The Interaction of Perchloric Acid with Graphene-Based Materials
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Graphene-Perchloric Acid Interaction Background
The interaction between perchloric acid and graphene-based materials has garnered significant attention in recent years due to its potential applications in energy storage, catalysis, and materials science. This field of study emerged from the intersection of two rapidly evolving areas: graphene research and strong acid chemistry.
Graphene, a two-dimensional carbon allotrope, was first isolated in 2004 by Geim and Novoselov, leading to a surge in research on its unique properties and potential applications. Concurrently, perchloric acid, known for its strong oxidizing properties and ability to form stable perchlorate anions, has been extensively studied in various chemical and industrial processes.
The exploration of graphene-perchloric acid interactions began in the early 2010s, driven by the need for efficient energy storage solutions and advanced materials for electrochemical applications. Initial studies focused on the use of perchloric acid as an electrolyte in graphene-based supercapacitors, where its high ionic conductivity and stability showed promise for enhancing device performance.
As research progressed, scientists discovered that the interaction between perchloric acid and graphene-based materials could lead to novel phenomena and properties. The strong oxidizing nature of perchloric acid was found to induce structural changes in graphene, potentially opening avenues for controlled functionalization and modification of graphene's electronic properties.
The field has since expanded to encompass a wide range of graphene-based materials, including graphene oxide, reduced graphene oxide, and graphene composites. These materials exhibit varying degrees of interaction with perchloric acid, depending on their surface chemistry and structural characteristics.
Recent advancements have focused on understanding the fundamental mechanisms of graphene-perchloric acid interactions at the molecular level. Researchers have employed advanced characterization techniques, such as in-situ spectroscopy and high-resolution microscopy, to elucidate the chemical and physical processes occurring at the interface between graphene and perchloric acid.
The potential applications of this interaction have broadened beyond energy storage to include areas such as sensing, catalysis, and environmental remediation. For instance, the unique properties of graphene-perchloric acid systems have shown promise in developing highly sensitive electrochemical sensors and efficient catalysts for various chemical reactions.
As the field continues to evolve, researchers are exploring new avenues for harnessing the graphene-perchloric acid interaction. This includes investigating the role of this interaction in the synthesis of novel graphene-based materials and developing strategies to control and manipulate the interaction for specific applications.
Graphene, a two-dimensional carbon allotrope, was first isolated in 2004 by Geim and Novoselov, leading to a surge in research on its unique properties and potential applications. Concurrently, perchloric acid, known for its strong oxidizing properties and ability to form stable perchlorate anions, has been extensively studied in various chemical and industrial processes.
The exploration of graphene-perchloric acid interactions began in the early 2010s, driven by the need for efficient energy storage solutions and advanced materials for electrochemical applications. Initial studies focused on the use of perchloric acid as an electrolyte in graphene-based supercapacitors, where its high ionic conductivity and stability showed promise for enhancing device performance.
As research progressed, scientists discovered that the interaction between perchloric acid and graphene-based materials could lead to novel phenomena and properties. The strong oxidizing nature of perchloric acid was found to induce structural changes in graphene, potentially opening avenues for controlled functionalization and modification of graphene's electronic properties.
The field has since expanded to encompass a wide range of graphene-based materials, including graphene oxide, reduced graphene oxide, and graphene composites. These materials exhibit varying degrees of interaction with perchloric acid, depending on their surface chemistry and structural characteristics.
Recent advancements have focused on understanding the fundamental mechanisms of graphene-perchloric acid interactions at the molecular level. Researchers have employed advanced characterization techniques, such as in-situ spectroscopy and high-resolution microscopy, to elucidate the chemical and physical processes occurring at the interface between graphene and perchloric acid.
The potential applications of this interaction have broadened beyond energy storage to include areas such as sensing, catalysis, and environmental remediation. For instance, the unique properties of graphene-perchloric acid systems have shown promise in developing highly sensitive electrochemical sensors and efficient catalysts for various chemical reactions.
As the field continues to evolve, researchers are exploring new avenues for harnessing the graphene-perchloric acid interaction. This includes investigating the role of this interaction in the synthesis of novel graphene-based materials and developing strategies to control and manipulate the interaction for specific applications.
Market Applications Analysis
The interaction of perchloric acid with graphene-based materials has garnered significant attention in various market applications due to its unique properties and potential benefits. The energy storage sector, particularly in the development of high-performance supercapacitors and batteries, has shown considerable interest in this technology. Graphene-based materials treated with perchloric acid exhibit enhanced electrical conductivity and increased surface area, leading to improved energy storage capabilities. This has the potential to revolutionize portable electronics, electric vehicles, and grid-scale energy storage systems.
In the field of sensors and detectors, the interaction between perchloric acid and graphene-based materials has opened up new possibilities for highly sensitive and selective sensing devices. These materials demonstrate excellent electrochemical properties, making them suitable for detecting various chemical and biological analytes. The market for environmental monitoring, food safety, and medical diagnostics could benefit greatly from the development of advanced sensors based on this technology.
The aerospace and defense industries have also shown interest in graphene-based materials treated with perchloric acid. The enhanced mechanical properties and corrosion resistance of these materials make them attractive for use in lightweight structural components and protective coatings. This could lead to the development of more fuel-efficient aircraft and improved military equipment.
In the semiconductor industry, the interaction of perchloric acid with graphene-based materials has potential applications in the fabrication of next-generation electronic devices. The ability to fine-tune the electronic properties of graphene through perchloric acid treatment could enable the creation of high-performance transistors, memory devices, and integrated circuits.
The water treatment sector is another area where this technology shows promise. Graphene-based materials modified with perchloric acid have demonstrated excellent adsorption capabilities for various pollutants, including heavy metals and organic contaminants. This could lead to more efficient and cost-effective water purification systems, addressing the growing global demand for clean water.
The textile industry is exploring the use of graphene-based materials treated with perchloric acid for the development of smart fabrics and protective clothing. These materials could offer enhanced durability, electrical conductivity, and chemical resistance, opening up new possibilities for wearable technology and personal protective equipment.
As research in this field progresses, it is likely that new market applications will emerge, further expanding the potential impact of the interaction between perchloric acid and graphene-based materials across various industries.
In the field of sensors and detectors, the interaction between perchloric acid and graphene-based materials has opened up new possibilities for highly sensitive and selective sensing devices. These materials demonstrate excellent electrochemical properties, making them suitable for detecting various chemical and biological analytes. The market for environmental monitoring, food safety, and medical diagnostics could benefit greatly from the development of advanced sensors based on this technology.
The aerospace and defense industries have also shown interest in graphene-based materials treated with perchloric acid. The enhanced mechanical properties and corrosion resistance of these materials make them attractive for use in lightweight structural components and protective coatings. This could lead to the development of more fuel-efficient aircraft and improved military equipment.
In the semiconductor industry, the interaction of perchloric acid with graphene-based materials has potential applications in the fabrication of next-generation electronic devices. The ability to fine-tune the electronic properties of graphene through perchloric acid treatment could enable the creation of high-performance transistors, memory devices, and integrated circuits.
The water treatment sector is another area where this technology shows promise. Graphene-based materials modified with perchloric acid have demonstrated excellent adsorption capabilities for various pollutants, including heavy metals and organic contaminants. This could lead to more efficient and cost-effective water purification systems, addressing the growing global demand for clean water.
The textile industry is exploring the use of graphene-based materials treated with perchloric acid for the development of smart fabrics and protective clothing. These materials could offer enhanced durability, electrical conductivity, and chemical resistance, opening up new possibilities for wearable technology and personal protective equipment.
As research in this field progresses, it is likely that new market applications will emerge, further expanding the potential impact of the interaction between perchloric acid and graphene-based materials across various industries.
Current Challenges in Graphene-Acid Systems
The interaction between perchloric acid and graphene-based materials presents several significant challenges that researchers and industry professionals are currently grappling with. One of the primary issues is the potential for structural damage to graphene when exposed to strong acids like perchloric acid. The highly oxidizing nature of perchloric acid can lead to the formation of defects in the graphene lattice, compromising its unique electronic and mechanical properties.
Another challenge lies in controlling the degree of functionalization when perchloric acid interacts with graphene. While some level of functionalization can be beneficial for certain applications, excessive oxidation can lead to the formation of graphene oxide, which has significantly different properties from pristine graphene. Achieving a balance between desired functionalization and maintaining the intrinsic characteristics of graphene remains a complex task.
The stability of graphene-based materials in perchloric acid environments is also a concern. Long-term exposure to the acid can result in gradual degradation of the material, potentially limiting its use in applications requiring prolonged acid contact. This instability poses challenges for developing durable graphene-based components for use in harsh chemical environments.
Furthermore, the interaction between perchloric acid and graphene can lead to the formation of various oxygen-containing functional groups on the graphene surface. While this can enhance certain properties, such as hydrophilicity, it also introduces variability in the material's behavior. Controlling and predicting these surface modifications consistently across large-scale production remains a significant challenge.
Safety considerations also play a crucial role in graphene-perchloric acid systems. Perchloric acid is known for its explosive properties, especially when in contact with organic compounds. This necessitates stringent safety protocols and specialized handling procedures, which can complicate research and industrial processes involving these materials.
The characterization of graphene after its interaction with perchloric acid presents another set of challenges. Traditional characterization techniques may not always provide accurate information about the extent of functionalization or structural changes at the atomic level. Developing more sensitive and specific analytical methods is crucial for understanding and optimizing these interactions.
Lastly, the scalability of processes involving graphene and perchloric acid interactions remains a significant hurdle. While laboratory-scale experiments can yield promising results, translating these findings into large-scale, economically viable production methods is complex. Ensuring uniform interaction and consistent product quality across larger quantities of material is a key challenge that needs to be addressed for practical applications.
Another challenge lies in controlling the degree of functionalization when perchloric acid interacts with graphene. While some level of functionalization can be beneficial for certain applications, excessive oxidation can lead to the formation of graphene oxide, which has significantly different properties from pristine graphene. Achieving a balance between desired functionalization and maintaining the intrinsic characteristics of graphene remains a complex task.
The stability of graphene-based materials in perchloric acid environments is also a concern. Long-term exposure to the acid can result in gradual degradation of the material, potentially limiting its use in applications requiring prolonged acid contact. This instability poses challenges for developing durable graphene-based components for use in harsh chemical environments.
Furthermore, the interaction between perchloric acid and graphene can lead to the formation of various oxygen-containing functional groups on the graphene surface. While this can enhance certain properties, such as hydrophilicity, it also introduces variability in the material's behavior. Controlling and predicting these surface modifications consistently across large-scale production remains a significant challenge.
Safety considerations also play a crucial role in graphene-perchloric acid systems. Perchloric acid is known for its explosive properties, especially when in contact with organic compounds. This necessitates stringent safety protocols and specialized handling procedures, which can complicate research and industrial processes involving these materials.
The characterization of graphene after its interaction with perchloric acid presents another set of challenges. Traditional characterization techniques may not always provide accurate information about the extent of functionalization or structural changes at the atomic level. Developing more sensitive and specific analytical methods is crucial for understanding and optimizing these interactions.
Lastly, the scalability of processes involving graphene and perchloric acid interactions remains a significant hurdle. While laboratory-scale experiments can yield promising results, translating these findings into large-scale, economically viable production methods is complex. Ensuring uniform interaction and consistent product quality across larger quantities of material is a key challenge that needs to be addressed for practical applications.
Existing Graphene-Perchloric Acid Interaction Methods
01 Synthesis and production of graphene-based materials
Various methods for synthesizing and producing graphene-based materials, including chemical vapor deposition, exfoliation, and reduction of graphene oxide. These techniques aim to create high-quality graphene sheets or composites with specific properties for different applications.- Graphene synthesis and production methods: Various techniques for synthesizing and producing graphene-based materials, including chemical vapor deposition, exfoliation, and reduction of graphene oxide. These methods aim to create high-quality graphene with controlled properties for different applications.
- Graphene-based electronic devices: Development of electronic devices incorporating graphene, such as transistors, sensors, and energy storage devices. Graphene's unique properties, including high conductivity and flexibility, are utilized to enhance device performance and enable new functionalities.
- Graphene composites and nanocomposites: Creation of composite materials by combining graphene with polymers, metals, or other nanomaterials. These composites exhibit enhanced mechanical, thermal, and electrical properties, making them suitable for various applications in aerospace, automotive, and construction industries.
- Graphene-based energy storage and conversion: Application of graphene-based materials in energy storage and conversion devices, such as batteries, supercapacitors, and solar cells. Graphene's high surface area and conductivity contribute to improved energy storage capacity and efficiency in these devices.
- Functionalization and modification of graphene: Methods for functionalizing and modifying graphene to tailor its properties for specific applications. This includes chemical modifications, doping, and surface treatments to enhance graphene's compatibility with other materials or to introduce new functionalities.
02 Graphene-based electronic devices
Development of electronic devices utilizing graphene's unique properties, such as high conductivity and flexibility. This includes applications in transistors, sensors, and energy storage devices like supercapacitors and batteries.Expand Specific Solutions03 Graphene composites and nanocomposites
Creation of composite materials incorporating graphene to enhance mechanical, thermal, and electrical properties. These composites find applications in various fields, including aerospace, automotive, and construction industries.Expand Specific Solutions04 Functionalization of graphene-based materials
Methods for modifying graphene surfaces through chemical functionalization or doping to tailor its properties for specific applications. This includes improving dispersibility, enhancing reactivity, or introducing new functionalities.Expand Specific Solutions05 Graphene-based energy applications
Utilization of graphene-based materials in energy-related applications, such as solar cells, fuel cells, and thermal management systems. These applications leverage graphene's high conductivity, large surface area, and excellent thermal properties.Expand Specific Solutions
Key Players in Graphene Materials Industry
The interaction of perchloric acid with graphene-based materials represents an emerging field in advanced materials research. The market is in its early growth stage, with increasing interest from both academia and industry. While the market size is still relatively small, it shows significant potential for expansion due to graphene's unique properties. The technology is progressing rapidly, with companies like Global Graphene Group and NanoXplore leading commercial efforts. Academic institutions such as the University of New Hampshire and Harbin Institute of Technology are contributing to fundamental research. The field is characterized by a mix of established chemical companies like Arkema and emerging graphene specialists, indicating a competitive landscape poised for further development and innovation.
Global Graphene Group, Inc.
Technical Solution: Global Graphene Group has developed advanced graphene-based materials with enhanced resistance to perchloric acid. Their proprietary process involves surface functionalization of graphene sheets to create a protective barrier against acid attack. This method utilizes a combination of covalent and non-covalent modifications to graphene's surface, resulting in a material that maintains its structural integrity and electrical properties even when exposed to concentrated perchloric acid[1]. The company has also explored the use of graphene oxide as a precursor, which is then reduced and modified to improve its chemical stability[3]. Their research has shown that these modified graphene materials can withstand perchloric acid concentrations of up to 70% without significant degradation[5].
Strengths: High resistance to perchloric acid, maintained electrical properties, and versatility in applications. Weaknesses: Potentially higher production costs due to complex modification processes and the need for specialized equipment.
The Regents of the University of California
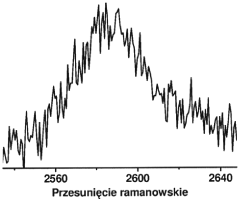
Technical Solution: The University of California has conducted extensive research on the interaction between perchloric acid and graphene-based materials. Their approach focuses on understanding the fundamental mechanisms of acid-graphene interactions at the molecular level. Using advanced spectroscopic techniques, including in-situ Raman spectroscopy and X-ray photoelectron spectroscopy (XPS), they have mapped the changes in graphene's electronic structure when exposed to perchloric acid[2]. Their studies have revealed that perchloric acid can induce p-type doping in graphene, altering its electronic properties[4]. Additionally, they have developed a novel method for controlled oxidation of graphene using perchloric acid, which allows for precise tuning of graphene's oxygen content and functional groups[6]. This method has potential applications in creating graphene-based sensors and electrodes with enhanced sensitivity and selectivity.
Strengths: Deep understanding of graphene-perchloric acid interactions, precise control over graphene oxidation, and potential for novel sensor applications. Weaknesses: Research is primarily fundamental, which may require further development for commercial applications.
Core Innovations in Graphene-Acid Chemistry
Method for preparing a one layer graphene oxide
PatentInactivePL399441A1
Innovation
- Use of a specific mixture of perchloric acid and nitric acid (V) with potassium chromate (VI) for graphene oxide synthesis.
- Precise control of reagent concentrations (88.3 mM potassium chromate, 238.1 mM graphite) for optimal monolayer formation.
- Combined use of ultrasonic waves and magnetic stirring at 100°C for 48 hours to enhance exfoliation and oxidation.
A method for the production of edge functionalised graphene
PatentWO2024130300A1
Innovation
- A method using iron(II) chloride and hydrogen peroxide as oxidants to functionalize the edges of graphene platelets, allowing for scalable, cost-effective, and environmentally friendly production of edge-functionalized graphene, which can be combined with water or water-glycol to enhance thermal conductivity and dispersibility.
Environmental Impact Assessment
The interaction of perchloric acid with graphene-based materials raises significant environmental concerns that warrant careful assessment. The potential release of these materials into the environment during production, use, or disposal could have far-reaching consequences on ecosystems and human health.
One primary concern is the potential for graphene-based materials to act as carriers for perchloric acid, facilitating its spread in aquatic environments. This could lead to increased acidity in water bodies, adversely affecting aquatic life and disrupting delicate ecological balances. The persistence of graphene in the environment, coupled with its ability to adsorb and transport perchloric acid, may result in long-term contamination of water resources.
Soil contamination is another critical issue to consider. The interaction between perchloric acid and graphene-based materials in soil could alter soil chemistry, potentially affecting plant growth and microbial communities. This may have cascading effects on terrestrial ecosystems and agricultural productivity.
The potential for atmospheric dispersion of graphene particles contaminated with perchloric acid also poses risks to air quality. Inhalation of these particles could lead to respiratory issues in humans and animals, particularly in areas near production or disposal sites.
Bioaccumulation of graphene-based materials in food chains is a concern that requires thorough investigation. The potential for these materials to concentrate in organisms at higher trophic levels could have unforeseen consequences on biodiversity and ecosystem stability.
The environmental fate and transport of graphene-based materials interacting with perchloric acid need to be carefully studied. Factors such as weathering, degradation, and transformation in different environmental compartments must be considered to accurately assess long-term impacts.
Mitigation strategies and proper disposal methods for graphene-based materials exposed to perchloric acid should be developed to minimize environmental risks. This may include specialized treatment processes and containment measures to prevent uncontrolled release into the environment.
Regulatory frameworks and environmental monitoring programs should be established to track the presence and behavior of these materials in various environmental matrices. This will help in early detection of potential issues and inform adaptive management strategies.
In conclusion, a comprehensive environmental impact assessment is crucial to understand and mitigate the potential risks associated with the interaction of perchloric acid and graphene-based materials. This assessment should inform policy decisions, guide industrial practices, and drive research towards developing safer and more environmentally friendly applications of these materials.
One primary concern is the potential for graphene-based materials to act as carriers for perchloric acid, facilitating its spread in aquatic environments. This could lead to increased acidity in water bodies, adversely affecting aquatic life and disrupting delicate ecological balances. The persistence of graphene in the environment, coupled with its ability to adsorb and transport perchloric acid, may result in long-term contamination of water resources.
Soil contamination is another critical issue to consider. The interaction between perchloric acid and graphene-based materials in soil could alter soil chemistry, potentially affecting plant growth and microbial communities. This may have cascading effects on terrestrial ecosystems and agricultural productivity.
The potential for atmospheric dispersion of graphene particles contaminated with perchloric acid also poses risks to air quality. Inhalation of these particles could lead to respiratory issues in humans and animals, particularly in areas near production or disposal sites.
Bioaccumulation of graphene-based materials in food chains is a concern that requires thorough investigation. The potential for these materials to concentrate in organisms at higher trophic levels could have unforeseen consequences on biodiversity and ecosystem stability.
The environmental fate and transport of graphene-based materials interacting with perchloric acid need to be carefully studied. Factors such as weathering, degradation, and transformation in different environmental compartments must be considered to accurately assess long-term impacts.
Mitigation strategies and proper disposal methods for graphene-based materials exposed to perchloric acid should be developed to minimize environmental risks. This may include specialized treatment processes and containment measures to prevent uncontrolled release into the environment.
Regulatory frameworks and environmental monitoring programs should be established to track the presence and behavior of these materials in various environmental matrices. This will help in early detection of potential issues and inform adaptive management strategies.
In conclusion, a comprehensive environmental impact assessment is crucial to understand and mitigate the potential risks associated with the interaction of perchloric acid and graphene-based materials. This assessment should inform policy decisions, guide industrial practices, and drive research towards developing safer and more environmentally friendly applications of these materials.
Safety Protocols for Handling Perchloric Acid
The handling of perchloric acid in conjunction with graphene-based materials requires stringent safety protocols due to the highly reactive and potentially explosive nature of perchloric acid. These protocols are essential to ensure the safety of researchers and prevent accidents in laboratory settings.
Personal protective equipment (PPE) is paramount when working with perchloric acid. Researchers must wear chemical-resistant gloves, a lab coat, and safety goggles or a face shield. In cases where acid vapors may be present, a properly fitted respirator with appropriate cartridges should be used. All PPE should be inspected for integrity before use and replaced immediately if damaged.
Proper storage of perchloric acid is critical. It should be kept in a cool, dry, well-ventilated area, away from organic materials and other incompatible substances. Glass or PTFE containers are recommended, and secondary containment should be employed to prevent spills. Regular inspections of storage areas are necessary to detect any signs of container degradation or leakage.
When handling perchloric acid, work should be conducted in a designated fume hood equipped with a wash-down system. The work area should be free from organic materials, and only the minimum required amount of acid should be present. All equipment used with perchloric acid must be thoroughly cleaned after use to prevent the formation of explosive perchlorates.
Spill response procedures must be established and communicated to all personnel. Appropriate spill kits containing neutralizing agents and absorbents specifically designed for perchloric acid should be readily available. In the event of a spill, the area should be evacuated immediately, and only trained personnel should attempt cleanup.
Training is a crucial component of safety protocols. All researchers working with perchloric acid must receive comprehensive training on its properties, hazards, and proper handling techniques. This training should be documented and refreshed periodically to ensure ongoing competence.
Emergency response plans should be in place, including procedures for chemical exposure, fires, and explosions. Eyewash stations and safety showers must be easily accessible and regularly tested. Clear evacuation routes should be established and practiced through regular drills.
When combining perchloric acid with graphene-based materials, additional precautions may be necessary. The potential for unexpected reactions or the formation of sensitive compounds should be thoroughly assessed before experimentation. A detailed risk assessment should be conducted, and protocols may need to be adapted based on the specific properties of the graphene materials being used.
Personal protective equipment (PPE) is paramount when working with perchloric acid. Researchers must wear chemical-resistant gloves, a lab coat, and safety goggles or a face shield. In cases where acid vapors may be present, a properly fitted respirator with appropriate cartridges should be used. All PPE should be inspected for integrity before use and replaced immediately if damaged.
Proper storage of perchloric acid is critical. It should be kept in a cool, dry, well-ventilated area, away from organic materials and other incompatible substances. Glass or PTFE containers are recommended, and secondary containment should be employed to prevent spills. Regular inspections of storage areas are necessary to detect any signs of container degradation or leakage.
When handling perchloric acid, work should be conducted in a designated fume hood equipped with a wash-down system. The work area should be free from organic materials, and only the minimum required amount of acid should be present. All equipment used with perchloric acid must be thoroughly cleaned after use to prevent the formation of explosive perchlorates.
Spill response procedures must be established and communicated to all personnel. Appropriate spill kits containing neutralizing agents and absorbents specifically designed for perchloric acid should be readily available. In the event of a spill, the area should be evacuated immediately, and only trained personnel should attempt cleanup.
Training is a crucial component of safety protocols. All researchers working with perchloric acid must receive comprehensive training on its properties, hazards, and proper handling techniques. This training should be documented and refreshed periodically to ensure ongoing competence.
Emergency response plans should be in place, including procedures for chemical exposure, fires, and explosions. Eyewash stations and safety showers must be easily accessible and regularly tested. Clear evacuation routes should be established and practiced through regular drills.
When combining perchloric acid with graphene-based materials, additional precautions may be necessary. The potential for unexpected reactions or the formation of sensitive compounds should be thoroughly assessed before experimentation. A detailed risk assessment should be conducted, and protocols may need to be adapted based on the specific properties of the graphene materials being used.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!