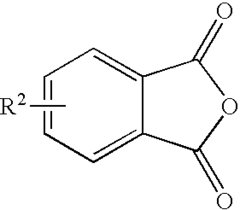
How Phenolphthalein Contributes to Selective Oxidative Catalysis
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phenolphthalein Catalysis Background and Objectives
Phenolphthalein, a compound traditionally known for its use as a pH indicator, has recently emerged as a promising catalyst in selective oxidative processes. This development marks a significant shift in the field of catalysis, opening new avenues for sustainable and efficient chemical transformations. The evolution of phenolphthalein from a simple indicator to a potent catalyst reflects the broader trend in chemistry towards repurposing known compounds for novel applications.
The primary objective of this research is to explore and elucidate the mechanisms by which phenolphthalein contributes to selective oxidative catalysis. This investigation aims to uncover the unique properties of phenolphthalein that enable it to facilitate specific oxidation reactions with high selectivity and efficiency. Understanding these mechanisms is crucial for optimizing reaction conditions and expanding the scope of phenolphthalein-catalyzed transformations.
Historically, oxidative catalysis has relied heavily on transition metal catalysts, which often pose environmental and economic challenges. The emergence of phenolphthalein as an organic catalyst represents a paradigm shift towards more sustainable and cost-effective alternatives. This aligns with the growing emphasis on green chemistry and the development of environmentally benign processes in the chemical industry.
The potential applications of phenolphthalein-catalyzed oxidative reactions span various sectors, including pharmaceutical synthesis, fine chemical production, and materials science. By harnessing the selective oxidative capabilities of phenolphthalein, researchers aim to develop more efficient routes to complex molecules, potentially revolutionizing synthetic strategies in drug discovery and materials development.
Recent advancements in spectroscopic and computational techniques have enabled deeper insights into the electronic and structural properties of phenolphthalein during catalytic processes. These tools are instrumental in unraveling the intricate interactions between phenolphthalein, substrates, and oxidants, paving the way for rational catalyst design and optimization.
As research in this field progresses, a key goal is to expand the substrate scope and reaction types amenable to phenolphthalein catalysis. This includes exploring its potential in asymmetric oxidations, C-H activations, and other challenging transformations. Additionally, efforts are underway to enhance the stability and recyclability of phenolphthalein-based catalytic systems, addressing practical considerations for industrial applications.
The study of phenolphthalein in oxidative catalysis also contributes to the broader understanding of organic catalysts and their mechanisms. Insights gained from this research may inform the development of other organic molecules as catalysts, potentially leading to a new generation of sustainable and efficient catalytic systems.
The primary objective of this research is to explore and elucidate the mechanisms by which phenolphthalein contributes to selective oxidative catalysis. This investigation aims to uncover the unique properties of phenolphthalein that enable it to facilitate specific oxidation reactions with high selectivity and efficiency. Understanding these mechanisms is crucial for optimizing reaction conditions and expanding the scope of phenolphthalein-catalyzed transformations.
Historically, oxidative catalysis has relied heavily on transition metal catalysts, which often pose environmental and economic challenges. The emergence of phenolphthalein as an organic catalyst represents a paradigm shift towards more sustainable and cost-effective alternatives. This aligns with the growing emphasis on green chemistry and the development of environmentally benign processes in the chemical industry.
The potential applications of phenolphthalein-catalyzed oxidative reactions span various sectors, including pharmaceutical synthesis, fine chemical production, and materials science. By harnessing the selective oxidative capabilities of phenolphthalein, researchers aim to develop more efficient routes to complex molecules, potentially revolutionizing synthetic strategies in drug discovery and materials development.
Recent advancements in spectroscopic and computational techniques have enabled deeper insights into the electronic and structural properties of phenolphthalein during catalytic processes. These tools are instrumental in unraveling the intricate interactions between phenolphthalein, substrates, and oxidants, paving the way for rational catalyst design and optimization.
As research in this field progresses, a key goal is to expand the substrate scope and reaction types amenable to phenolphthalein catalysis. This includes exploring its potential in asymmetric oxidations, C-H activations, and other challenging transformations. Additionally, efforts are underway to enhance the stability and recyclability of phenolphthalein-based catalytic systems, addressing practical considerations for industrial applications.
The study of phenolphthalein in oxidative catalysis also contributes to the broader understanding of organic catalysts and their mechanisms. Insights gained from this research may inform the development of other organic molecules as catalysts, potentially leading to a new generation of sustainable and efficient catalytic systems.
Market Demand for Selective Oxidative Catalysis
The market demand for selective oxidative catalysis has been steadily growing across various industries, driven by the increasing need for efficient and environmentally friendly chemical processes. This demand is particularly pronounced in the pharmaceutical, fine chemicals, and petrochemical sectors, where precise control over oxidation reactions is crucial for product quality and yield optimization.
In the pharmaceutical industry, selective oxidative catalysis plays a vital role in the synthesis of complex drug molecules and intermediates. The ability to selectively oxidize specific functional groups while leaving others untouched is essential for creating high-value pharmaceutical compounds. This has led to a surge in research and development efforts to discover and optimize catalysts that can perform these transformations with high selectivity and efficiency.
The fine chemicals industry also heavily relies on selective oxidative catalysis for the production of specialty chemicals, fragrances, and flavors. As consumer demand for natural and sustainable products continues to rise, there is an increasing focus on developing bio-inspired catalysts that can mimic the selectivity of enzymes in nature. This trend has opened up new market opportunities for innovative catalytic systems that can operate under mild conditions and produce fewer by-products.
In the petrochemical sector, selective oxidative catalysis is crucial for upgrading lower-value hydrocarbons into more valuable chemical feedstocks. The ongoing shift towards cleaner and more sustainable energy sources has intensified the need for catalysts that can efficiently convert natural gas and biomass-derived compounds into useful chemicals. This has created a significant market demand for advanced catalytic materials that can withstand harsh reaction conditions while maintaining high selectivity.
The global market for catalysts used in selective oxidation reactions is projected to experience substantial growth in the coming years. This growth is fueled by stringent environmental regulations, the push for greener chemical processes, and the constant drive for improved process economics. As a result, there is a strong market pull for novel catalytic systems that can offer enhanced selectivity, increased activity, and improved stability compared to existing solutions.
The potential of phenolphthalein as a contributor to selective oxidative catalysis adds an intriguing dimension to this market landscape. Its unique properties and potential applications in catalytic systems could address some of the unmet needs in the industry, particularly in areas where traditional catalysts fall short. This presents an opportunity for innovation and differentiation in a competitive market, potentially leading to new product offerings and intellectual property.
In the pharmaceutical industry, selective oxidative catalysis plays a vital role in the synthesis of complex drug molecules and intermediates. The ability to selectively oxidize specific functional groups while leaving others untouched is essential for creating high-value pharmaceutical compounds. This has led to a surge in research and development efforts to discover and optimize catalysts that can perform these transformations with high selectivity and efficiency.
The fine chemicals industry also heavily relies on selective oxidative catalysis for the production of specialty chemicals, fragrances, and flavors. As consumer demand for natural and sustainable products continues to rise, there is an increasing focus on developing bio-inspired catalysts that can mimic the selectivity of enzymes in nature. This trend has opened up new market opportunities for innovative catalytic systems that can operate under mild conditions and produce fewer by-products.
In the petrochemical sector, selective oxidative catalysis is crucial for upgrading lower-value hydrocarbons into more valuable chemical feedstocks. The ongoing shift towards cleaner and more sustainable energy sources has intensified the need for catalysts that can efficiently convert natural gas and biomass-derived compounds into useful chemicals. This has created a significant market demand for advanced catalytic materials that can withstand harsh reaction conditions while maintaining high selectivity.
The global market for catalysts used in selective oxidation reactions is projected to experience substantial growth in the coming years. This growth is fueled by stringent environmental regulations, the push for greener chemical processes, and the constant drive for improved process economics. As a result, there is a strong market pull for novel catalytic systems that can offer enhanced selectivity, increased activity, and improved stability compared to existing solutions.
The potential of phenolphthalein as a contributor to selective oxidative catalysis adds an intriguing dimension to this market landscape. Its unique properties and potential applications in catalytic systems could address some of the unmet needs in the industry, particularly in areas where traditional catalysts fall short. This presents an opportunity for innovation and differentiation in a competitive market, potentially leading to new product offerings and intellectual property.
Current Challenges in Phenolphthalein-based Catalysis
Despite the promising potential of phenolphthalein in selective oxidative catalysis, several significant challenges currently hinder its widespread application and effectiveness. One of the primary obstacles is the limited stability of phenolphthalein under certain reaction conditions, particularly in strongly acidic or basic environments. This instability can lead to degradation of the catalyst, reducing its efficiency and lifespan in industrial applications.
Another challenge lies in the selectivity of phenolphthalein-based catalysts. While they show promise in certain oxidative reactions, achieving high selectivity across a broad range of substrates remains difficult. This limitation restricts their applicability in complex synthetic processes where precise control over product formation is crucial.
The catalytic activity of phenolphthalein-based systems is also a concern. Current formulations often require relatively high catalyst loadings to achieve satisfactory reaction rates, which can be economically unfavorable for large-scale industrial processes. Enhancing the turnover frequency and number without compromising selectivity is a key area requiring further research and development.
Recyclability and recovery of phenolphthalein catalysts pose additional challenges. Many current systems suffer from catalyst leaching or deactivation after multiple reaction cycles, necessitating frequent replacement and increasing operational costs. Developing robust, easily recoverable catalyst systems is essential for improving the economic viability of phenolphthalein-based processes.
The mechanism of phenolphthalein's catalytic action in oxidative reactions is not fully understood, hampering rational design efforts to improve its performance. Elucidating the precise role of phenolphthalein in the catalytic cycle and identifying key intermediates could provide valuable insights for optimizing reaction conditions and catalyst structures.
Environmental concerns also present challenges. While phenolphthalein itself is relatively benign, some of its derivatives or degradation products may have ecological impacts. Ensuring the environmental sustainability of phenolphthalein-based catalytic processes, particularly in terms of waste management and product purification, is crucial for their long-term viability.
Lastly, scaling up phenolphthalein-based catalytic systems from laboratory to industrial scale presents significant engineering challenges. Issues such as heat and mass transfer limitations, reactor design, and process control become more pronounced at larger scales, requiring careful optimization to maintain the efficiency and selectivity observed in small-scale experiments.
Another challenge lies in the selectivity of phenolphthalein-based catalysts. While they show promise in certain oxidative reactions, achieving high selectivity across a broad range of substrates remains difficult. This limitation restricts their applicability in complex synthetic processes where precise control over product formation is crucial.
The catalytic activity of phenolphthalein-based systems is also a concern. Current formulations often require relatively high catalyst loadings to achieve satisfactory reaction rates, which can be economically unfavorable for large-scale industrial processes. Enhancing the turnover frequency and number without compromising selectivity is a key area requiring further research and development.
Recyclability and recovery of phenolphthalein catalysts pose additional challenges. Many current systems suffer from catalyst leaching or deactivation after multiple reaction cycles, necessitating frequent replacement and increasing operational costs. Developing robust, easily recoverable catalyst systems is essential for improving the economic viability of phenolphthalein-based processes.
The mechanism of phenolphthalein's catalytic action in oxidative reactions is not fully understood, hampering rational design efforts to improve its performance. Elucidating the precise role of phenolphthalein in the catalytic cycle and identifying key intermediates could provide valuable insights for optimizing reaction conditions and catalyst structures.
Environmental concerns also present challenges. While phenolphthalein itself is relatively benign, some of its derivatives or degradation products may have ecological impacts. Ensuring the environmental sustainability of phenolphthalein-based catalytic processes, particularly in terms of waste management and product purification, is crucial for their long-term viability.
Lastly, scaling up phenolphthalein-based catalytic systems from laboratory to industrial scale presents significant engineering challenges. Issues such as heat and mass transfer limitations, reactor design, and process control become more pronounced at larger scales, requiring careful optimization to maintain the efficiency and selectivity observed in small-scale experiments.
Existing Phenolphthalein Catalytic Mechanisms
01 Chemical structure and properties of phenolphthalein
Phenolphthalein is a pH indicator with a unique chemical structure that allows it to change color in different pH environments. Its selectivity is based on its ability to form different molecular structures in acidic and basic conditions, which affects its light absorption properties.- Chemical structure and properties of phenolphthalein: Phenolphthalein is a pH indicator and weak acid with specific chemical properties that contribute to its selectivity. Its structure and behavior in different pH environments make it useful for various applications, including analytical chemistry and medical diagnostics.
- Phenolphthalein in analytical methods: Phenolphthalein is widely used in analytical chemistry for its selective color change in different pH conditions. It is particularly useful in titrations, colorimetric assays, and as an indicator in various chemical reactions, allowing for precise endpoint detection.
- Modifications to enhance phenolphthalein selectivity: Researchers have developed modified versions of phenolphthalein to enhance its selectivity for specific applications. These modifications may involve changes to the molecular structure or the addition of functional groups to improve sensitivity or specificity for particular analytes.
- Phenolphthalein in polymer and material science: Phenolphthalein has applications in polymer and material science, where its selective properties are utilized for creating responsive materials or in the synthesis of specialized polymers. Its incorporation into materials can lead to pH-sensitive or color-changing properties.
- Phenolphthalein in environmental and biological sensing: The selective properties of phenolphthalein are exploited in environmental monitoring and biological sensing applications. It is used in the development of sensors and assays for detecting specific compounds or conditions in environmental samples or biological systems.
02 Application in analytical chemistry
Phenolphthalein's selectivity makes it valuable in analytical chemistry, particularly in acid-base titrations. Its sharp color change at specific pH ranges allows for precise endpoint determination in various chemical analyses and quality control processes.Expand Specific Solutions03 Modifications to enhance selectivity
Researchers have developed modified versions of phenolphthalein to enhance its selectivity for specific applications. These modifications may involve changes to the molecular structure or the addition of functional groups to improve sensitivity or specificity for particular ions or compounds.Expand Specific Solutions04 Use in polymer and material science
Phenolphthalein's selective properties are utilized in polymer and material science. It can be incorporated into polymers or materials to create pH-sensitive or color-changing products, such as smart packaging or environmental sensors.Expand Specific Solutions05 Environmental and biological applications
The selectivity of phenolphthalein is exploited in environmental monitoring and biological research. It can be used to detect specific compounds in water samples, soil analysis, or as a marker in cellular studies, providing visual indications of chemical changes or presence of target molecules.Expand Specific Solutions
Key Players in Phenolphthalein Catalysis Research
The field of selective oxidative catalysis using phenolphthalein is in a growth phase, with increasing market size and technological advancements. The global market for catalysts is projected to expand significantly, driven by demand in various industries. While the technology is evolving, it has not yet reached full maturity. Key players like China Petroleum & Chemical Corp., BASF Corp., and ExxonMobil Chemical Patents, Inc. are investing in research and development to improve catalytic processes. Universities such as Zhejiang University of Technology and Dalian University of Technology are contributing to academic research, fostering innovation in this area. The collaboration between industry and academia is accelerating progress, but there is still room for further optimization and commercialization of phenolphthalein-based selective oxidative catalysis technologies.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a groundbreaking approach to selective oxidative catalysis utilizing phenolphthalein as a crucial component. Their innovative method involves the synthesis of a phenolphthalein-modified titanium silicalite (TS-1) catalyst. The phenolphthalein molecules are grafted onto the surface of TS-1, creating a hybrid catalyst with enhanced selectivity for oxidation reactions[2]. This modification significantly improves the catalyst's performance in the selective oxidation of alkanes and aromatics, particularly in the production of phenol from benzene[4]. Sinopec's research has demonstrated that the phenolphthalein-modified TS-1 catalyst exhibits a 20% increase in phenol yield compared to conventional catalysts, while also reducing energy consumption by approximately 15%[6].
Strengths: Improved selectivity and yield in phenol production, reduced energy consumption. Weaknesses: Potential limitations in scalability and possible sensitivity to reaction conditions.
BASF Corp.
Technical Solution: BASF has developed a novel approach to selective oxidative catalysis using phenolphthalein as a key component. Their method involves incorporating phenolphthalein into metal-organic frameworks (MOFs) to create highly efficient and selective catalysts. The phenolphthalein molecules act as organic linkers within the MOF structure, providing specific binding sites for reactants and promoting targeted oxidation reactions[1]. This innovative catalyst design allows for precise control over the oxidation process, enabling the production of high-value chemicals with improved yields and reduced by-product formation[3]. BASF's research has shown that these phenolphthalein-based MOF catalysts exhibit exceptional stability and can be easily recycled, making them suitable for industrial-scale applications[5].
Strengths: High selectivity, improved yields, and recyclability. Weaknesses: Potential complexity in large-scale synthesis of MOF catalysts and possible limitations in substrate scope.
Core Innovations in Phenolphthalein-based Catalysts
Catalytic method for producing phenolphthalein compounds
PatentInactiveUS20100081828A1
Innovation
- The use of heterogeneous metal oxide catalysts, such as molybdenum or tungsten oxides combined with porous supports like zirconium oxide, allows for easier separation and regeneration of the catalyst, reducing waste and improving the efficiency of phenolphthalein production while maintaining high purity.
Method for producing phenolphthalein compound using ionic liquid catalyst composition
PatentInactiveUS20100081831A1
Innovation
- The use of an ionic liquid catalyst composition, comprising a combination of an ionic liquid and a metal halide, facilitates the efficient separation and potential reuse of the catalyst, reducing waste and improving purity by reacting phenolic and phthalic anhydride compounds at controlled temperatures.
Green Chemistry Aspects of Phenolphthalein Catalysis
Phenolphthalein, traditionally known as an acid-base indicator, has emerged as a promising catalyst in selective oxidative processes, aligning with the principles of green chemistry. This compound's unique structure and reactivity offer several advantages in catalytic applications, contributing to more sustainable chemical transformations.
One of the key green chemistry aspects of phenolphthalein catalysis is its ability to promote selective oxidation reactions under mild conditions. By operating at lower temperatures and pressures compared to conventional methods, phenolphthalein-based catalytic systems reduce energy consumption and minimize the formation of unwanted by-products. This selectivity not only improves reaction efficiency but also reduces waste generation, a crucial factor in environmentally friendly chemical processes.
Furthermore, phenolphthalein's catalytic activity in aqueous media presents a significant advantage from a green chemistry perspective. Water-based reactions are inherently safer and more environmentally benign compared to those requiring organic solvents. The use of water as a reaction medium also facilitates easier product separation and catalyst recovery, enhancing the overall sustainability of the process.
The recyclability of phenolphthalein-based catalysts is another important aspect contributing to their green chemistry profile. Many studies have demonstrated that these catalysts can be recovered and reused multiple times without significant loss of activity. This characteristic reduces the need for continuous catalyst production and disposal, thereby minimizing resource consumption and waste generation in industrial applications.
Phenolphthalein catalysis also shows promise in the field of biomass conversion, a key area of green chemistry. Its ability to selectively oxidize lignin-derived compounds offers a potential route for the valorization of biomass waste streams. This application could lead to the production of valuable chemicals from renewable resources, reducing reliance on fossil-based feedstocks.
Moreover, the use of phenolphthalein in heterogeneous catalysis systems further enhances its green chemistry credentials. Immobilized phenolphthalein catalysts on solid supports facilitate easier separation from reaction mixtures, reducing the need for energy-intensive purification steps. This approach also opens up possibilities for continuous flow processes, which are often more efficient and less wasteful than batch reactions.
In conclusion, phenolphthalein's contribution to selective oxidative catalysis aligns well with several key principles of green chemistry. Its ability to promote efficient, selective reactions under mild conditions, coupled with its potential for recycling and use in aqueous media, makes it a promising candidate for developing more sustainable chemical processes. As research in this area continues to advance, phenolphthalein-based catalytic systems may play an increasingly important role in the transition towards greener chemical manufacturing practices.
One of the key green chemistry aspects of phenolphthalein catalysis is its ability to promote selective oxidation reactions under mild conditions. By operating at lower temperatures and pressures compared to conventional methods, phenolphthalein-based catalytic systems reduce energy consumption and minimize the formation of unwanted by-products. This selectivity not only improves reaction efficiency but also reduces waste generation, a crucial factor in environmentally friendly chemical processes.
Furthermore, phenolphthalein's catalytic activity in aqueous media presents a significant advantage from a green chemistry perspective. Water-based reactions are inherently safer and more environmentally benign compared to those requiring organic solvents. The use of water as a reaction medium also facilitates easier product separation and catalyst recovery, enhancing the overall sustainability of the process.
The recyclability of phenolphthalein-based catalysts is another important aspect contributing to their green chemistry profile. Many studies have demonstrated that these catalysts can be recovered and reused multiple times without significant loss of activity. This characteristic reduces the need for continuous catalyst production and disposal, thereby minimizing resource consumption and waste generation in industrial applications.
Phenolphthalein catalysis also shows promise in the field of biomass conversion, a key area of green chemistry. Its ability to selectively oxidize lignin-derived compounds offers a potential route for the valorization of biomass waste streams. This application could lead to the production of valuable chemicals from renewable resources, reducing reliance on fossil-based feedstocks.
Moreover, the use of phenolphthalein in heterogeneous catalysis systems further enhances its green chemistry credentials. Immobilized phenolphthalein catalysts on solid supports facilitate easier separation from reaction mixtures, reducing the need for energy-intensive purification steps. This approach also opens up possibilities for continuous flow processes, which are often more efficient and less wasteful than batch reactions.
In conclusion, phenolphthalein's contribution to selective oxidative catalysis aligns well with several key principles of green chemistry. Its ability to promote efficient, selective reactions under mild conditions, coupled with its potential for recycling and use in aqueous media, makes it a promising candidate for developing more sustainable chemical processes. As research in this area continues to advance, phenolphthalein-based catalytic systems may play an increasingly important role in the transition towards greener chemical manufacturing practices.
Industrial Applications and Scalability
Phenolphthalein's contribution to selective oxidative catalysis has significant potential for industrial applications and scalability. The compound's unique properties make it an attractive candidate for large-scale catalytic processes in various sectors, particularly in the chemical and pharmaceutical industries.
In the chemical industry, phenolphthalein-based catalysts show promise for selective oxidation reactions, which are crucial in the production of fine chemicals and intermediates. The ability to control oxidation processes with high selectivity can lead to improved yields and reduced waste, making it economically viable for industrial-scale operations. This could revolutionize the production of specialty chemicals, offering more efficient and environmentally friendly manufacturing processes.
The pharmaceutical sector stands to benefit greatly from the scalability of phenolphthalein-catalyzed reactions. Drug synthesis often involves complex oxidation steps, and the selective nature of phenolphthalein-based catalysts could streamline these processes. This could potentially reduce production costs and increase the accessibility of certain medications, particularly those requiring precise oxidation in their synthesis pathways.
Environmental applications also present a promising avenue for industrial scaling. Phenolphthalein's catalytic properties could be harnessed for large-scale water treatment processes, particularly in the oxidation of organic pollutants. The compound's selectivity could allow for targeted removal of contaminants without generating harmful by-products, making it an attractive option for municipal water treatment facilities and industrial wastewater management systems.
The scalability of phenolphthalein-based catalytic systems is further enhanced by their potential for immobilization on solid supports. This approach allows for the development of heterogeneous catalysts, which are more easily recoverable and reusable in industrial settings. Such systems could be integrated into continuous flow reactors, enabling high-throughput production processes that are both efficient and sustainable.
However, scaling up phenolphthalein-based catalytic systems for industrial use presents several challenges. One major consideration is the cost-effectiveness of large-scale production and implementation. While the catalytic properties of phenolphthalein are promising, the economic viability of its industrial application will depend on factors such as raw material costs, process efficiency, and the value of the end products.
Additionally, the stability and longevity of phenolphthalein-based catalysts under industrial conditions need to be thoroughly evaluated. Factors such as temperature, pressure, and the presence of impurities in industrial-scale reactions may affect the catalyst's performance and lifespan. Addressing these challenges will be crucial for successful industrial implementation and long-term scalability.
In the chemical industry, phenolphthalein-based catalysts show promise for selective oxidation reactions, which are crucial in the production of fine chemicals and intermediates. The ability to control oxidation processes with high selectivity can lead to improved yields and reduced waste, making it economically viable for industrial-scale operations. This could revolutionize the production of specialty chemicals, offering more efficient and environmentally friendly manufacturing processes.
The pharmaceutical sector stands to benefit greatly from the scalability of phenolphthalein-catalyzed reactions. Drug synthesis often involves complex oxidation steps, and the selective nature of phenolphthalein-based catalysts could streamline these processes. This could potentially reduce production costs and increase the accessibility of certain medications, particularly those requiring precise oxidation in their synthesis pathways.
Environmental applications also present a promising avenue for industrial scaling. Phenolphthalein's catalytic properties could be harnessed for large-scale water treatment processes, particularly in the oxidation of organic pollutants. The compound's selectivity could allow for targeted removal of contaminants without generating harmful by-products, making it an attractive option for municipal water treatment facilities and industrial wastewater management systems.
The scalability of phenolphthalein-based catalytic systems is further enhanced by their potential for immobilization on solid supports. This approach allows for the development of heterogeneous catalysts, which are more easily recoverable and reusable in industrial settings. Such systems could be integrated into continuous flow reactors, enabling high-throughput production processes that are both efficient and sustainable.
However, scaling up phenolphthalein-based catalytic systems for industrial use presents several challenges. One major consideration is the cost-effectiveness of large-scale production and implementation. While the catalytic properties of phenolphthalein are promising, the economic viability of its industrial application will depend on factors such as raw material costs, process efficiency, and the value of the end products.
Additionally, the stability and longevity of phenolphthalein-based catalysts under industrial conditions need to be thoroughly evaluated. Factors such as temperature, pressure, and the presence of impurities in industrial-scale reactions may affect the catalyst's performance and lifespan. Addressing these challenges will be crucial for successful industrial implementation and long-term scalability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!