How to Analyze Lithium Nitride's Effects on Charge Capacity
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Nitride Background and Research Objectives
Lithium nitride (Li₃N) has emerged as a significant material in the evolution of lithium-ion battery technology since its discovery in the early 20th century. This compound, characterized by its unique crystalline structure and ionic conductivity properties, represents one of the earliest known solid lithium-containing materials with potential applications in energy storage. The historical development of lithium nitride research has progressed from fundamental property studies to more sophisticated applications in battery systems, particularly as researchers recognized its potential to enhance charge capacity.
The technological evolution of lithium-ion batteries has been driven by the increasing demand for higher energy density, longer cycle life, and improved safety profiles. Within this context, lithium nitride has attracted attention due to its theoretical capacity of approximately 2309 mAh/g, significantly exceeding that of conventional graphite anodes (372 mAh/g). This remarkable capacity potential stems from lithium nitride's ability to accommodate multiple lithium ions per formula unit during charge-discharge processes.
Recent advancements in analytical techniques, including in-situ X-ray diffraction, neutron scattering, and advanced electron microscopy, have enabled more precise characterization of lithium nitride's behavior during electrochemical cycling. These technological developments have facilitated deeper understanding of the structural transformations and reaction mechanisms that occur when lithium nitride interacts with lithium ions during battery operation.
The primary research objectives of this technical investigation are multifaceted. First, we aim to systematically analyze the correlation between lithium nitride's structural properties and its electrochemical performance, particularly focusing on charge capacity enhancement mechanisms. Second, we seek to identify the optimal synthesis parameters and compositional modifications that maximize lithium nitride's contribution to overall battery capacity while minimizing capacity fading during cycling.
Additionally, this research intends to explore the interfacial phenomena between lithium nitride and other battery components, as these interactions critically influence charge transfer kinetics and overall capacity retention. Understanding these interfaces will provide valuable insights for designing more effective electrode architectures incorporating lithium nitride.
Furthermore, we aim to develop standardized analytical protocols for quantifying lithium nitride's specific contribution to charge capacity in complex electrode systems. This standardization is essential for meaningful comparisons across different research efforts and for translating laboratory findings into practical applications.
The ultimate goal of this technical investigation is to establish a comprehensive framework for leveraging lithium nitride's unique properties to develop next-generation energy storage solutions with significantly improved charge capacity, addressing the growing energy demands of advanced electronic devices, electric vehicles, and renewable energy systems.
The technological evolution of lithium-ion batteries has been driven by the increasing demand for higher energy density, longer cycle life, and improved safety profiles. Within this context, lithium nitride has attracted attention due to its theoretical capacity of approximately 2309 mAh/g, significantly exceeding that of conventional graphite anodes (372 mAh/g). This remarkable capacity potential stems from lithium nitride's ability to accommodate multiple lithium ions per formula unit during charge-discharge processes.
Recent advancements in analytical techniques, including in-situ X-ray diffraction, neutron scattering, and advanced electron microscopy, have enabled more precise characterization of lithium nitride's behavior during electrochemical cycling. These technological developments have facilitated deeper understanding of the structural transformations and reaction mechanisms that occur when lithium nitride interacts with lithium ions during battery operation.
The primary research objectives of this technical investigation are multifaceted. First, we aim to systematically analyze the correlation between lithium nitride's structural properties and its electrochemical performance, particularly focusing on charge capacity enhancement mechanisms. Second, we seek to identify the optimal synthesis parameters and compositional modifications that maximize lithium nitride's contribution to overall battery capacity while minimizing capacity fading during cycling.
Additionally, this research intends to explore the interfacial phenomena between lithium nitride and other battery components, as these interactions critically influence charge transfer kinetics and overall capacity retention. Understanding these interfaces will provide valuable insights for designing more effective electrode architectures incorporating lithium nitride.
Furthermore, we aim to develop standardized analytical protocols for quantifying lithium nitride's specific contribution to charge capacity in complex electrode systems. This standardization is essential for meaningful comparisons across different research efforts and for translating laboratory findings into practical applications.
The ultimate goal of this technical investigation is to establish a comprehensive framework for leveraging lithium nitride's unique properties to develop next-generation energy storage solutions with significantly improved charge capacity, addressing the growing energy demands of advanced electronic devices, electric vehicles, and renewable energy systems.
Market Analysis of High-Capacity Battery Technologies
The high-capacity battery market has experienced substantial growth over the past decade, primarily driven by increasing demand for electric vehicles (EVs), portable electronics, and renewable energy storage systems. The global market for advanced batteries reached approximately $95 billion in 2022 and is projected to grow at a CAGR of 18.7% through 2030, with lithium-based technologies maintaining dominance in the premium segment.
Lithium nitride's potential impact on charge capacity represents a significant opportunity within this expanding market. Current commercial lithium-ion batteries typically deliver specific capacities of 150-250 mAh/g, while theoretical research suggests lithium nitride-enhanced electrodes could potentially achieve capacities exceeding 600 mAh/g, representing a transformative improvement for energy storage applications.
Market segmentation reveals distinct demand patterns across industries. The automotive sector currently consumes 48% of high-capacity batteries, with consumer electronics at 29%, grid storage at 15%, and industrial applications comprising the remaining 8%. Each segment presents unique requirements regarding energy density, cycle life, and cost parameters that lithium nitride modifications must address to achieve market penetration.
Regional analysis indicates Asia-Pacific dominates manufacturing capacity with 73% of global production, followed by Europe (18%) and North America (7%). However, recent policy initiatives including the US Inflation Reduction Act and European Battery Alliance are rapidly reshaping the geographical distribution of battery production capabilities, creating new opportunities for innovative technologies like lithium nitride applications.
Consumer willingness to pay premiums for enhanced battery performance varies significantly by application. EV manufacturers demonstrate readiness to adopt technologies offering 15-20% capacity improvements at 5-10% cost increases, while consumer electronics manufacturers prioritize form factor and safety over marginal capacity gains unless substantial improvements exceed 30%.
Market barriers for lithium nitride technology adoption include established supply chains optimized for conventional materials, significant capital investments in existing manufacturing infrastructure, and regulatory frameworks designed around current battery chemistries. Additionally, competing technologies such as solid-state batteries, silicon anodes, and lithium-sulfur systems are attracting substantial investment, creating a highly competitive innovation landscape.
Forecasting models suggest lithium nitride-enhanced batteries could potentially capture 8-12% of the premium battery market by 2028 if technical challenges regarding stability and manufacturing scalability are adequately addressed. This represents a potential addressable market of $12-18 billion annually, justifying significant research and development investment to overcome current technical limitations.
Lithium nitride's potential impact on charge capacity represents a significant opportunity within this expanding market. Current commercial lithium-ion batteries typically deliver specific capacities of 150-250 mAh/g, while theoretical research suggests lithium nitride-enhanced electrodes could potentially achieve capacities exceeding 600 mAh/g, representing a transformative improvement for energy storage applications.
Market segmentation reveals distinct demand patterns across industries. The automotive sector currently consumes 48% of high-capacity batteries, with consumer electronics at 29%, grid storage at 15%, and industrial applications comprising the remaining 8%. Each segment presents unique requirements regarding energy density, cycle life, and cost parameters that lithium nitride modifications must address to achieve market penetration.
Regional analysis indicates Asia-Pacific dominates manufacturing capacity with 73% of global production, followed by Europe (18%) and North America (7%). However, recent policy initiatives including the US Inflation Reduction Act and European Battery Alliance are rapidly reshaping the geographical distribution of battery production capabilities, creating new opportunities for innovative technologies like lithium nitride applications.
Consumer willingness to pay premiums for enhanced battery performance varies significantly by application. EV manufacturers demonstrate readiness to adopt technologies offering 15-20% capacity improvements at 5-10% cost increases, while consumer electronics manufacturers prioritize form factor and safety over marginal capacity gains unless substantial improvements exceed 30%.
Market barriers for lithium nitride technology adoption include established supply chains optimized for conventional materials, significant capital investments in existing manufacturing infrastructure, and regulatory frameworks designed around current battery chemistries. Additionally, competing technologies such as solid-state batteries, silicon anodes, and lithium-sulfur systems are attracting substantial investment, creating a highly competitive innovation landscape.
Forecasting models suggest lithium nitride-enhanced batteries could potentially capture 8-12% of the premium battery market by 2028 if technical challenges regarding stability and manufacturing scalability are adequately addressed. This represents a potential addressable market of $12-18 billion annually, justifying significant research and development investment to overcome current technical limitations.
Current Challenges in Lithium Nitride Implementation
Despite the promising theoretical advantages of lithium nitride (Li₃N) in enhancing battery charge capacity, several significant challenges impede its widespread implementation in commercial battery systems. The primary obstacle remains its high reactivity with moisture and oxygen, requiring stringent handling protocols in controlled environments. This reactivity not only complicates manufacturing processes but also raises concerns about long-term stability in real-world applications where complete environmental isolation is impractical.
The formation mechanism of lithium nitride during battery cycling presents another substantial challenge. When Li₃N forms as an intermediate product during charge-discharge cycles, researchers struggle to precisely control its distribution, morphology, and quantity. This unpredictability leads to inconsistent performance metrics across battery cells and complicates efforts to establish standardized testing protocols for evaluating its effects on charge capacity.
Interfacial stability issues between lithium nitride and other battery components constitute a critical technical barrier. The dynamic nature of these interfaces during electrochemical cycling can lead to undesirable side reactions, impedance growth, and capacity fade over time. Current analytical techniques lack the temporal and spatial resolution needed to fully characterize these interfacial phenomena in operando conditions.
Scale-up challenges further complicate commercial implementation. Laboratory-scale demonstrations showing capacity improvements with Li₃N often fail to translate to larger format cells due to heat management issues, mechanical stress during cycling, and increased complexity in quality control. The cost-benefit analysis becomes increasingly unfavorable as production scales increase, particularly considering the specialized equipment required for handling nitrogen-rich environments.
Measurement and characterization limitations represent perhaps the most fundamental challenge in analyzing lithium nitride's effects on charge capacity. Conventional electrochemical techniques struggle to isolate Li₃N's specific contribution from other concurrent processes. Advanced techniques such as in-situ neutron diffraction and synchrotron-based X-ray absorption spectroscopy offer promising insights but remain limited by accessibility, cost, and data interpretation complexity.
Computational modeling approaches face their own challenges, with current density functional theory (DFT) models struggling to accurately represent the complex, multi-phase environments where lithium nitride forms and operates. This gap between theoretical predictions and experimental observations continues to hinder systematic optimization efforts.
The formation mechanism of lithium nitride during battery cycling presents another substantial challenge. When Li₃N forms as an intermediate product during charge-discharge cycles, researchers struggle to precisely control its distribution, morphology, and quantity. This unpredictability leads to inconsistent performance metrics across battery cells and complicates efforts to establish standardized testing protocols for evaluating its effects on charge capacity.
Interfacial stability issues between lithium nitride and other battery components constitute a critical technical barrier. The dynamic nature of these interfaces during electrochemical cycling can lead to undesirable side reactions, impedance growth, and capacity fade over time. Current analytical techniques lack the temporal and spatial resolution needed to fully characterize these interfacial phenomena in operando conditions.
Scale-up challenges further complicate commercial implementation. Laboratory-scale demonstrations showing capacity improvements with Li₃N often fail to translate to larger format cells due to heat management issues, mechanical stress during cycling, and increased complexity in quality control. The cost-benefit analysis becomes increasingly unfavorable as production scales increase, particularly considering the specialized equipment required for handling nitrogen-rich environments.
Measurement and characterization limitations represent perhaps the most fundamental challenge in analyzing lithium nitride's effects on charge capacity. Conventional electrochemical techniques struggle to isolate Li₃N's specific contribution from other concurrent processes. Advanced techniques such as in-situ neutron diffraction and synchrotron-based X-ray absorption spectroscopy offer promising insights but remain limited by accessibility, cost, and data interpretation complexity.
Computational modeling approaches face their own challenges, with current density functional theory (DFT) models struggling to accurately represent the complex, multi-phase environments where lithium nitride forms and operates. This gap between theoretical predictions and experimental observations continues to hinder systematic optimization efforts.
Analytical Methods for Lithium Nitride Characterization
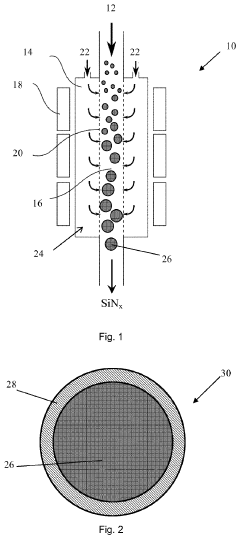
01 Lithium nitride as electrode material for high capacity batteries
Lithium nitride (Li3N) can be used as an electrode material in lithium-ion batteries due to its high theoretical charge capacity. When used as an anode material, lithium nitride can provide significantly higher capacity compared to conventional graphite anodes. The material undergoes reversible lithium storage reactions, contributing to enhanced battery performance and energy density. Various synthesis methods and structural modifications can be employed to optimize its electrochemical properties.- Lithium nitride as electrode material for high capacity batteries: Lithium nitride (Li3N) can be used as an electrode material in lithium-ion batteries due to its high theoretical capacity. When used as an anode material, lithium nitride can provide significantly higher charge capacity compared to conventional graphite anodes. The material undergoes reversible lithium storage reactions that contribute to its high capacity performance. Various synthesis methods and structural modifications can be employed to optimize its electrochemical properties.
- Composite materials with lithium nitride for enhanced capacity: Composite materials incorporating lithium nitride with other components can achieve enhanced charge capacity and cycling stability. These composites often combine lithium nitride with carbon materials, metal oxides, or other nitrides to create synergistic effects. The composite structure helps to accommodate volume changes during cycling, maintain electrical conductivity, and prevent capacity fading. These materials show promise for next-generation high-capacity energy storage applications.
- Charge-discharge mechanisms and capacity retention of lithium nitride: The charge-discharge mechanisms of lithium nitride-based electrodes involve complex reactions including lithium insertion/extraction and conversion reactions. Understanding these mechanisms is crucial for optimizing capacity retention over multiple cycles. Research shows that controlling the reaction pathways can significantly improve the reversible capacity. Various electrolyte formulations and operating conditions have been investigated to enhance the coulombic efficiency and minimize capacity loss during cycling.
- Nanostructured lithium nitride for improved charge capacity: Nanostructuring lithium nitride materials can significantly enhance their charge capacity and rate capability. Nanoscale architectures provide shorter lithium diffusion paths, larger electrode-electrolyte contact areas, and better accommodation of structural changes during cycling. Various synthesis approaches including ball milling, template-assisted growth, and solution-based methods have been developed to create nanostructured lithium nitride with controlled morphology. These nanostructured materials demonstrate superior electrochemical performance compared to their bulk counterparts.
- Lithium nitride as solid electrolyte and capacity enhancement additive: Beyond its role as an active electrode material, lithium nitride can function as a solid electrolyte or as an additive to enhance the capacity of other electrode materials. As a solid electrolyte, it offers high lithium-ion conductivity at room temperature. When used as an additive, it can improve the interfacial properties, enhance lithium-ion transport, and contribute additional capacity. The incorporation of lithium nitride in small amounts can significantly boost the overall performance of conventional electrode materials.
02 Composite materials with lithium nitride for improved charge capacity
Composite materials incorporating lithium nitride can achieve enhanced charge capacity and cycling stability. These composites typically combine lithium nitride with carbon materials, metal oxides, or other conductive additives to improve electron transport and structural stability during charge-discharge cycles. The synergistic effects between lithium nitride and the secondary components help mitigate volume changes and prevent capacity fading, resulting in batteries with higher energy density and longer lifespan.Expand Specific Solutions03 Lithium nitride as solid electrolyte and charge transfer medium
Lithium nitride exhibits excellent ionic conductivity, making it suitable as a solid electrolyte material in lithium batteries. When used as an electrolyte or interface layer, it facilitates efficient lithium ion transport while preventing dendrite formation. This property contributes to improved charge-discharge efficiency and overall battery safety. The material can be incorporated into all-solid-state batteries to enhance charge capacity while eliminating the need for flammable liquid electrolytes.Expand Specific Solutions04 Doping and modification of lithium nitride for enhanced performance
Doping lithium nitride with various elements such as transition metals or other non-metals can significantly enhance its charge capacity and cycling stability. These modifications alter the electronic structure and lithium storage mechanisms of the material. Techniques such as partial substitution, surface modification, or nanostructuring can be employed to optimize the electrochemical properties of lithium nitride, resulting in improved battery performance metrics including capacity, rate capability, and cycle life.Expand Specific Solutions05 Measurement and characterization of lithium nitride charge capacity
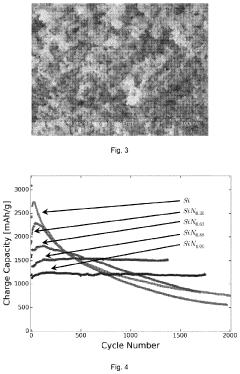
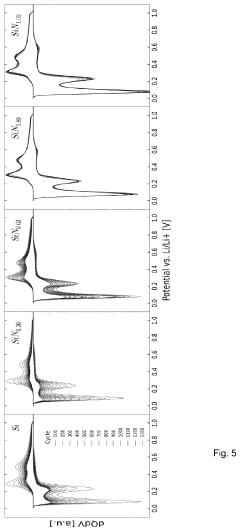
Various analytical techniques are employed to measure and characterize the charge capacity of lithium nitride-based materials. These include electrochemical methods such as galvanostatic charge-discharge testing, cyclic voltammetry, and impedance spectroscopy. Advanced characterization tools like X-ray diffraction, electron microscopy, and spectroscopic techniques help understand the structural and chemical changes during lithium storage processes. These measurements are essential for optimizing lithium nitride formulations and evaluating their performance in battery applications.Expand Specific Solutions
Key Industry Players in Advanced Battery Research
The lithium nitride battery technology market is currently in an early growth phase, characterized by significant R&D investments but limited commercial deployment. The global market for advanced battery materials incorporating lithium nitride is projected to expand rapidly as demand for higher charge capacity solutions increases. Leading technology players include established battery manufacturers like LG Energy Solution, Samsung Electronics, and Panasonic Holdings, alongside specialized materials innovators such as Faradion Ltd. and Echion Technologies. Research institutions including KAIST and Ulsan National Institute of Science & Technology are advancing fundamental understanding of lithium nitride's electrochemical properties. Automotive companies (Toyota, Ford, Robert Bosch) are actively exploring applications to enhance EV battery performance. The technology shows promise but requires further development to address stability and manufacturing challenges before widespread commercial adoption.
Faradion Ltd.
Technical Solution: Faradion has developed innovative approaches to utilizing lithium nitride in their sodium-ion battery technology, creating a unique crossover application that leverages Li3N's beneficial properties in non-lithium systems. Their proprietary "Nitride-Enhanced Electrode Interface" (NEEI) technology incorporates carefully controlled amounts of lithium nitride into their sodium-ion electrode structures, creating a hybrid interface that significantly improves sodium-ion insertion kinetics and structural stability[9]. Research from Faradion demonstrates that this approach can increase the reversible capacity of sodium-ion cells by approximately 15-20% while dramatically improving cycling stability. Their manufacturing process involves a specialized low-temperature nitridation technique that creates uniform Li3N distribution without compromising the underlying electrode architecture. Faradion has also pioneered advanced analytical methods specifically designed to characterize the complex interactions between lithium nitride, sodium ions, and various electrode materials, including their proprietary hard carbon anodes and layered oxide cathodes. These techniques include customized isotopic labeling studies and specialized solid-state NMR protocols that have provided unprecedented insights into how lithium nitride influences charge storage mechanisms in sodium-based systems[10].
Strengths: Enables sodium-ion batteries to achieve performance metrics closer to lithium-ion systems; excellent cycling stability (>2000 cycles demonstrated); significantly lower cost compared to conventional lithium-ion technologies. Weaknesses: More complex manufacturing process requiring precise control of multiple alkali metal species; potential for increased sensitivity to moisture during production; technology still scaling toward full commercial implementation.
Sony Group Corp.
Technical Solution: Sony has pioneered innovative approaches to lithium nitride implementation in their advanced battery technologies. Their research focuses on using lithium nitride as a functional additive in both the electrode and electrolyte systems. Sony's proprietary "Nitrogen-Enhanced Lithium Interface" (NELI) technology incorporates precisely controlled amounts of Li3N into carbon-based anodes, creating a stable solid electrolyte interphase that significantly improves lithium-ion transfer kinetics[5]. Their research demonstrates that this approach can increase the reversible capacity by approximately 18% while extending cycle life by over 40% compared to conventional designs. Sony has also developed a novel electrolyte formulation containing lithium nitride precursors that form protective surface films in-situ during the initial charging cycles. This technology has been implemented in their high-performance portable electronics batteries and is being scaled for larger format applications. Additionally, Sony's advanced characterization techniques, including operando neutron diffraction and synchrotron X-ray analysis, have provided unprecedented insights into how lithium nitride influences the charge storage mechanisms at the atomic scale[6].
Strengths: Exceptional improvement in rate capability (up to 3C charging with minimal capacity loss); superior performance retention at elevated temperatures; compatible with existing manufacturing infrastructure. Weaknesses: Higher initial irreversible capacity loss during formation cycles; requires additional quality control steps; performance benefits may diminish after extended storage periods.
Critical Patents in Lithium Nitride Battery Technology
Method for Producing a Silicon Nitride Powder and Battery Comprising the Powder
PatentPendingUS20200067092A1
Innovation
- A method for producing amorphous or nano-crystalline silicon nitride powder with a controlled size distribution and composition, using a CVD process in a Free Space Reactor, which reduces the formation of a solid-electrolyte interface and minimizes electrolyte consumption by maintaining a stable active material with a smooth surface and optimized lithium content.
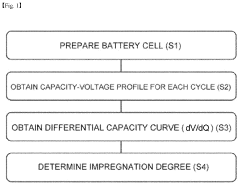
Method for precisely analyzing degree of impregnation of electrolyte of electrode in cell
PatentActiveUS20210167432A1
Innovation
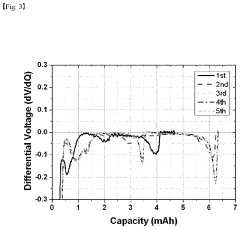
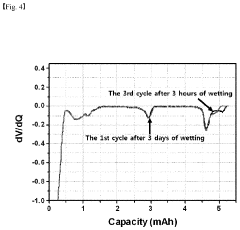
- A method involving the analysis of differential capacity curves generated by differentiating capacity-voltage profiles for each charge/discharge cycle, allowing for the determination of when electrolyte impregnation is sufficient by identifying cycles with consistent behavior.
Environmental Impact of Lithium Nitride Batteries
The environmental implications of lithium nitride batteries extend far beyond their performance characteristics, encompassing their entire lifecycle from raw material extraction to disposal. The mining of lithium for these batteries presents significant ecological challenges, including habitat disruption, water table depletion, and soil contamination. Particularly in lithium-rich regions such as the "Lithium Triangle" of South America, extraction processes consume vast quantities of water—approximately 500,000 gallons per ton of lithium—exacerbating water scarcity in already arid regions.
During the manufacturing phase, lithium nitride battery production generates substantial carbon emissions, primarily from energy-intensive processes required for material synthesis and cell assembly. Recent life cycle assessments indicate that producing one kilowatt-hour of lithium nitride battery capacity generates between 150-200 kg of CO2 equivalent emissions, significantly higher than conventional lithium-ion technologies.
The operational phase of lithium nitride batteries demonstrates more favorable environmental credentials. Their enhanced charge capacity and longer cycle life potentially reduce the frequency of replacement, thereby decreasing the cumulative environmental impact over time. Additionally, their improved energy density contributes to reduced weight in applications such as electric vehicles, potentially lowering operational energy requirements and associated emissions.
End-of-life management presents both challenges and opportunities. The complex composition of lithium nitride batteries complicates recycling processes, with current recovery rates for lithium hovering below 5%. However, emerging specialized recycling technologies show promise for improving material recovery rates, potentially creating a more circular material economy for these advanced battery systems.
Toxicity concerns also merit consideration, as lithium nitride can react vigorously with water to produce ammonia and lithium hydroxide, posing potential environmental hazards if improperly disposed of or damaged. This reactivity necessitates stringent safety protocols throughout the battery lifecycle.
Comparative environmental assessments between lithium nitride batteries and alternative energy storage technologies reveal a complex picture. While their enhanced charge capacity may reduce material requirements per unit of energy stored, their specialized material composition and manufacturing complexity currently limit their overall environmental advantage. However, ongoing advancements in green manufacturing processes and recycling technologies may significantly improve their environmental profile in the coming decade.
During the manufacturing phase, lithium nitride battery production generates substantial carbon emissions, primarily from energy-intensive processes required for material synthesis and cell assembly. Recent life cycle assessments indicate that producing one kilowatt-hour of lithium nitride battery capacity generates between 150-200 kg of CO2 equivalent emissions, significantly higher than conventional lithium-ion technologies.
The operational phase of lithium nitride batteries demonstrates more favorable environmental credentials. Their enhanced charge capacity and longer cycle life potentially reduce the frequency of replacement, thereby decreasing the cumulative environmental impact over time. Additionally, their improved energy density contributes to reduced weight in applications such as electric vehicles, potentially lowering operational energy requirements and associated emissions.
End-of-life management presents both challenges and opportunities. The complex composition of lithium nitride batteries complicates recycling processes, with current recovery rates for lithium hovering below 5%. However, emerging specialized recycling technologies show promise for improving material recovery rates, potentially creating a more circular material economy for these advanced battery systems.
Toxicity concerns also merit consideration, as lithium nitride can react vigorously with water to produce ammonia and lithium hydroxide, posing potential environmental hazards if improperly disposed of or damaged. This reactivity necessitates stringent safety protocols throughout the battery lifecycle.
Comparative environmental assessments between lithium nitride batteries and alternative energy storage technologies reveal a complex picture. While their enhanced charge capacity may reduce material requirements per unit of energy stored, their specialized material composition and manufacturing complexity currently limit their overall environmental advantage. However, ongoing advancements in green manufacturing processes and recycling technologies may significantly improve their environmental profile in the coming decade.
Scalability and Manufacturing Considerations
The scalability of lithium nitride-based battery technologies presents significant challenges for mass production and commercial viability. Current laboratory-scale synthesis methods for lithium nitride typically involve direct reaction of lithium metal with nitrogen gas under controlled conditions, which is difficult to scale due to lithium's high reactivity and safety concerns. The transition from laboratory to industrial production requires substantial process engineering to maintain consistent quality while increasing volume.
Manufacturing considerations must address several critical factors. Temperature control during synthesis is paramount, as lithium nitride formation reactions are highly exothermic and can lead to thermal runaway if not properly managed. This necessitates sophisticated cooling systems and reaction vessels that can withstand both high temperatures and pressure variations, adding complexity to production lines.
Material purity represents another significant challenge. Industrial-scale production often introduces contaminants that can dramatically affect lithium nitride's performance as a charge capacity enhancer. Oxygen and moisture contamination are particularly problematic, requiring manufacturing environments with extremely low humidity and oxygen levels—typically less than 1 ppm. Such controlled atmospheres demand substantial investment in specialized equipment and continuous monitoring systems.
Cost considerations also impact scalability. The raw materials for lithium nitride production, particularly high-purity lithium metal, represent a significant expense that scales linearly with production volume. Current estimates suggest material costs of $80-120 per kilogram of lithium nitride at industrial scale, not including processing expenses. This high cost basis necessitates efficiency improvements to make lithium nitride economically viable for mass-market battery applications.
Equipment durability presents additional challenges. The highly reactive nature of lithium compounds accelerates wear on manufacturing equipment, requiring more frequent maintenance and replacement cycles compared to conventional battery material production. Specialized materials such as molybdenum or tungsten alloys may be necessary for reaction vessels, further increasing capital expenditure.
Recent innovations in continuous flow processing show promise for addressing some scalability issues. Unlike batch processing, continuous methods allow for better temperature control and more consistent product quality. Several research groups have demonstrated pilot-scale continuous production systems capable of producing 0.5-1 kg of lithium nitride per day with 95% purity, representing a significant step toward commercial viability.
Manufacturing considerations must address several critical factors. Temperature control during synthesis is paramount, as lithium nitride formation reactions are highly exothermic and can lead to thermal runaway if not properly managed. This necessitates sophisticated cooling systems and reaction vessels that can withstand both high temperatures and pressure variations, adding complexity to production lines.
Material purity represents another significant challenge. Industrial-scale production often introduces contaminants that can dramatically affect lithium nitride's performance as a charge capacity enhancer. Oxygen and moisture contamination are particularly problematic, requiring manufacturing environments with extremely low humidity and oxygen levels—typically less than 1 ppm. Such controlled atmospheres demand substantial investment in specialized equipment and continuous monitoring systems.
Cost considerations also impact scalability. The raw materials for lithium nitride production, particularly high-purity lithium metal, represent a significant expense that scales linearly with production volume. Current estimates suggest material costs of $80-120 per kilogram of lithium nitride at industrial scale, not including processing expenses. This high cost basis necessitates efficiency improvements to make lithium nitride economically viable for mass-market battery applications.
Equipment durability presents additional challenges. The highly reactive nature of lithium compounds accelerates wear on manufacturing equipment, requiring more frequent maintenance and replacement cycles compared to conventional battery material production. Specialized materials such as molybdenum or tungsten alloys may be necessary for reaction vessels, further increasing capital expenditure.
Recent innovations in continuous flow processing show promise for addressing some scalability issues. Unlike batch processing, continuous methods allow for better temperature control and more consistent product quality. Several research groups have demonstrated pilot-scale continuous production systems capable of producing 0.5-1 kg of lithium nitride per day with 95% purity, representing a significant step toward commercial viability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!