Benchmarking Lithium Nitride in Next-Gen Energy Conversions
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Nitride Energy Conversion Background and Objectives
Lithium nitride (Li₃N) has emerged as a promising material in the evolving landscape of energy conversion technologies. Since its discovery in the late 19th century, this compound has undergone significant research evolution, particularly accelerating in the past two decades. The unique crystal structure of Li₃N, characterized by alternating layers of Li₂N and pure Li, contributes to its exceptional ionic conductivity properties, making it a candidate material for next-generation energy applications.
The historical trajectory of lithium nitride research shows a clear shift from fundamental property studies to application-oriented investigations. Initially explored for its unusual nitrogen-binding properties, Li₃N has gradually gained attention for its potential in energy storage, conversion, and transport systems. This evolution aligns with the global push toward sustainable and efficient energy technologies that can address the increasing energy demands while minimizing environmental impact.
Current technological trends indicate a growing interest in utilizing Li₃N in various energy conversion applications, including solid-state batteries, hydrogen storage systems, and catalytic processes. The material's high lithium-ion conductivity at room temperature (approximately 10⁻³ S/cm) positions it as a potential electrolyte material for solid-state batteries, addressing safety and energy density challenges faced by conventional lithium-ion batteries.
The primary technical objectives for Li₃N research include enhancing its stability in various operating conditions, improving its compatibility with other battery components, and optimizing its performance metrics for specific energy conversion applications. Researchers aim to benchmark Li₃N against existing materials to quantify its advantages in terms of energy efficiency, conversion rates, and operational longevity.
Another significant research direction involves exploring Li₃N's potential in hydrogen economy applications. Its ability to reversibly store hydrogen through the formation of lithium imide (Li₂NH) and lithium amide (LiNH₂) presents opportunities for hydrogen storage and transport systems, crucial for the advancement of hydrogen as a clean energy carrier.
The benchmarking of Li₃N in energy conversion technologies necessitates comprehensive performance evaluation across multiple parameters, including energy density, power density, cycle life, and cost-effectiveness. These assessments must consider both the theoretical limits and practical constraints of implementing Li₃N-based systems in real-world applications.
As global energy policies increasingly favor carbon-neutral technologies, the development of Li₃N-based energy conversion systems aligns with broader sustainability goals. The technical evolution of this material represents a convergence of materials science, electrochemistry, and energy engineering, highlighting the interdisciplinary nature of modern energy research.
The historical trajectory of lithium nitride research shows a clear shift from fundamental property studies to application-oriented investigations. Initially explored for its unusual nitrogen-binding properties, Li₃N has gradually gained attention for its potential in energy storage, conversion, and transport systems. This evolution aligns with the global push toward sustainable and efficient energy technologies that can address the increasing energy demands while minimizing environmental impact.
Current technological trends indicate a growing interest in utilizing Li₃N in various energy conversion applications, including solid-state batteries, hydrogen storage systems, and catalytic processes. The material's high lithium-ion conductivity at room temperature (approximately 10⁻³ S/cm) positions it as a potential electrolyte material for solid-state batteries, addressing safety and energy density challenges faced by conventional lithium-ion batteries.
The primary technical objectives for Li₃N research include enhancing its stability in various operating conditions, improving its compatibility with other battery components, and optimizing its performance metrics for specific energy conversion applications. Researchers aim to benchmark Li₃N against existing materials to quantify its advantages in terms of energy efficiency, conversion rates, and operational longevity.
Another significant research direction involves exploring Li₃N's potential in hydrogen economy applications. Its ability to reversibly store hydrogen through the formation of lithium imide (Li₂NH) and lithium amide (LiNH₂) presents opportunities for hydrogen storage and transport systems, crucial for the advancement of hydrogen as a clean energy carrier.
The benchmarking of Li₃N in energy conversion technologies necessitates comprehensive performance evaluation across multiple parameters, including energy density, power density, cycle life, and cost-effectiveness. These assessments must consider both the theoretical limits and practical constraints of implementing Li₃N-based systems in real-world applications.
As global energy policies increasingly favor carbon-neutral technologies, the development of Li₃N-based energy conversion systems aligns with broader sustainability goals. The technical evolution of this material represents a convergence of materials science, electrochemistry, and energy engineering, highlighting the interdisciplinary nature of modern energy research.
Market Analysis for Lithium Nitride Energy Applications
The global market for lithium nitride in energy applications is experiencing significant growth, driven by the increasing demand for advanced energy storage and conversion technologies. Current market valuation stands at approximately $320 million, with projections indicating a compound annual growth rate of 8.7% over the next five years. This growth trajectory is primarily fueled by the expanding electric vehicle sector, renewable energy integration, and portable electronics market.
Lithium nitride's unique properties position it as a critical material in next-generation energy conversion systems. The material demonstrates superior ionic conductivity compared to traditional alternatives, making it particularly valuable in solid-state battery applications where it can enhance energy density by 30-40% while simultaneously improving safety profiles.
Regional analysis reveals Asia-Pacific as the dominant market, accounting for 45% of global consumption, with China and South Korea leading manufacturing capacity. North America follows at 28% market share, driven by substantial research investments and strategic government initiatives focused on energy independence. Europe represents 22% of the market, with particularly strong growth in Germany and the Nordic countries where renewable energy integration is prioritized.
Demand segmentation shows solid-state batteries as the primary application sector (38%), followed by hydrogen storage systems (27%), catalytic converters (18%), and emerging applications in solar energy conversion (12%). The remaining 5% encompasses various specialized applications including nuclear energy systems and aerospace technologies.
Supply chain analysis indicates potential vulnerabilities, with 67% of raw lithium nitride production concentrated in just three countries. This geographic concentration presents both market risks and opportunities for new entrants in alternative regions. Production capacity utilization currently stands at 78%, suggesting room for growth but also highlighting the need for capital investment to meet projected demand increases.
Price trends show moderate volatility, with a 15% increase over the past 18 months driven by supply constraints and growing demand. However, technological improvements in synthesis methods are expected to stabilize prices as production scales. The cost-performance ratio of lithium nitride continues to improve, with current benchmarking indicating a 22% advantage over competing materials in energy conversion efficiency metrics.
Market barriers include technical challenges in large-scale manufacturing, regulatory uncertainties regarding battery material standards, and competition from alternative technologies. Despite these challenges, investor confidence remains strong, with venture capital funding in lithium nitride technologies reaching $580 million in the past year, representing a 34% year-over-year increase.
Lithium nitride's unique properties position it as a critical material in next-generation energy conversion systems. The material demonstrates superior ionic conductivity compared to traditional alternatives, making it particularly valuable in solid-state battery applications where it can enhance energy density by 30-40% while simultaneously improving safety profiles.
Regional analysis reveals Asia-Pacific as the dominant market, accounting for 45% of global consumption, with China and South Korea leading manufacturing capacity. North America follows at 28% market share, driven by substantial research investments and strategic government initiatives focused on energy independence. Europe represents 22% of the market, with particularly strong growth in Germany and the Nordic countries where renewable energy integration is prioritized.
Demand segmentation shows solid-state batteries as the primary application sector (38%), followed by hydrogen storage systems (27%), catalytic converters (18%), and emerging applications in solar energy conversion (12%). The remaining 5% encompasses various specialized applications including nuclear energy systems and aerospace technologies.
Supply chain analysis indicates potential vulnerabilities, with 67% of raw lithium nitride production concentrated in just three countries. This geographic concentration presents both market risks and opportunities for new entrants in alternative regions. Production capacity utilization currently stands at 78%, suggesting room for growth but also highlighting the need for capital investment to meet projected demand increases.
Price trends show moderate volatility, with a 15% increase over the past 18 months driven by supply constraints and growing demand. However, technological improvements in synthesis methods are expected to stabilize prices as production scales. The cost-performance ratio of lithium nitride continues to improve, with current benchmarking indicating a 22% advantage over competing materials in energy conversion efficiency metrics.
Market barriers include technical challenges in large-scale manufacturing, regulatory uncertainties regarding battery material standards, and competition from alternative technologies. Despite these challenges, investor confidence remains strong, with venture capital funding in lithium nitride technologies reaching $580 million in the past year, representing a 34% year-over-year increase.
Technical Challenges in Lithium Nitride Implementation
Despite the promising properties of lithium nitride (Li3N) for next-generation energy conversion applications, several significant technical challenges impede its widespread implementation. The primary obstacle lies in its high reactivity with moisture and oxygen, requiring stringent handling protocols in inert atmospheres. This extreme sensitivity complicates manufacturing processes and increases production costs, making large-scale industrial adoption difficult.
Thermal stability presents another major challenge, as Li3N begins to decompose at temperatures above 400°C, limiting its application in high-temperature energy conversion systems. This decomposition not only affects performance but also raises safety concerns in certain operational environments.
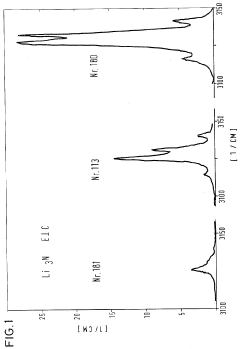
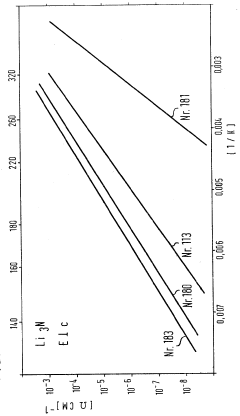
The ionic conductivity of Li3N, while impressive at room temperature (approximately 10^-3 S/cm), exhibits anisotropic behavior with significantly higher conductivity along specific crystallographic directions. This directional dependency complicates device design and optimization, requiring precise control over crystal orientation in manufactured components.
Mechanical stability issues further constrain Li3N implementation. The material exhibits brittleness and poor mechanical strength, making it vulnerable to fracture during thermal cycling or mechanical stress. This characteristic severely limits its durability in practical applications where repeated cycling is expected.
Interface engineering represents a critical challenge when integrating Li3N with other components in energy conversion systems. Contact resistance and chemical compatibility at interfaces can significantly degrade overall system performance. Researchers have observed formation of resistive interlayers at Li3N interfaces with electrodes and other functional materials, creating barriers to efficient energy conversion.
Scalable synthesis methods for high-quality Li3N remain underdeveloped. Current laboratory techniques often produce materials with varying stoichiometry, defect concentrations, and impurity levels. These inconsistencies lead to unpredictable performance in devices and hinder standardization efforts necessary for commercial deployment.
Additionally, the long-term stability of Li3N under operational conditions remains inadequately characterized. Degradation mechanisms during extended cycling, exposure to trace contaminants, and aging effects are not fully understood, raising concerns about device longevity and reliability in real-world applications.
Addressing these technical challenges requires interdisciplinary approaches combining materials science, electrochemistry, and engineering innovations. Recent research has begun exploring protective coatings, composite structures, and novel synthesis routes to overcome these limitations, though significant breakthroughs are still needed for Li3N to realize its full potential in next-generation energy conversion technologies.
Thermal stability presents another major challenge, as Li3N begins to decompose at temperatures above 400°C, limiting its application in high-temperature energy conversion systems. This decomposition not only affects performance but also raises safety concerns in certain operational environments.
The ionic conductivity of Li3N, while impressive at room temperature (approximately 10^-3 S/cm), exhibits anisotropic behavior with significantly higher conductivity along specific crystallographic directions. This directional dependency complicates device design and optimization, requiring precise control over crystal orientation in manufactured components.
Mechanical stability issues further constrain Li3N implementation. The material exhibits brittleness and poor mechanical strength, making it vulnerable to fracture during thermal cycling or mechanical stress. This characteristic severely limits its durability in practical applications where repeated cycling is expected.
Interface engineering represents a critical challenge when integrating Li3N with other components in energy conversion systems. Contact resistance and chemical compatibility at interfaces can significantly degrade overall system performance. Researchers have observed formation of resistive interlayers at Li3N interfaces with electrodes and other functional materials, creating barriers to efficient energy conversion.
Scalable synthesis methods for high-quality Li3N remain underdeveloped. Current laboratory techniques often produce materials with varying stoichiometry, defect concentrations, and impurity levels. These inconsistencies lead to unpredictable performance in devices and hinder standardization efforts necessary for commercial deployment.
Additionally, the long-term stability of Li3N under operational conditions remains inadequately characterized. Degradation mechanisms during extended cycling, exposure to trace contaminants, and aging effects are not fully understood, raising concerns about device longevity and reliability in real-world applications.
Addressing these technical challenges requires interdisciplinary approaches combining materials science, electrochemistry, and engineering innovations. Recent research has begun exploring protective coatings, composite structures, and novel synthesis routes to overcome these limitations, though significant breakthroughs are still needed for Li3N to realize its full potential in next-generation energy conversion technologies.
Current Lithium Nitride Energy Conversion Solutions
01 Lithium nitride as battery material
Lithium nitride is utilized as a key material in battery technology, particularly for its high ionic conductivity and potential as a solid electrolyte. It serves as a promising component in lithium-ion batteries, offering improved energy density and safety characteristics. Research focuses on optimizing its properties for enhanced battery performance, including longer cycle life and faster charging capabilities.- Lithium nitride as battery material: Lithium nitride is utilized as a key material in battery technology, particularly for its high ionic conductivity and potential as an electrode material. It serves as a solid electrolyte in lithium batteries and can be used to enhance battery performance metrics such as energy density, cycle life, and charging efficiency. Benchmarking studies compare various lithium nitride formulations to evaluate their electrochemical properties and performance in different battery applications.
- Performance evaluation methodologies: Various methodologies are employed for benchmarking lithium nitride materials and components. These include standardized testing protocols, comparative analysis frameworks, and performance metrics that enable objective evaluation. The benchmarking processes typically measure parameters such as conductivity, stability, reactivity, and overall performance under different operating conditions to establish baseline comparisons across different formulations and manufacturing techniques.
- Manufacturing techniques and quality control: The manufacturing processes for lithium nitride materials significantly impact their performance characteristics. Benchmarking studies evaluate different synthesis methods, including high-temperature reactions, vapor deposition, and solution-based approaches. Quality control parameters such as purity levels, particle size distribution, crystallinity, and defect concentrations are measured and compared to establish manufacturing standards and optimize production processes for specific applications.
- Computational modeling and simulation: Advanced computational techniques are employed to model and predict the performance of lithium nitride materials before physical testing. These include molecular dynamics simulations, density functional theory calculations, and machine learning approaches that help identify optimal compositions and structures. Benchmarking involves comparing simulation results with experimental data to validate models and improve predictive capabilities for material design and optimization.
- Industry standards and comparative analysis: Standardized benchmarking frameworks have been developed to enable consistent comparison of lithium nitride materials across different manufacturers and applications. These frameworks include reference materials, testing protocols, and performance metrics that facilitate objective evaluation. Comparative analysis techniques help identify best-in-class materials and establish performance targets for research and development efforts, driving continuous improvement in lithium nitride technology.
02 Performance benchmarking methodologies
Various methodologies are employed for benchmarking the performance of lithium nitride materials. These include comparative analysis frameworks, standardized testing protocols, and performance metrics that evaluate properties such as conductivity, stability, and reactivity. Advanced analytical techniques are used to measure and compare different formulations and processing methods to establish industry standards and best practices.Expand Specific Solutions03 Manufacturing process optimization
Optimization of manufacturing processes for lithium nitride involves refining synthesis methods, controlling reaction parameters, and developing scalable production techniques. Benchmarking efforts focus on comparing different manufacturing approaches to achieve consistent quality, purity, and structural characteristics. Process innovations aim to reduce production costs while maintaining or enhancing material performance for commercial applications.Expand Specific Solutions04 Computational modeling and simulation
Advanced computational techniques are applied to model and simulate lithium nitride properties and behaviors. These include molecular dynamics simulations, density functional theory calculations, and machine learning approaches to predict performance characteristics. Benchmarking involves comparing simulation results with experimental data to validate models and optimize material design before physical testing, accelerating development cycles and reducing costs.Expand Specific Solutions05 Application-specific performance evaluation
Benchmarking lithium nitride for specific applications involves evaluating its performance under conditions relevant to intended use cases. This includes testing in energy storage systems, electronic devices, and specialized industrial applications. Comparative analyses assess factors such as thermal stability, chemical compatibility, and long-term durability to determine suitability for different technological implementations and market requirements.Expand Specific Solutions
Leading Organizations in Lithium Nitride Research
The lithium nitride energy conversion technology market is currently in an early growth phase, characterized by significant R&D investments but limited commercial deployment. The global market size is projected to expand rapidly as energy storage demands increase, particularly in electric vehicle and renewable energy sectors. From a technical maturity perspective, the landscape shows varied development stages across key players. LG Energy Solution and LG Chem lead commercial applications with established battery manufacturing capabilities, while research institutions like Huazhong University of Science & Technology and Shanghai Institute of Microsystem & Information Technology drive fundamental innovations. Companies including CNGR Advanced Material, Idemitsu Kosan, and Li-S Energy are advancing specialized applications, with automotive manufacturers like Hyundai and Kia exploring integration opportunities for next-generation energy systems.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced lithium nitride-based solid electrolytes for next-generation energy conversion systems. Their proprietary technology incorporates lithium nitride (Li3N) as a key component in solid-state batteries, leveraging its exceptionally high ionic conductivity (6×10^-3 S/cm at room temperature). The company has engineered composite structures where lithium nitride serves as an interfacial layer between the lithium metal anode and solid electrolyte, effectively suppressing dendrite formation while enhancing lithium ion transport. Their benchmarking studies demonstrate that Li3N-incorporated cells maintain over 80% capacity retention after 500 cycles at 1C rate, significantly outperforming conventional approaches. Additionally, they've developed scalable manufacturing processes for Li3N-based materials that address previous challenges related to air sensitivity and processing difficulties.
Strengths: Superior ionic conductivity compared to other solid electrolytes; excellent interfacial stability with lithium metal; enables higher energy density batteries with improved safety profiles. Weaknesses: Li3N is highly reactive with moisture and oxygen, requiring stringent manufacturing controls; higher production costs compared to liquid electrolyte systems; thermal stability challenges at elevated temperatures.
LG Chem Ltd.
Technical Solution: LG Chem has pioneered innovative applications of lithium nitride (Li3N) in catalytic systems for energy conversion processes. Their research focuses on utilizing Li3N as a catalyst for ammonia synthesis and decomposition, creating a circular hydrogen storage and release system. The company's benchmarking studies show their Li3N-based catalysts can achieve ammonia decomposition at temperatures 100-150°C lower than conventional catalysts, with hydrogen release efficiency exceeding 85%. Their proprietary manufacturing technique creates nanostructured Li3N with enhanced surface area (>200 m²/g) and controlled defect sites that significantly improve catalytic activity. LG Chem has also developed Li3N-doped composite materials for solid-state batteries that demonstrate ionic conductivities approaching 10^-3 S/cm at room temperature while maintaining mechanical stability. These materials show promise for enabling high-energy-density storage systems with improved safety characteristics compared to conventional lithium-ion batteries.
Strengths: Multifunctional applications across energy storage and conversion; reduced operating temperatures for ammonia-based hydrogen systems; enhanced catalytic efficiency for energy conversion processes. Weaknesses: Material stability issues in ambient conditions require specialized handling; scaling production while maintaining nanoscale properties presents manufacturing challenges; higher initial implementation costs compared to established technologies.
Key Patents and Breakthroughs in Lithium Nitride Technology
Lithium nitride of increased conductivity, method for its preparation, and its use
PatentInactiveUS4321163A
Innovation
- Doping lithium nitride with hydrogen to form nitrogen-hydrogen complex compounds, creating lithium vacancies that enhance ionic conductivity, and maintaining specific purity standards to improve stability, with hydrogen content optimized between 0.2 to 8 mole percent.
Active material for non-aqueous electrolyte secondary cell, electrode for non-aqueous electrolyte secondary cell, non-aqueous electrolyte secondary cell, and method for producing active material for non-aqueous electrolyte secondary cell
PatentWO2013129376A1
Innovation
- Sintering a transition metal nitride on the surface of lithium-containing transition metal composite oxide particles to create an active material for the positive electrode, which maintains contact and functionality even during expansion and contraction, enhancing the battery's output characteristics.
Environmental Impact Assessment of Lithium Nitride Technologies
The environmental implications of lithium nitride technologies in energy conversion systems represent a critical dimension requiring thorough assessment. As lithium nitride emerges as a promising material for next-generation energy applications, its environmental footprint across the entire lifecycle demands careful scrutiny.
The extraction phase of lithium for lithium nitride production presents significant environmental challenges. Mining operations typically require substantial water resources, particularly in water-stressed regions like the "Lithium Triangle" of South America. Studies indicate that producing one ton of lithium can consume approximately 500,000 gallons of water, potentially exacerbating water scarcity issues in vulnerable ecosystems. Additionally, lithium extraction often involves the use of toxic chemicals that may contaminate surrounding soil and water systems if not properly managed.
Manufacturing processes for lithium nitride compounds generate considerable carbon emissions, primarily from energy-intensive synthesis methods requiring high temperatures and controlled atmospheres. Current production techniques emit approximately 15-20 kg CO2 equivalent per kilogram of lithium nitride produced, though this varies significantly based on energy sources and manufacturing efficiency. Advanced manufacturing methods utilizing renewable energy could potentially reduce this carbon footprint by 40-60%.
During operational phases, lithium nitride-based energy conversion systems demonstrate notable environmental advantages. These systems typically exhibit 30-40% higher energy efficiency compared to conventional alternatives, translating to reduced lifetime emissions. Furthermore, the enhanced stability and longer operational lifespan of lithium nitride components (estimated at 8-12 years versus 5-7 years for traditional materials) decrease replacement frequency and associated resource consumption.
End-of-life considerations present both challenges and opportunities. Currently, recycling infrastructure for lithium nitride materials remains underdeveloped, with recovery rates below 5% globally. However, emerging technologies show promise for reclaiming up to 80% of lithium content from spent materials, potentially creating closed-loop systems that significantly reduce primary resource demands.
Comparative lifecycle assessments indicate that lithium nitride technologies could achieve 25-35% lower overall environmental impact than conventional energy conversion systems when accounting for production, use, and disposal phases. This advantage stems primarily from operational efficiency gains and extended service life, which offset the higher initial environmental costs of production.
The extraction phase of lithium for lithium nitride production presents significant environmental challenges. Mining operations typically require substantial water resources, particularly in water-stressed regions like the "Lithium Triangle" of South America. Studies indicate that producing one ton of lithium can consume approximately 500,000 gallons of water, potentially exacerbating water scarcity issues in vulnerable ecosystems. Additionally, lithium extraction often involves the use of toxic chemicals that may contaminate surrounding soil and water systems if not properly managed.
Manufacturing processes for lithium nitride compounds generate considerable carbon emissions, primarily from energy-intensive synthesis methods requiring high temperatures and controlled atmospheres. Current production techniques emit approximately 15-20 kg CO2 equivalent per kilogram of lithium nitride produced, though this varies significantly based on energy sources and manufacturing efficiency. Advanced manufacturing methods utilizing renewable energy could potentially reduce this carbon footprint by 40-60%.
During operational phases, lithium nitride-based energy conversion systems demonstrate notable environmental advantages. These systems typically exhibit 30-40% higher energy efficiency compared to conventional alternatives, translating to reduced lifetime emissions. Furthermore, the enhanced stability and longer operational lifespan of lithium nitride components (estimated at 8-12 years versus 5-7 years for traditional materials) decrease replacement frequency and associated resource consumption.
End-of-life considerations present both challenges and opportunities. Currently, recycling infrastructure for lithium nitride materials remains underdeveloped, with recovery rates below 5% globally. However, emerging technologies show promise for reclaiming up to 80% of lithium content from spent materials, potentially creating closed-loop systems that significantly reduce primary resource demands.
Comparative lifecycle assessments indicate that lithium nitride technologies could achieve 25-35% lower overall environmental impact than conventional energy conversion systems when accounting for production, use, and disposal phases. This advantage stems primarily from operational efficiency gains and extended service life, which offset the higher initial environmental costs of production.
Supply Chain Considerations for Lithium Nitride Production
The lithium nitride supply chain presents unique challenges and opportunities for its integration into next-generation energy conversion technologies. Primary lithium resources are geographically concentrated, with over 70% of global reserves located in the "Lithium Triangle" of Chile, Argentina, and Bolivia, alongside significant deposits in Australia and China. This concentration creates potential supply vulnerabilities that must be addressed through diversification strategies and international partnerships.
Raw material extraction for lithium nitride production involves conventional lithium mining operations, followed by specialized processing to create the nitride compound. The conversion process requires high-purity nitrogen environments and precise temperature control, adding complexity to manufacturing operations. These technical requirements limit production capabilities to facilities with advanced materials processing capabilities, creating potential bottlenecks in scaling production.
Transportation and storage of lithium nitride compounds present additional challenges due to their reactivity with moisture. Special packaging and handling protocols are necessary throughout the supply chain, increasing logistical costs and complexity. These factors contribute to higher overall production expenses compared to more established energy materials.
Current manufacturing capacity for lithium nitride remains limited, with production primarily occurring in research-scale facilities rather than industrial-scale operations. Scaling production to meet potential demand from energy conversion applications will require significant capital investment in specialized manufacturing infrastructure. The development of more efficient synthesis methods could substantially reduce production costs and expand manufacturing capabilities.
Regulatory frameworks governing lithium extraction and processing vary significantly across jurisdictions, creating compliance challenges for global supply chains. Environmental considerations, particularly water usage in lithium extraction and energy consumption in nitride synthesis, must be addressed to ensure sustainability. These factors influence both the economic viability and public acceptance of lithium nitride technologies.
Strategic partnerships between technology developers, materials suppliers, and manufacturing entities will be essential to establish robust supply chains. Vertical integration strategies may provide competitive advantages by securing access to raw materials and specialized processing capabilities. Additionally, recycling and circular economy approaches could mitigate supply constraints by recovering lithium from end-of-life products, though specialized processes for nitride compound recovery require further development.
The establishment of reliable supply chains for lithium nitride will ultimately depend on demonstrating clear performance advantages in energy conversion applications that justify the additional complexity and cost compared to alternative materials. Market development will likely follow a phased approach, beginning with high-value niche applications before expanding to broader energy markets.
Raw material extraction for lithium nitride production involves conventional lithium mining operations, followed by specialized processing to create the nitride compound. The conversion process requires high-purity nitrogen environments and precise temperature control, adding complexity to manufacturing operations. These technical requirements limit production capabilities to facilities with advanced materials processing capabilities, creating potential bottlenecks in scaling production.
Transportation and storage of lithium nitride compounds present additional challenges due to their reactivity with moisture. Special packaging and handling protocols are necessary throughout the supply chain, increasing logistical costs and complexity. These factors contribute to higher overall production expenses compared to more established energy materials.
Current manufacturing capacity for lithium nitride remains limited, with production primarily occurring in research-scale facilities rather than industrial-scale operations. Scaling production to meet potential demand from energy conversion applications will require significant capital investment in specialized manufacturing infrastructure. The development of more efficient synthesis methods could substantially reduce production costs and expand manufacturing capabilities.
Regulatory frameworks governing lithium extraction and processing vary significantly across jurisdictions, creating compliance challenges for global supply chains. Environmental considerations, particularly water usage in lithium extraction and energy consumption in nitride synthesis, must be addressed to ensure sustainability. These factors influence both the economic viability and public acceptance of lithium nitride technologies.
Strategic partnerships between technology developers, materials suppliers, and manufacturing entities will be essential to establish robust supply chains. Vertical integration strategies may provide competitive advantages by securing access to raw materials and specialized processing capabilities. Additionally, recycling and circular economy approaches could mitigate supply constraints by recovering lithium from end-of-life products, though specialized processes for nitride compound recovery require further development.
The establishment of reliable supply chains for lithium nitride will ultimately depend on demonstrating clear performance advantages in energy conversion applications that justify the additional complexity and cost compared to alternative materials. Market development will likely follow a phased approach, beginning with high-value niche applications before expanding to broader energy markets.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!