
Methods and compositions for reducing precipitation from acid solutions
PatentInactiveCA2108702C
Innovation
- Incorporating at least one oxime, such as acetaldoxime, into the acid solution to preferentially react with sulfide ions, preventing their reaction with metal ions and thus reducing the precipitation of metal sulfides like ferrous sulfide.
Metal recovery method
PatentWO2021105215A1
Innovation
- A method that adjusts the pH of acid solutions to precipitate iron and chrome, followed by dewatering and recovery, with nickel recovered through ion exchange and electrowinning, using precipitation agents like sodium hydroxide that do not contaminate metals, and incorporating acid retardation to remove excess acid and fluorides, allowing for direct return of metals to metal production plants.
Safety Considerations in Perchloric Acid Handling
Handling perchloric acid requires strict adherence to safety protocols due to its highly reactive and potentially explosive nature. When working with perchloric acid in metal precipitation processes, several critical safety considerations must be addressed to minimize risks and ensure a secure laboratory environment.
Personal protective equipment (PPE) is essential when handling perchloric acid. This includes chemical-resistant gloves, a lab coat, and safety goggles or a face shield. A fume hood specifically designed for perchloric acid use is crucial to prevent the accumulation of potentially explosive perchlorate salts. These specialized fume hoods are equipped with a wash-down system to remove any residual acid or salts.
Storage of perchloric acid demands particular attention. It should be kept in a cool, well-ventilated area, away from combustible materials and other chemicals. Glass or PTFE containers are recommended for storage, as perchloric acid can react with many metals. Regular inspections of storage areas are necessary to detect any signs of leakage or degradation.
Proper waste disposal is critical when working with perchloric acid. Neutralization and dilution procedures must be followed carefully before disposal. Organic materials should never be mixed with perchloric acid waste, as this can lead to the formation of explosive compounds.
Training and education are paramount for all personnel working with perchloric acid. This includes understanding the specific hazards associated with the acid, proper handling techniques, and emergency response procedures. Regular safety drills and refresher courses should be conducted to maintain a high level of awareness and preparedness.
Emergency response planning is essential when working with perchloric acid. This includes having readily available spill kits, eyewash stations, and safety showers. A detailed emergency response plan should be in place, outlining steps to be taken in case of spills, fires, or explosions.
When using perchloric acid for metal precipitation, additional precautions are necessary. The reaction vessel should be properly sized to prevent overflow, and temperature control is crucial to avoid overheating. Slow addition of reagents and constant monitoring of the reaction are important to prevent sudden, potentially dangerous reactions.
Regular maintenance and inspection of equipment used with perchloric acid are vital. This includes checking for signs of corrosion, ensuring proper functioning of safety features, and replacing any compromised components promptly. Dedicated equipment for perchloric acid use should be clearly labeled and not used for other purposes to prevent cross-contamination.
Personal protective equipment (PPE) is essential when handling perchloric acid. This includes chemical-resistant gloves, a lab coat, and safety goggles or a face shield. A fume hood specifically designed for perchloric acid use is crucial to prevent the accumulation of potentially explosive perchlorate salts. These specialized fume hoods are equipped with a wash-down system to remove any residual acid or salts.
Storage of perchloric acid demands particular attention. It should be kept in a cool, well-ventilated area, away from combustible materials and other chemicals. Glass or PTFE containers are recommended for storage, as perchloric acid can react with many metals. Regular inspections of storage areas are necessary to detect any signs of leakage or degradation.
Proper waste disposal is critical when working with perchloric acid. Neutralization and dilution procedures must be followed carefully before disposal. Organic materials should never be mixed with perchloric acid waste, as this can lead to the formation of explosive compounds.
Training and education are paramount for all personnel working with perchloric acid. This includes understanding the specific hazards associated with the acid, proper handling techniques, and emergency response procedures. Regular safety drills and refresher courses should be conducted to maintain a high level of awareness and preparedness.
Emergency response planning is essential when working with perchloric acid. This includes having readily available spill kits, eyewash stations, and safety showers. A detailed emergency response plan should be in place, outlining steps to be taken in case of spills, fires, or explosions.
When using perchloric acid for metal precipitation, additional precautions are necessary. The reaction vessel should be properly sized to prevent overflow, and temperature control is crucial to avoid overheating. Slow addition of reagents and constant monitoring of the reaction are important to prevent sudden, potentially dangerous reactions.
Regular maintenance and inspection of equipment used with perchloric acid are vital. This includes checking for signs of corrosion, ensuring proper functioning of safety features, and replacing any compromised components promptly. Dedicated equipment for perchloric acid use should be clearly labeled and not used for other purposes to prevent cross-contamination.
Environmental Impact of Perchloric Acid Use
The use of perchloric acid in metal precipitation processes has significant environmental implications that warrant careful consideration. The primary concern stems from the potential release of perchlorate ions into the environment, which can persist for extended periods and pose risks to ecosystems and human health.
Perchlorate contamination in soil and water bodies can lead to adverse effects on plant growth and development. Studies have shown that perchlorate can interfere with iodine uptake in plants, potentially affecting their metabolism and overall health. This impact on vegetation can cascade through the food chain, affecting herbivores and higher trophic levels.
Aquatic ecosystems are particularly vulnerable to perchlorate contamination. Fish and amphibians exposed to elevated levels of perchlorate may experience disruptions in thyroid function, which can impair growth, development, and reproductive capabilities. This can lead to population declines and imbalances in aquatic communities.
Human health is another critical concern associated with perchlorate contamination. Perchlorate can interfere with iodine uptake in the thyroid gland, potentially leading to thyroid disorders and related health issues. Pregnant women and developing fetuses are especially susceptible to these effects, as thyroid hormones play a crucial role in fetal development.
The persistence of perchlorate in the environment exacerbates these concerns. Unlike some other contaminants, perchlorate does not readily degrade under normal environmental conditions, leading to long-term contamination of affected areas. This persistence necessitates extensive and costly remediation efforts when contamination occurs.
Proper handling and disposal of perchloric acid and its byproducts are essential to mitigate these environmental risks. Industrial facilities using perchloric acid must implement stringent waste management protocols to prevent releases into the environment. This includes appropriate treatment of wastewater and proper disposal of solid waste containing perchlorate.
Regulatory bodies have recognized the environmental risks associated with perchlorate and have implemented guidelines and standards for its use and disposal. In some regions, strict limits have been placed on perchlorate levels in drinking water and soil, driving the development of more efficient treatment technologies and alternative processes that reduce or eliminate perchloric acid use.
Research into alternative precipitation methods and less environmentally harmful acids is ongoing, aiming to reduce reliance on perchloric acid in metal processing. These efforts focus on developing processes that maintain efficiency in metal precipitation while minimizing environmental impact, aligning with broader sustainability goals in the chemical and metallurgical industries.
Perchlorate contamination in soil and water bodies can lead to adverse effects on plant growth and development. Studies have shown that perchlorate can interfere with iodine uptake in plants, potentially affecting their metabolism and overall health. This impact on vegetation can cascade through the food chain, affecting herbivores and higher trophic levels.
Aquatic ecosystems are particularly vulnerable to perchlorate contamination. Fish and amphibians exposed to elevated levels of perchlorate may experience disruptions in thyroid function, which can impair growth, development, and reproductive capabilities. This can lead to population declines and imbalances in aquatic communities.
Human health is another critical concern associated with perchlorate contamination. Perchlorate can interfere with iodine uptake in the thyroid gland, potentially leading to thyroid disorders and related health issues. Pregnant women and developing fetuses are especially susceptible to these effects, as thyroid hormones play a crucial role in fetal development.
The persistence of perchlorate in the environment exacerbates these concerns. Unlike some other contaminants, perchlorate does not readily degrade under normal environmental conditions, leading to long-term contamination of affected areas. This persistence necessitates extensive and costly remediation efforts when contamination occurs.
Proper handling and disposal of perchloric acid and its byproducts are essential to mitigate these environmental risks. Industrial facilities using perchloric acid must implement stringent waste management protocols to prevent releases into the environment. This includes appropriate treatment of wastewater and proper disposal of solid waste containing perchlorate.
Regulatory bodies have recognized the environmental risks associated with perchlorate and have implemented guidelines and standards for its use and disposal. In some regions, strict limits have been placed on perchlorate levels in drinking water and soil, driving the development of more efficient treatment technologies and alternative processes that reduce or eliminate perchloric acid use.
Research into alternative precipitation methods and less environmentally harmful acids is ongoing, aiming to reduce reliance on perchloric acid in metal processing. These efforts focus on developing processes that maintain efficiency in metal precipitation while minimizing environmental impact, aligning with broader sustainability goals in the chemical and metallurgical industries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!