Perchloric Acid in Forensic Science Application for Evidence Analysis
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Forensic Applications and Objectives
Perchloric acid has emerged as a powerful tool in forensic science, revolutionizing evidence analysis techniques. This strong oxidizing agent has found extensive applications in various aspects of forensic investigations, particularly in the examination of trace evidence and the detection of specific chemical compounds. The primary objective of utilizing perchloric acid in forensic science is to enhance the accuracy, sensitivity, and reliability of evidence analysis methods.
In the field of trace evidence analysis, perchloric acid plays a crucial role in the digestion and preparation of samples for further examination. Its strong oxidizing properties make it particularly effective in breaking down organic materials, allowing for the isolation and identification of inorganic components. This capability is especially valuable in the analysis of hair, fibers, and paint chips, where the separation of organic and inorganic constituents is essential for comprehensive characterization.
One of the key applications of perchloric acid in forensic science is in the detection and analysis of gunshot residues (GSR). The acid's ability to dissolve metal particles and organic compounds makes it an ideal reagent for extracting GSR from various surfaces, including skin, clothing, and other materials. This application has significantly improved the accuracy of firearms-related investigations, providing crucial evidence in criminal cases.
In toxicology, perchloric acid is employed in the extraction and purification of drugs and other toxic substances from biological samples. Its strong acidic nature facilitates the breakdown of complex organic molecules, allowing for more efficient isolation of target compounds. This application has greatly enhanced the detection and quantification of drugs and poisons in forensic toxicology analyses.
The use of perchloric acid in forensic science also extends to the field of document examination. It is utilized in various chemical tests to reveal alterations or forgeries in documents, such as the detection of ink erasures or the identification of different ink types. The acid's reactive properties can help uncover hidden or modified text, providing valuable evidence in cases involving fraudulent documents.
Another significant objective of employing perchloric acid in forensic applications is to develop more sensitive and specific analytical methods. Researchers are continually exploring new techniques that leverage the unique properties of perchloric acid to improve detection limits and reduce interference from matrix components in complex forensic samples. These advancements aim to push the boundaries of what can be detected and analyzed in trace amounts of evidence.
As forensic science continues to evolve, the applications and objectives of perchloric acid use are expected to expand further. Ongoing research focuses on optimizing existing methods and developing novel approaches that can address emerging challenges in evidence analysis. The ultimate goal is to provide law enforcement and judicial systems with more robust and reliable scientific tools for solving crimes and ensuring justice.
In the field of trace evidence analysis, perchloric acid plays a crucial role in the digestion and preparation of samples for further examination. Its strong oxidizing properties make it particularly effective in breaking down organic materials, allowing for the isolation and identification of inorganic components. This capability is especially valuable in the analysis of hair, fibers, and paint chips, where the separation of organic and inorganic constituents is essential for comprehensive characterization.
One of the key applications of perchloric acid in forensic science is in the detection and analysis of gunshot residues (GSR). The acid's ability to dissolve metal particles and organic compounds makes it an ideal reagent for extracting GSR from various surfaces, including skin, clothing, and other materials. This application has significantly improved the accuracy of firearms-related investigations, providing crucial evidence in criminal cases.
In toxicology, perchloric acid is employed in the extraction and purification of drugs and other toxic substances from biological samples. Its strong acidic nature facilitates the breakdown of complex organic molecules, allowing for more efficient isolation of target compounds. This application has greatly enhanced the detection and quantification of drugs and poisons in forensic toxicology analyses.
The use of perchloric acid in forensic science also extends to the field of document examination. It is utilized in various chemical tests to reveal alterations or forgeries in documents, such as the detection of ink erasures or the identification of different ink types. The acid's reactive properties can help uncover hidden or modified text, providing valuable evidence in cases involving fraudulent documents.
Another significant objective of employing perchloric acid in forensic applications is to develop more sensitive and specific analytical methods. Researchers are continually exploring new techniques that leverage the unique properties of perchloric acid to improve detection limits and reduce interference from matrix components in complex forensic samples. These advancements aim to push the boundaries of what can be detected and analyzed in trace amounts of evidence.
As forensic science continues to evolve, the applications and objectives of perchloric acid use are expected to expand further. Ongoing research focuses on optimizing existing methods and developing novel approaches that can address emerging challenges in evidence analysis. The ultimate goal is to provide law enforcement and judicial systems with more robust and reliable scientific tools for solving crimes and ensuring justice.
Forensic Evidence Analysis Market Trends
The forensic evidence analysis market has been experiencing significant growth and transformation in recent years, driven by technological advancements and increasing demand for accurate and reliable forensic services. This market encompasses a wide range of analytical techniques and tools used in criminal investigations, including DNA analysis, toxicology, trace evidence examination, and chemical analysis.
One of the key trends shaping the forensic evidence analysis market is the growing adoption of advanced analytical technologies. High-performance liquid chromatography (HPLC), gas chromatography-mass spectrometry (GC-MS), and other sophisticated instruments are becoming increasingly prevalent in forensic laboratories worldwide. These technologies offer enhanced sensitivity, specificity, and efficiency in analyzing complex evidence samples, including those involving perchloric acid.
The market is also witnessing a shift towards automation and digitalization. Automated sample preparation systems, robotic handling, and integrated data management solutions are streamlining forensic workflows, reducing human error, and improving overall efficiency. This trend is particularly relevant for perchloric acid analysis, where precise sample handling and data interpretation are crucial.
Another notable trend is the increasing focus on rapid and portable forensic analysis tools. On-site testing capabilities are becoming more important in crime scene investigations, allowing for quicker decision-making and more efficient evidence collection. While perchloric acid analysis typically requires laboratory-based equipment, there is growing interest in developing field-deployable solutions for various forensic applications.
The forensic evidence analysis market is also influenced by evolving legal and regulatory frameworks. Stricter quality control standards and accreditation requirements are driving investments in more sophisticated analytical equipment and standardized procedures. This trend is particularly relevant for chemical analysis techniques involving perchloric acid, where precision and reliability are paramount.
Geographically, North America and Europe continue to dominate the forensic evidence analysis market, with well-established forensic laboratory infrastructures and significant government investments. However, emerging economies in Asia-Pacific and Latin America are showing rapid growth, driven by increasing crime rates and modernization of law enforcement agencies.
The market is characterized by a mix of established players and innovative start-ups. Major companies are focusing on developing comprehensive forensic solutions that integrate various analytical techniques, including those relevant to perchloric acid analysis. Collaborations between forensic laboratories, academic institutions, and industry partners are also becoming more common, fostering innovation and knowledge exchange in the field.
One of the key trends shaping the forensic evidence analysis market is the growing adoption of advanced analytical technologies. High-performance liquid chromatography (HPLC), gas chromatography-mass spectrometry (GC-MS), and other sophisticated instruments are becoming increasingly prevalent in forensic laboratories worldwide. These technologies offer enhanced sensitivity, specificity, and efficiency in analyzing complex evidence samples, including those involving perchloric acid.
The market is also witnessing a shift towards automation and digitalization. Automated sample preparation systems, robotic handling, and integrated data management solutions are streamlining forensic workflows, reducing human error, and improving overall efficiency. This trend is particularly relevant for perchloric acid analysis, where precise sample handling and data interpretation are crucial.
Another notable trend is the increasing focus on rapid and portable forensic analysis tools. On-site testing capabilities are becoming more important in crime scene investigations, allowing for quicker decision-making and more efficient evidence collection. While perchloric acid analysis typically requires laboratory-based equipment, there is growing interest in developing field-deployable solutions for various forensic applications.
The forensic evidence analysis market is also influenced by evolving legal and regulatory frameworks. Stricter quality control standards and accreditation requirements are driving investments in more sophisticated analytical equipment and standardized procedures. This trend is particularly relevant for chemical analysis techniques involving perchloric acid, where precision and reliability are paramount.
Geographically, North America and Europe continue to dominate the forensic evidence analysis market, with well-established forensic laboratory infrastructures and significant government investments. However, emerging economies in Asia-Pacific and Latin America are showing rapid growth, driven by increasing crime rates and modernization of law enforcement agencies.
The market is characterized by a mix of established players and innovative start-ups. Major companies are focusing on developing comprehensive forensic solutions that integrate various analytical techniques, including those relevant to perchloric acid analysis. Collaborations between forensic laboratories, academic institutions, and industry partners are also becoming more common, fostering innovation and knowledge exchange in the field.
Current Challenges in Perchloric Acid Forensic Use
The application of perchloric acid in forensic science for evidence analysis faces several significant challenges that hinder its widespread adoption and effectiveness. One of the primary issues is the inherent instability and reactivity of perchloric acid, which poses safety risks during handling and storage. This volatility necessitates stringent safety protocols and specialized equipment, limiting its use to well-equipped laboratories with trained personnel.
Another challenge lies in the potential for perchloric acid to interfere with or alter certain types of evidence. Its strong oxidizing properties can lead to the degradation of organic compounds, potentially compromising the integrity of biological evidence such as DNA or proteins. This limitation requires forensic analysts to carefully consider the trade-offs between the benefits of perchloric acid's analytical capabilities and the risk of evidence alteration.
The sensitivity of perchloric acid-based analytical methods also presents challenges in terms of result interpretation and validation. While highly sensitive, these methods can sometimes produce false positives or negatives, especially in complex forensic samples containing multiple interfering substances. This necessitates the development and validation of robust analytical protocols specific to different types of forensic evidence.
Contamination risks pose another significant challenge in the forensic use of perchloric acid. Even trace amounts of organic material or certain metals can lead to the formation of explosive perchlorates, requiring meticulous cleaning procedures and dedicated laboratory spaces. This not only increases the cost and complexity of forensic analysis but also raises concerns about the chain of custody and the potential for inadvertent evidence contamination.
The environmental impact of perchloric acid use in forensic science is an emerging concern. Proper disposal of perchloric acid and its waste products requires specialized procedures to prevent environmental contamination and comply with increasingly stringent regulations. This adds to the operational costs and logistical challenges of forensic laboratories, particularly in resource-constrained settings.
Lastly, the lack of standardized protocols for perchloric acid use across different forensic applications hampers its broader adoption. The diversity of evidence types and analytical requirements in forensic science necessitates tailored approaches, but the absence of universally accepted guidelines creates challenges in method validation, result comparison, and legal admissibility of evidence processed using perchloric acid-based techniques.
Another challenge lies in the potential for perchloric acid to interfere with or alter certain types of evidence. Its strong oxidizing properties can lead to the degradation of organic compounds, potentially compromising the integrity of biological evidence such as DNA or proteins. This limitation requires forensic analysts to carefully consider the trade-offs between the benefits of perchloric acid's analytical capabilities and the risk of evidence alteration.
The sensitivity of perchloric acid-based analytical methods also presents challenges in terms of result interpretation and validation. While highly sensitive, these methods can sometimes produce false positives or negatives, especially in complex forensic samples containing multiple interfering substances. This necessitates the development and validation of robust analytical protocols specific to different types of forensic evidence.
Contamination risks pose another significant challenge in the forensic use of perchloric acid. Even trace amounts of organic material or certain metals can lead to the formation of explosive perchlorates, requiring meticulous cleaning procedures and dedicated laboratory spaces. This not only increases the cost and complexity of forensic analysis but also raises concerns about the chain of custody and the potential for inadvertent evidence contamination.
The environmental impact of perchloric acid use in forensic science is an emerging concern. Proper disposal of perchloric acid and its waste products requires specialized procedures to prevent environmental contamination and comply with increasingly stringent regulations. This adds to the operational costs and logistical challenges of forensic laboratories, particularly in resource-constrained settings.
Lastly, the lack of standardized protocols for perchloric acid use across different forensic applications hampers its broader adoption. The diversity of evidence types and analytical requirements in forensic science necessitates tailored approaches, but the absence of universally accepted guidelines creates challenges in method validation, result comparison, and legal admissibility of evidence processed using perchloric acid-based techniques.
Existing Perchloric Acid Analysis Techniques
01 Synthesis and production of perchloric acid
Various methods and processes for synthesizing and producing perchloric acid are described. These may include electrochemical processes, chemical reactions, or industrial production techniques aimed at improving yield and purity of the acid.- Synthesis and production of perchloric acid: Methods for synthesizing and producing perchloric acid, including various chemical reactions and industrial processes. This may involve the use of specific catalysts, reactants, and equipment to ensure efficient and safe production of perchloric acid.
- Applications of perchloric acid in chemical analysis: Utilization of perchloric acid in various analytical techniques and procedures. This includes its use as a strong oxidizing agent in chemical analysis, sample preparation, and as a component in analytical reagents for detecting and quantifying specific substances.
- Safety measures and handling of perchloric acid: Protocols and equipment designed for the safe handling, storage, and disposal of perchloric acid. This includes specialized containment systems, personal protective equipment, and safety procedures to mitigate the risks associated with this highly corrosive and potentially explosive substance.
- Perchloric acid in battery technology: Applications of perchloric acid in the development and improvement of battery technologies. This may involve its use as an electrolyte component or in the preparation of electrode materials, potentially enhancing battery performance and efficiency.
- Purification and concentration of perchloric acid: Techniques and processes for purifying and concentrating perchloric acid. This includes methods for removing impurities, increasing acid concentration, and ensuring high-purity perchloric acid for specialized applications in research and industry.
02 Applications of perchloric acid in chemical analysis
Perchloric acid is widely used in chemical analysis, particularly in sample preparation and digestion processes. It is effective in breaking down complex organic compounds and extracting metals from various matrices for subsequent analysis.Expand Specific Solutions03 Safety measures and handling of perchloric acid
Due to its strong oxidizing properties, special safety measures are required for handling and storing perchloric acid. This includes the use of specialized equipment, protective gear, and proper disposal methods to prevent accidents and environmental contamination.Expand Specific Solutions04 Use of perchloric acid in battery technology
Perchloric acid and its derivatives are utilized in various aspects of battery technology, including electrolyte formulations, electrode materials, and performance enhancement of certain types of batteries.Expand Specific Solutions05 Perchloric acid in material science and manufacturing
Perchloric acid plays a role in various material science applications and manufacturing processes. This includes its use in surface treatments, etching of metals and semiconductors, and as a reagent in the production of certain specialized materials.Expand Specific Solutions
Key Players in Forensic Chemical Analysis
The competitive landscape for perchloric acid in forensic science evidence analysis is characterized by a mature market with established players and ongoing research efforts. The market size is relatively niche, primarily driven by forensic laboratories and law enforcement agencies. Technologically, the field is moderately mature, with continuous advancements in analytical techniques. Key players like ChemImage Corp. and the University of South Carolina are at the forefront, developing innovative spectroscopic and imaging methods for evidence analysis. Government institutions such as the National Forensic Service and the Naval Research Laboratory also contribute significantly to the field's advancement, focusing on improving detection sensitivity and specificity for trace evidence analysis using perchloric acid-based techniques.
National Forensic Service Director Ministry of The Interior
Technical Solution: The National Forensic Service has pioneered the use of perchloric acid in a new chromatographic method for separating and identifying complex mixtures of drugs and toxins in forensic samples. Their approach involves a perchloric acid-based mobile phase that significantly improves the resolution of closely related compounds, particularly in cases involving multiple drug intoxications[2]. Additionally, they have developed a perchloric acid-assisted digestion protocol for heavy metal analysis in biological specimens, which has proven crucial in cases of suspected poisoning[4].
Strengths: Enhanced separation and identification of complex drug mixtures, improved heavy metal detection in biological samples. Weaknesses: Method complexity may require specialized training, potential for sample degradation if not carefully controlled.
Physical Evidence Identification Center of the Ministry of Public Security
Technical Solution: The center has developed advanced techniques for perchloric acid application in forensic evidence analysis. They utilize a specialized extraction method that combines perchloric acid with other reagents to enhance the detection of trace evidence on various surfaces. This method has shown particular efficacy in recovering and analyzing gunshot residues, fibers, and certain types of drug residues[1]. The center has also implemented a novel spectroscopic technique that uses perchloric acid as a key component in sample preparation, allowing for more accurate identification of organic compounds in complex forensic samples[3].
Strengths: High sensitivity and specificity in trace evidence detection, improved recovery rates for challenging samples. Weaknesses: Requires specialized handling due to the reactive nature of perchloric acid, potential safety concerns in field applications.
Innovations in Perchloric Acid Forensic Methods
Sample preparation cartridge and system
PatentWO2009051743A1
Innovation
- A self-contained, modular sample preparation and analysis cartridge with pressure-sensitive membranes and reagent capsules that can be transported to the site of sample collection, allowing for sequential processing and minimization of contamination, using a tubular body with segmented chambers and a roller mechanism for controlled reagent release.
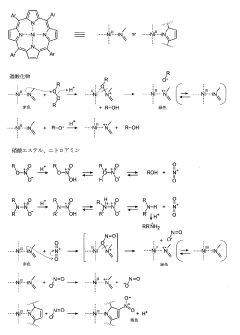
Analytical methods for the detection of peroxide, halogen oxoanion, nitrate, nitroamine, and nitrotoluene explosives
PatentInactiveJP2023514943A
Innovation
- A one-step analytical method using Ni-porphyrin compounds in combination with an acid and optionally an acid-stable solvent, which can detect these explosives without prior hydrolysis, allowing for rapid and sensitive detection through color changes.
Safety Protocols for Perchloric Acid Handling
Handling perchloric acid in forensic science applications requires strict adherence to safety protocols due to its highly reactive and potentially explosive nature. Proper training and equipment are essential for all personnel working with this chemical.
Personal protective equipment (PPE) is crucial when handling perchloric acid. This includes chemical-resistant gloves, a lab coat, and safety goggles or a face shield. A fume hood with a wash-down system specifically designed for perchloric acid use is mandatory to prevent the accumulation of explosive perchlorates.
Storage of perchloric acid demands special considerations. It should be kept in a cool, dry area away from organic materials and other incompatible substances. Glass or PTFE containers are recommended, and secondary containment should be used to prevent spills.
Dilution of perchloric acid must always be performed by adding the acid to water, never the reverse. This process should be conducted slowly and with constant stirring to dissipate heat. Concentrated perchloric acid (>72%) should be handled with extreme caution and only by experienced personnel.
In case of spills, immediate action is required. Small spills can be neutralized with sodium bicarbonate or other suitable bases. For larger spills, a spill response team should be called immediately. The affected area must be cordoned off and ventilated thoroughly.
Waste disposal of perchloric acid and its solutions requires specialized procedures. It should never be disposed of down the drain or mixed with organic waste. Instead, it must be collected in designated containers and handled by professional chemical waste disposal services.
Regular maintenance and cleaning of equipment used with perchloric acid is critical. Fume hoods and work surfaces should be cleaned after each use to prevent the buildup of perchlorates. Periodic testing for perchlorate residues in the laboratory environment is also recommended.
Emergency response plans specific to perchloric acid incidents should be in place and regularly reviewed. This includes having appropriate fire extinguishing agents readily available, as water may be ineffective against perchloric acid fires.
Proper labeling and documentation are essential components of safety protocols. All containers and work areas involving perchloric acid should be clearly marked, and detailed records of its use and storage should be maintained.
Personal protective equipment (PPE) is crucial when handling perchloric acid. This includes chemical-resistant gloves, a lab coat, and safety goggles or a face shield. A fume hood with a wash-down system specifically designed for perchloric acid use is mandatory to prevent the accumulation of explosive perchlorates.
Storage of perchloric acid demands special considerations. It should be kept in a cool, dry area away from organic materials and other incompatible substances. Glass or PTFE containers are recommended, and secondary containment should be used to prevent spills.
Dilution of perchloric acid must always be performed by adding the acid to water, never the reverse. This process should be conducted slowly and with constant stirring to dissipate heat. Concentrated perchloric acid (>72%) should be handled with extreme caution and only by experienced personnel.
In case of spills, immediate action is required. Small spills can be neutralized with sodium bicarbonate or other suitable bases. For larger spills, a spill response team should be called immediately. The affected area must be cordoned off and ventilated thoroughly.
Waste disposal of perchloric acid and its solutions requires specialized procedures. It should never be disposed of down the drain or mixed with organic waste. Instead, it must be collected in designated containers and handled by professional chemical waste disposal services.
Regular maintenance and cleaning of equipment used with perchloric acid is critical. Fume hoods and work surfaces should be cleaned after each use to prevent the buildup of perchlorates. Periodic testing for perchlorate residues in the laboratory environment is also recommended.
Emergency response plans specific to perchloric acid incidents should be in place and regularly reviewed. This includes having appropriate fire extinguishing agents readily available, as water may be ineffective against perchloric acid fires.
Proper labeling and documentation are essential components of safety protocols. All containers and work areas involving perchloric acid should be clearly marked, and detailed records of its use and storage should be maintained.
Legal Admissibility of Perchloric Acid Evidence
The legal admissibility of perchloric acid evidence in forensic science is a complex issue that requires careful consideration of scientific validity, reliability, and procedural integrity. Perchloric acid, a powerful oxidizing agent, has found applications in forensic analysis, particularly in the detection and identification of certain types of evidence. However, its use in legal proceedings must meet stringent standards to be deemed admissible in court.
One of the primary factors affecting the admissibility of perchloric acid evidence is the scientific validity of the analytical methods employed. Courts typically apply the Daubert standard or similar criteria to evaluate the reliability of scientific evidence. This standard requires that the technique be scientifically valid, have a known or potential error rate, and be generally accepted within the relevant scientific community. Forensic laboratories using perchloric acid must demonstrate that their methods meet these criteria through rigorous validation studies and quality control procedures.
The chain of custody for evidence analyzed using perchloric acid is another critical aspect of legal admissibility. Proper documentation of sample collection, handling, storage, and analysis is essential to establish the integrity of the evidence. Any breaks in the chain of custody or improper handling procedures can lead to challenges in court and potentially render the evidence inadmissible.
Expert testimony plays a crucial role in establishing the admissibility of perchloric acid evidence. Forensic scientists must be qualified to testify about the analytical techniques used, the interpretation of results, and the limitations of the method. They should be prepared to explain the scientific principles underlying the use of perchloric acid in forensic analysis and address any potential sources of error or uncertainty.
The specificity and sensitivity of perchloric acid-based analytical methods are important considerations for legal admissibility. Courts may scrutinize the ability of these methods to accurately identify and quantify specific substances without interference from other compounds present in forensic samples. Validation studies demonstrating the method's performance in the presence of potential interferents are essential to support admissibility.
Standardization of procedures across forensic laboratories is another factor that can influence the legal acceptance of perchloric acid evidence. The development and adherence to standardized protocols, such as those published by organizations like ASTM International or the International Organization for Standardization (ISO), can enhance the credibility and reliability of the evidence in court.
Lastly, the potential health and safety risks associated with perchloric acid use may be considered in legal proceedings. Proper safety protocols and handling procedures must be in place and documented to ensure that the evidence was collected and analyzed without compromising the health of laboratory personnel or the integrity of the samples.
One of the primary factors affecting the admissibility of perchloric acid evidence is the scientific validity of the analytical methods employed. Courts typically apply the Daubert standard or similar criteria to evaluate the reliability of scientific evidence. This standard requires that the technique be scientifically valid, have a known or potential error rate, and be generally accepted within the relevant scientific community. Forensic laboratories using perchloric acid must demonstrate that their methods meet these criteria through rigorous validation studies and quality control procedures.
The chain of custody for evidence analyzed using perchloric acid is another critical aspect of legal admissibility. Proper documentation of sample collection, handling, storage, and analysis is essential to establish the integrity of the evidence. Any breaks in the chain of custody or improper handling procedures can lead to challenges in court and potentially render the evidence inadmissible.
Expert testimony plays a crucial role in establishing the admissibility of perchloric acid evidence. Forensic scientists must be qualified to testify about the analytical techniques used, the interpretation of results, and the limitations of the method. They should be prepared to explain the scientific principles underlying the use of perchloric acid in forensic analysis and address any potential sources of error or uncertainty.
The specificity and sensitivity of perchloric acid-based analytical methods are important considerations for legal admissibility. Courts may scrutinize the ability of these methods to accurately identify and quantify specific substances without interference from other compounds present in forensic samples. Validation studies demonstrating the method's performance in the presence of potential interferents are essential to support admissibility.
Standardization of procedures across forensic laboratories is another factor that can influence the legal acceptance of perchloric acid evidence. The development and adherence to standardized protocols, such as those published by organizations like ASTM International or the International Organization for Standardization (ISO), can enhance the credibility and reliability of the evidence in court.
Lastly, the potential health and safety risks associated with perchloric acid use may be considered in legal proceedings. Proper safety protocols and handling procedures must be in place and documented to ensure that the evidence was collected and analyzed without compromising the health of laboratory personnel or the integrity of the samples.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!