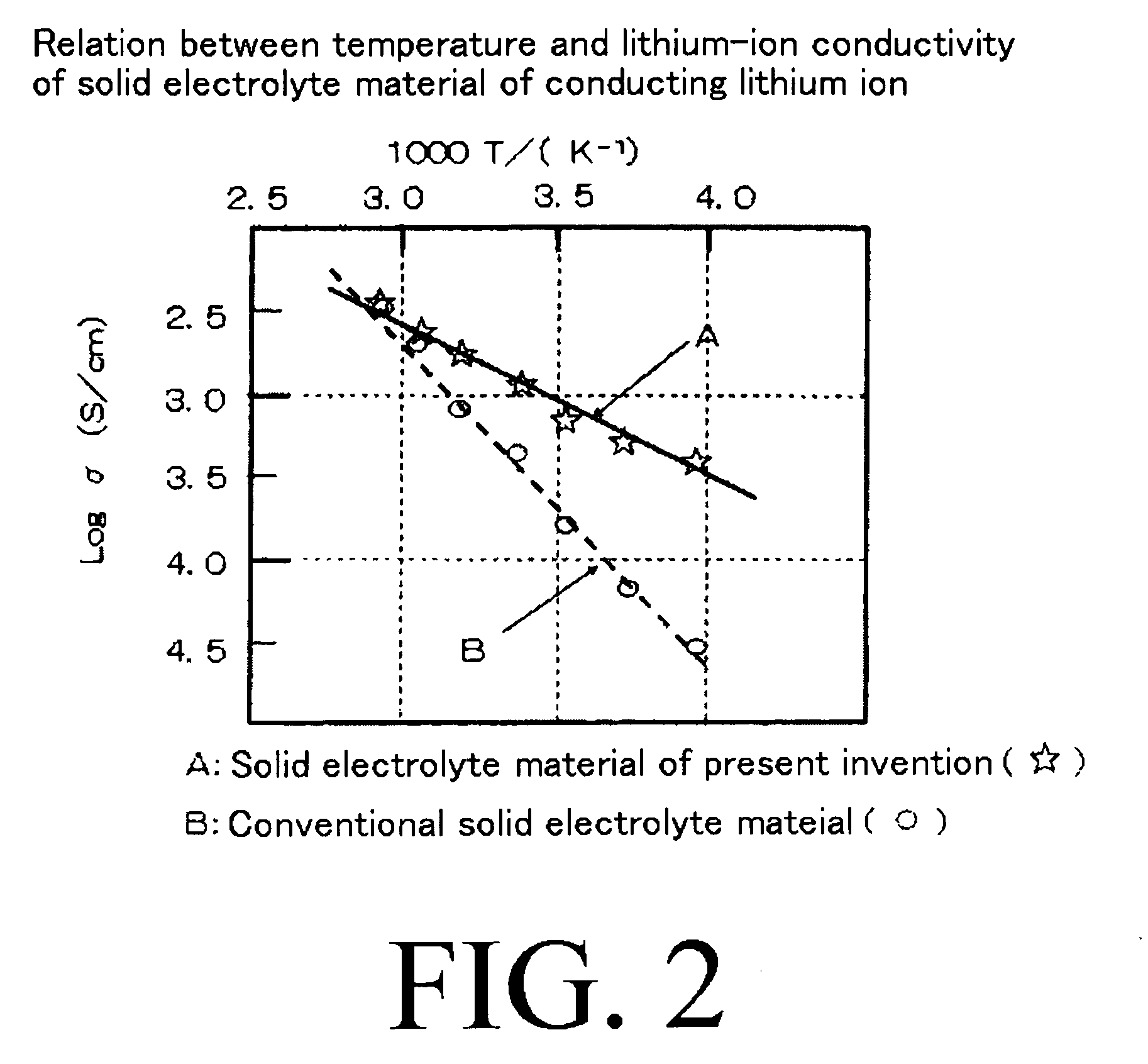
Lithium Nitride as Electrolyte: Ion Conductivity Analysis
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Li3N Electrolyte Background and Objectives
Lithium nitride (Li3N) has emerged as a promising solid-state electrolyte material for next-generation energy storage systems due to its exceptional lithium-ion conductivity properties. The development of Li3N as an electrolyte material can be traced back to the 1970s when researchers first discovered its high ionic conductivity at room temperature. Since then, significant advancements have been made in understanding its structural properties and ion transport mechanisms, positioning Li3N as a potential candidate for solid-state batteries.
The evolution of Li3N research has been characterized by several key milestones. Initially, the focus was on understanding its basic crystal structure and intrinsic conductivity properties. Later research shifted toward addressing stability issues, particularly its reactivity with electrode materials and sensitivity to moisture and air. Recent technological advances have enabled more sophisticated synthesis methods and characterization techniques, allowing for better control over material properties and performance optimization.
The primary technical objective of investigating Li3N as an electrolyte is to harness its exceptionally high lithium-ion conductivity, which approaches 10^-3 S/cm at room temperature—a value comparable to liquid electrolytes but without their safety concerns. This conductivity arises from the unique layered structure of Li3N, consisting of Li2N layers separated by Li+ ions, which facilitates rapid lithium-ion diffusion through the material.
Additional objectives include enhancing the electrochemical stability window of Li3N, which is currently limited to approximately 0.7-2.1V vs. Li/Li+. This narrow window restricts its application with high-voltage cathode materials. Researchers aim to extend this stability range through various strategies, including doping with other elements, creating composite structures, or developing protective interface layers.
Improving the mechanical properties and environmental stability of Li3N represents another critical goal. Pure Li3N is relatively soft and reactive with atmospheric components, limiting its practical application. Engineering approaches to address these challenges include the development of composite materials that maintain high ionic conductivity while enhancing mechanical strength and chemical stability.
The long-term vision for Li3N-based electrolytes extends beyond traditional lithium-ion batteries to include next-generation technologies such as lithium-sulfur and lithium-air batteries, where solid-state electrolytes could solve many existing challenges. Understanding the fundamental ion conductivity mechanisms in Li3N is expected to provide insights applicable to the broader field of solid-state ionics and contribute to the development of other advanced energy storage materials.
The evolution of Li3N research has been characterized by several key milestones. Initially, the focus was on understanding its basic crystal structure and intrinsic conductivity properties. Later research shifted toward addressing stability issues, particularly its reactivity with electrode materials and sensitivity to moisture and air. Recent technological advances have enabled more sophisticated synthesis methods and characterization techniques, allowing for better control over material properties and performance optimization.
The primary technical objective of investigating Li3N as an electrolyte is to harness its exceptionally high lithium-ion conductivity, which approaches 10^-3 S/cm at room temperature—a value comparable to liquid electrolytes but without their safety concerns. This conductivity arises from the unique layered structure of Li3N, consisting of Li2N layers separated by Li+ ions, which facilitates rapid lithium-ion diffusion through the material.
Additional objectives include enhancing the electrochemical stability window of Li3N, which is currently limited to approximately 0.7-2.1V vs. Li/Li+. This narrow window restricts its application with high-voltage cathode materials. Researchers aim to extend this stability range through various strategies, including doping with other elements, creating composite structures, or developing protective interface layers.
Improving the mechanical properties and environmental stability of Li3N represents another critical goal. Pure Li3N is relatively soft and reactive with atmospheric components, limiting its practical application. Engineering approaches to address these challenges include the development of composite materials that maintain high ionic conductivity while enhancing mechanical strength and chemical stability.
The long-term vision for Li3N-based electrolytes extends beyond traditional lithium-ion batteries to include next-generation technologies such as lithium-sulfur and lithium-air batteries, where solid-state electrolytes could solve many existing challenges. Understanding the fundamental ion conductivity mechanisms in Li3N is expected to provide insights applicable to the broader field of solid-state ionics and contribute to the development of other advanced energy storage materials.
Market Analysis for Solid-State Battery Electrolytes
The solid-state battery electrolyte market is experiencing unprecedented growth, driven by increasing demand for safer, higher-energy-density batteries across multiple industries. Current market valuations place the global solid-state battery market at approximately $0.5 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 34.2% through 2030, potentially reaching $3.4 billion by the end of the decade.
Lithium nitride-based electrolytes represent a specialized segment within this expanding market, currently occupying a relatively small but growing share. Their superior ionic conductivity properties—reaching 10^-3 to 10^-2 S/cm at room temperature—position them as particularly promising candidates for next-generation battery technologies.
The market demand for solid-state electrolytes is primarily segmented across three major application sectors. The electric vehicle industry constitutes the largest market share at approximately 45%, driven by automotive manufacturers' aggressive electrification roadmaps and safety concerns regarding conventional lithium-ion batteries. Consumer electronics follows at 30%, with manufacturers seeking higher energy density solutions for portable devices. The remaining 25% encompasses energy storage systems, aerospace applications, and medical devices.
Regional analysis reveals Asia-Pacific as the dominant market for solid-state electrolyte development, accounting for 52% of global research and commercial activities. Japan and South Korea lead in patent filings related to lithium nitride electrolytes, while China demonstrates the fastest growth rate in manufacturing capacity. North America represents 28% of the market, with significant research contributions from national laboratories and university consortia, particularly in advanced lithium nitride synthesis techniques.
Market barriers for lithium nitride electrolytes include high production costs, estimated at 8-10 times that of conventional liquid electrolytes, and scalability challenges in maintaining consistent ion conductivity properties during mass production. Additionally, the material's sensitivity to moisture necessitates specialized handling and manufacturing environments, increasing production complexity.
Customer adoption analysis indicates that battery manufacturers require ionic conductivity exceeding 10^-4 S/cm at room temperature as a minimum threshold for commercial viability, a benchmark that advanced lithium nitride formulations have successfully achieved. However, cycle life requirements of >1,000 cycles remain challenging for current lithium nitride electrolyte implementations.
The competitive landscape features established materials science corporations investing in lithium nitride research alongside specialized startups focused exclusively on solid-state electrolyte development. Strategic partnerships between electrolyte developers and battery manufacturers have increased by 65% since 2020, indicating growing commercial interest in lithium nitride and related technologies.
Lithium nitride-based electrolytes represent a specialized segment within this expanding market, currently occupying a relatively small but growing share. Their superior ionic conductivity properties—reaching 10^-3 to 10^-2 S/cm at room temperature—position them as particularly promising candidates for next-generation battery technologies.
The market demand for solid-state electrolytes is primarily segmented across three major application sectors. The electric vehicle industry constitutes the largest market share at approximately 45%, driven by automotive manufacturers' aggressive electrification roadmaps and safety concerns regarding conventional lithium-ion batteries. Consumer electronics follows at 30%, with manufacturers seeking higher energy density solutions for portable devices. The remaining 25% encompasses energy storage systems, aerospace applications, and medical devices.
Regional analysis reveals Asia-Pacific as the dominant market for solid-state electrolyte development, accounting for 52% of global research and commercial activities. Japan and South Korea lead in patent filings related to lithium nitride electrolytes, while China demonstrates the fastest growth rate in manufacturing capacity. North America represents 28% of the market, with significant research contributions from national laboratories and university consortia, particularly in advanced lithium nitride synthesis techniques.
Market barriers for lithium nitride electrolytes include high production costs, estimated at 8-10 times that of conventional liquid electrolytes, and scalability challenges in maintaining consistent ion conductivity properties during mass production. Additionally, the material's sensitivity to moisture necessitates specialized handling and manufacturing environments, increasing production complexity.
Customer adoption analysis indicates that battery manufacturers require ionic conductivity exceeding 10^-4 S/cm at room temperature as a minimum threshold for commercial viability, a benchmark that advanced lithium nitride formulations have successfully achieved. However, cycle life requirements of >1,000 cycles remain challenging for current lithium nitride electrolyte implementations.
The competitive landscape features established materials science corporations investing in lithium nitride research alongside specialized startups focused exclusively on solid-state electrolyte development. Strategic partnerships between electrolyte developers and battery manufacturers have increased by 65% since 2020, indicating growing commercial interest in lithium nitride and related technologies.
Current Status and Challenges in Li3N Conductivity
Lithium nitride (Li3N) has emerged as a promising solid-state electrolyte material due to its high ionic conductivity at room temperature, reaching approximately 10^-3 S/cm. This conductivity level positions Li3N among the most conductive lithium-ion solid electrolytes discovered to date. The material exhibits a unique crystal structure with alternating layers of Li2N and pure Li, which creates fast ion diffusion pathways primarily through a vacancy-mediated mechanism in the Li2N layers.
Despite its promising conductivity, the current state of Li3N as an electrolyte faces several significant challenges. The most critical limitation is its narrow electrochemical stability window, typically around 0.7-2.1V vs. Li/Li+. This narrow window severely restricts its application in high-voltage lithium batteries, as it undergoes decomposition when in contact with common cathode materials operating above 3V.
Another major challenge is Li3N's high chemical reactivity with moisture and oxygen in ambient conditions. The material rapidly degrades when exposed to air, forming lithium hydroxide and ammonia, which necessitates stringent handling protocols in inert atmospheres. This reactivity significantly complicates manufacturing processes and increases production costs for Li3N-based devices.
Mechanical properties present additional hurdles, as Li3N exhibits relatively poor mechanical strength and is prone to fracture under pressure. This characteristic limits its application in battery configurations requiring good mechanical stability, particularly in designs where electrode volume changes during cycling could induce stress on the electrolyte layer.
Recent research has focused on addressing these limitations through various approaches. Composite strategies involving the combination of Li3N with more stable materials have shown promise in expanding the electrochemical window while maintaining reasonable conductivity. Doping with elements such as Mg, Al, and Si has demonstrated improvements in both stability and conductivity in laboratory settings.
Interface engineering represents another active research direction, with protective layers being developed to mitigate the reactivity between Li3N and electrode materials. These interfaces aim to prevent direct contact while allowing efficient ion transport across boundaries.
The geographical distribution of Li3N research shows concentration in East Asia (particularly Japan and China), North America, and Europe, with collaborative international efforts increasing in recent years. Academic institutions lead fundamental research, while industrial R&D focuses on practical applications and manufacturing solutions to overcome the material's inherent limitations.
Despite its promising conductivity, the current state of Li3N as an electrolyte faces several significant challenges. The most critical limitation is its narrow electrochemical stability window, typically around 0.7-2.1V vs. Li/Li+. This narrow window severely restricts its application in high-voltage lithium batteries, as it undergoes decomposition when in contact with common cathode materials operating above 3V.
Another major challenge is Li3N's high chemical reactivity with moisture and oxygen in ambient conditions. The material rapidly degrades when exposed to air, forming lithium hydroxide and ammonia, which necessitates stringent handling protocols in inert atmospheres. This reactivity significantly complicates manufacturing processes and increases production costs for Li3N-based devices.
Mechanical properties present additional hurdles, as Li3N exhibits relatively poor mechanical strength and is prone to fracture under pressure. This characteristic limits its application in battery configurations requiring good mechanical stability, particularly in designs where electrode volume changes during cycling could induce stress on the electrolyte layer.
Recent research has focused on addressing these limitations through various approaches. Composite strategies involving the combination of Li3N with more stable materials have shown promise in expanding the electrochemical window while maintaining reasonable conductivity. Doping with elements such as Mg, Al, and Si has demonstrated improvements in both stability and conductivity in laboratory settings.
Interface engineering represents another active research direction, with protective layers being developed to mitigate the reactivity between Li3N and electrode materials. These interfaces aim to prevent direct contact while allowing efficient ion transport across boundaries.
The geographical distribution of Li3N research shows concentration in East Asia (particularly Japan and China), North America, and Europe, with collaborative international efforts increasing in recent years. Academic institutions lead fundamental research, while industrial R&D focuses on practical applications and manufacturing solutions to overcome the material's inherent limitations.
Current Approaches to Enhance Li3N Ionic Conductivity
01 Lithium nitride-based solid electrolytes for batteries
Lithium nitride (Li3N) and its derivatives serve as solid electrolytes in lithium batteries due to their high ionic conductivity. These materials facilitate lithium ion transport while blocking electron flow, making them suitable for all-solid-state batteries. Various compositions and structural modifications of lithium nitride compounds have been developed to enhance ionic conductivity while maintaining electrochemical stability, enabling improved battery performance and safety.- Lithium nitride-based solid electrolytes for batteries: Lithium nitride (Li3N) and its derivatives serve as solid electrolytes in lithium-ion batteries due to their high ionic conductivity. These materials facilitate lithium ion transport while blocking electron flow, making them suitable for all-solid-state batteries. The incorporation of lithium nitride in solid electrolytes enhances battery safety by eliminating flammable liquid electrolytes while maintaining high energy density and long cycle life.
- Doping strategies to enhance lithium nitride conductivity: Various doping strategies are employed to enhance the ionic conductivity of lithium nitride materials. Introducing elements such as magnesium, aluminum, or transition metals into the lithium nitride structure can create defects that facilitate faster lithium ion movement. These dopants modify the crystal structure and electronic properties, resulting in improved room-temperature ionic conductivity and electrochemical stability, which are critical for practical battery applications.
- Composite materials incorporating lithium nitride: Composite materials that incorporate lithium nitride with other components show enhanced ionic conductivity and mechanical properties. These composites often combine lithium nitride with polymers, ceramics, or other inorganic materials to create hybrid electrolytes with improved performance characteristics. The synergistic effects between lithium nitride and the secondary components result in electrolytes with better interfacial contact with electrodes, reduced grain boundary resistance, and enhanced overall battery performance.
- Thin film and interface engineering of lithium nitride conductors: Thin film lithium nitride conductors and interface engineering techniques are developed to optimize ion transport in solid-state batteries. These approaches focus on creating ultra-thin lithium nitride layers with controlled crystallinity and orientation to maximize ionic conductivity. Interface engineering between lithium nitride and electrode materials reduces contact resistance and improves electrochemical stability. Various deposition methods including sputtering, pulsed laser deposition, and atomic layer deposition are used to create these optimized structures.
- Novel lithium nitride-based structures for enhanced conductivity: Novel structures based on lithium nitride chemistry are being developed to achieve unprecedented levels of ionic conductivity. These include nanostructured materials, hierarchical architectures, and crystallographically engineered compounds that facilitate rapid lithium ion transport. Research focuses on controlling grain boundaries, creating preferential ion conduction pathways, and developing materials with three-dimensional ion conduction networks. These advanced structures aim to overcome the conductivity limitations of conventional lithium nitride while maintaining its advantages.
02 Doping strategies to enhance lithium nitride conductivity
Doping lithium nitride with various elements such as magnesium, aluminum, or transition metals significantly improves its ionic conductivity. These dopants create defects in the crystal structure that facilitate lithium ion movement. The concentration and type of dopant can be optimized to achieve conductivity values approaching those of liquid electrolytes while maintaining the mechanical and thermal stability advantages of solid materials.Expand Specific Solutions03 Fabrication methods for lithium nitride ion conductors
Various fabrication techniques have been developed to produce high-quality lithium nitride ion conductors, including solid-state reactions, ball milling, and thin film deposition methods. Processing parameters such as temperature, pressure, and atmosphere significantly affect the microstructure and resulting ionic conductivity. Advanced manufacturing approaches like atomic layer deposition and solution-based methods enable precise control over composition and morphology to optimize ion transport properties.Expand Specific Solutions04 Composite and interface engineering for lithium nitride conductors
Composite structures combining lithium nitride with other materials like polymers or ceramics can overcome limitations of single-phase conductors. These composites often exhibit enhanced mechanical properties and improved interfacial stability with electrodes. Interface engineering techniques reduce resistance at grain boundaries and electrode-electrolyte interfaces, which are critical bottlenecks for overall ionic conductivity in practical devices. Surface modifications and buffer layers help maintain stable interfaces during cycling.Expand Specific Solutions05 Applications of lithium nitride ion conductors beyond batteries
Beyond traditional battery applications, lithium nitride ion conductors find use in electrochromic devices, sensors, and energy conversion systems. Their unique combination of ionic conductivity and chemical stability makes them suitable for specialized applications like lithium isotope separation, nuclear technology, and hydrogen storage systems. Emerging applications include their use in flexible electronics and as components in solid-state capacitors where their ion transport properties provide advantages over conventional materials.Expand Specific Solutions
Leading Research Groups and Companies in Solid Electrolytes
The lithium nitride electrolyte market is in an early growth phase, characterized by intensive R&D activities rather than widespread commercialization. The global solid-state battery market, where this technology fits, is projected to reach $8-10 billion by 2030, with lithium nitride electrolytes representing a specialized segment. Leading automotive manufacturers (Toyota, BMW) and battery specialists (LG Energy Solution, Samsung SDI) are driving innovation alongside research institutions (University of Maryland, Kyoto University, NIMS). Toyota appears particularly advanced with multiple patents, while companies like PolyPlus Battery and Resonac are developing complementary technologies. The technology remains at TRL 4-6, with challenges in scaling production and improving room-temperature conductivity before mass commercialization becomes viable.
Toyota Motor Corp.
Technical Solution: Toyota has developed advanced lithium nitride-based solid electrolytes for next-generation batteries. Their approach involves creating composite materials that combine Li3N with other lithium-containing compounds to enhance ionic conductivity. Toyota's research has demonstrated that lithium nitride exhibits exceptionally high lithium-ion conductivity (>10^-3 S/cm at room temperature) when properly synthesized and incorporated into their proprietary solid-state battery architecture. The company has implemented specialized synthesis methods to control the crystallinity and grain boundary properties of Li3N, which are critical factors affecting ion transport. Their technology also addresses the reactivity issues of Li3N by developing protective interface layers that prevent unwanted side reactions while maintaining high ionic conductivity pathways.
Strengths: Toyota's approach achieves superior ionic conductivity compared to conventional solid electrolytes, enabling faster charging capabilities. Their protective interface technology effectively mitigates the high reactivity of Li3N with electrode materials. Weaknesses: The manufacturing process remains complex and costly for mass production, and long-term stability under various operating conditions still presents challenges.
National Institute for Materials Science IAI
Technical Solution: The National Institute for Materials Science (NIMS) has conducted extensive fundamental research on lithium nitride as an ionic conductor for solid-state batteries. Their approach combines advanced computational modeling with experimental validation to understand the atomic-scale mechanisms of lithium ion transport in Li3N structures. NIMS researchers have developed specialized synthesis methods that allow precise control over crystal orientation and defect concentration in Li3N, factors that significantly impact ionic conductivity. Their studies have revealed that lithium nitride can achieve exceptionally high ionic conductivity (up to 1.2×10^-3 S/cm at room temperature) when properly synthesized with optimized microstructure. NIMS has pioneered the use of advanced characterization techniques including in-situ neutron diffraction and synchrotron X-ray analysis to directly observe lithium ion migration pathways in Li3N under various conditions. This fundamental understanding has led to the development of novel doping strategies and composite formulations that enhance the stability of Li3N while maintaining its superior ionic conductivity. Their research has also explored the integration of Li3N into hierarchical structures with protective surface layers to mitigate its reactivity issues while preserving fast ion transport channels.
Strengths: NIMS' fundamental research provides deep scientific understanding of ion transport mechanisms in Li3N, enabling rational design of improved materials. Their characterization techniques offer unprecedented insights into structure-property relationships. Weaknesses: As a research institute, their focus is more on fundamental understanding rather than commercial implementation, and translating their findings to industrial scale production presents additional challenges.
Key Patents and Research on Li3N Electrolyte Materials
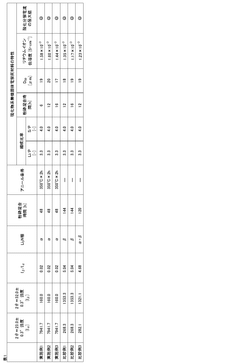
Lithium nitride composition for sulfide-based inorganic solid electrolyte material
PatentWO2020203046A1
Innovation
- A lithium nitride composition with a high proportion of α-type lithium nitride is used as a raw material to improve the lithium ion conductivity of sulfide-based inorganic solid electrolyte materials, achieved by controlling the diffraction intensity ratio of α-type to β-type lithium nitride phases and employing a manufacturing method involving nitriding, mechanical treatment, and annealing of lithium nitride foil.
Solid electrolyte material of conducting lithium ion, battery device using the solid electrolyte material and all-solid lithium secondary battery provided with the battery device
PatentActiveUS20090087751A1
Innovation
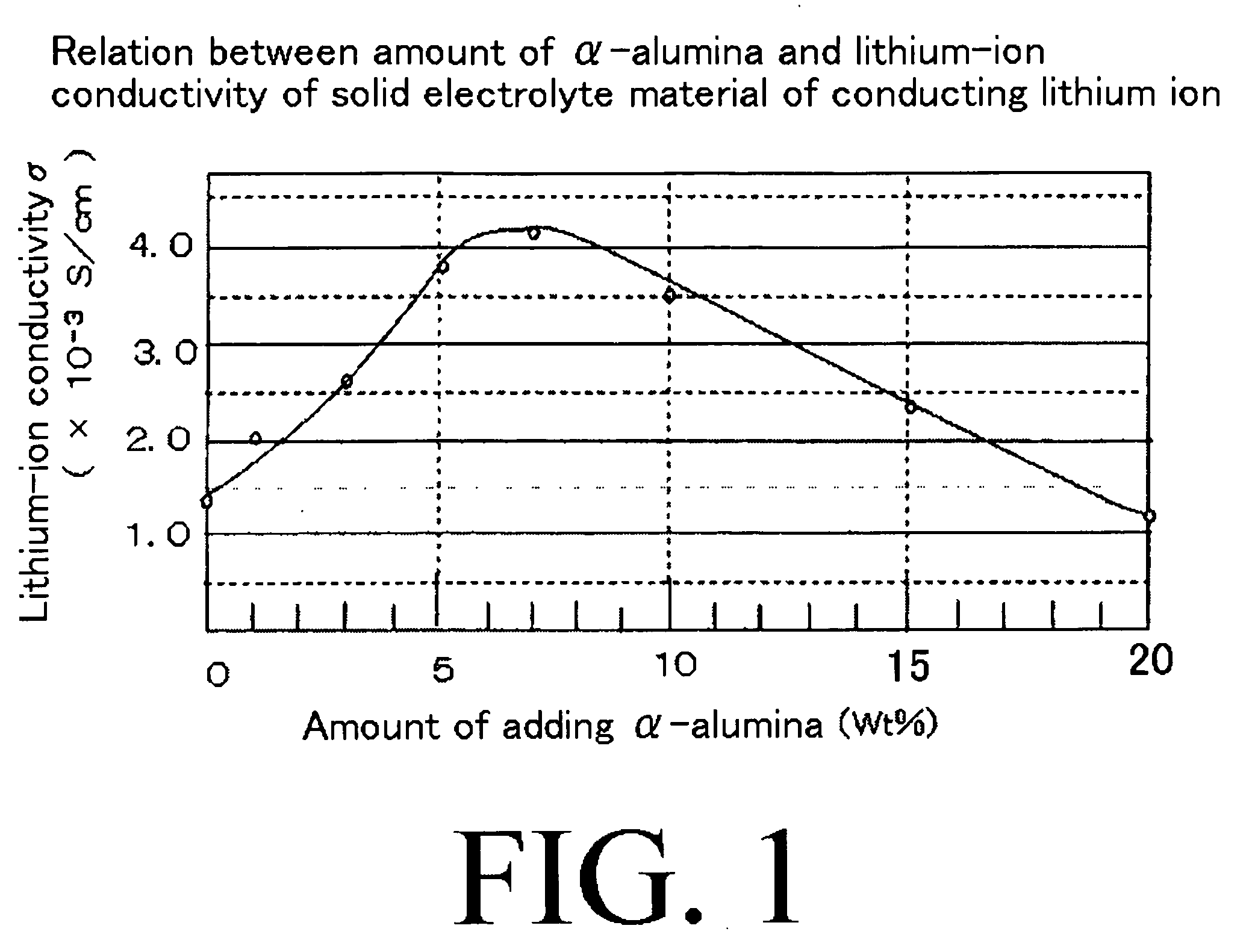
- A solid electrolyte material with superior lithium-ion conductivity is developed by mixing sulfide-based lithium-ion conductors with α-alumina, which forms new ion-conducting paths and includes an organic binder to improve strength and maintain conductivity, used in an all-solid lithium secondary battery configuration.
Safety and Stability Assessment of Li3N Electrolytes
Safety assessment of Li3N electrolytes reveals significant challenges despite their promising ion conductivity properties. The primary concern lies in the material's high reactivity with moisture and air, which can lead to the formation of ammonia gas and lithium hydroxide. This reaction not only degrades the electrolyte performance but also creates potential safety hazards in battery systems, particularly when exposed to ambient conditions during manufacturing or after cell damage.
Thermal stability testing indicates that Li3N begins to decompose at temperatures above 380°C, which provides a reasonable safety margin for normal battery operation. However, under abuse conditions such as short circuits or overcharging, the rapid temperature increase may trigger decomposition reactions, potentially compromising cell integrity. Calorimetric studies have demonstrated that the enthalpy of decomposition is substantial enough to contribute to thermal runaway scenarios if proper thermal management systems are not implemented.
Chemical compatibility with common electrode materials presents another critical challenge. Research has shown that Li3N can react with transition metal oxides commonly used in cathode materials, forming complex nitrides and potentially releasing oxygen, which could exacerbate thermal events. Interface stability studies using techniques such as X-ray photoelectron spectroscopy (XPS) and impedance spectroscopy reveal the formation of interfacial layers that may increase resistance over time.
Long-term cycling tests demonstrate that Li3N electrolytes can develop microcracks and increased porosity after repeated lithium ion transport, potentially creating pathways for dendrite growth. This structural degradation accelerates when cells are operated at higher current densities or elevated temperatures, limiting practical application in high-power devices. Computational modeling suggests that incorporating stabilizing additives or creating composite structures may mitigate some of these issues.
Environmental impact assessments indicate that while Li3N itself is not directly toxic, its decomposition products require careful handling and disposal protocols. The material's high reactivity necessitates specialized manufacturing environments with stringent moisture and oxygen controls, significantly increasing production costs and complexity compared to conventional liquid electrolytes.
Recent safety innovations have focused on encapsulation strategies to protect Li3N from environmental exposure, including core-shell architectures and protective coating technologies. These approaches show promise in laboratory settings but face challenges in scaling to commercial production. Regulatory compliance testing indicates that additional engineering controls will be necessary before Li3N-based batteries can meet transportation and consumer electronics safety standards.
Thermal stability testing indicates that Li3N begins to decompose at temperatures above 380°C, which provides a reasonable safety margin for normal battery operation. However, under abuse conditions such as short circuits or overcharging, the rapid temperature increase may trigger decomposition reactions, potentially compromising cell integrity. Calorimetric studies have demonstrated that the enthalpy of decomposition is substantial enough to contribute to thermal runaway scenarios if proper thermal management systems are not implemented.
Chemical compatibility with common electrode materials presents another critical challenge. Research has shown that Li3N can react with transition metal oxides commonly used in cathode materials, forming complex nitrides and potentially releasing oxygen, which could exacerbate thermal events. Interface stability studies using techniques such as X-ray photoelectron spectroscopy (XPS) and impedance spectroscopy reveal the formation of interfacial layers that may increase resistance over time.
Long-term cycling tests demonstrate that Li3N electrolytes can develop microcracks and increased porosity after repeated lithium ion transport, potentially creating pathways for dendrite growth. This structural degradation accelerates when cells are operated at higher current densities or elevated temperatures, limiting practical application in high-power devices. Computational modeling suggests that incorporating stabilizing additives or creating composite structures may mitigate some of these issues.
Environmental impact assessments indicate that while Li3N itself is not directly toxic, its decomposition products require careful handling and disposal protocols. The material's high reactivity necessitates specialized manufacturing environments with stringent moisture and oxygen controls, significantly increasing production costs and complexity compared to conventional liquid electrolytes.
Recent safety innovations have focused on encapsulation strategies to protect Li3N from environmental exposure, including core-shell architectures and protective coating technologies. These approaches show promise in laboratory settings but face challenges in scaling to commercial production. Regulatory compliance testing indicates that additional engineering controls will be necessary before Li3N-based batteries can meet transportation and consumer electronics safety standards.
Manufacturing Scalability and Cost Analysis
The scalability of lithium nitride (Li₃N) electrolyte manufacturing represents a critical factor in determining its commercial viability for next-generation battery technologies. Current production methods primarily rely on direct nitridation of lithium metal under nitrogen atmosphere at elevated temperatures (600-800°C), which presents significant challenges for mass production due to lithium's high reactivity with oxygen and moisture.
Industrial-scale manufacturing of Li₃N electrolytes faces several technical hurdles that impact cost structures. The requirement for strictly controlled inert atmospheres throughout the production process necessitates specialized equipment and facilities, substantially increasing capital expenditure. Preliminary cost analyses indicate that manufacturing infrastructure for Li₃N electrolytes requires approximately 30-40% higher investment compared to conventional liquid electrolyte production lines.
Raw material costs constitute another significant factor in the economic equation. While nitrogen is abundant and inexpensive, high-purity lithium metal remains costly at $80-100/kg. The synthesis process currently achieves only moderate yields (70-85%), resulting in material utilization inefficiencies that further elevate production costs. Calculations suggest that material costs alone contribute approximately $45-60 per kWh of battery capacity when using Li₃N as the primary electrolyte.
Energy consumption during the high-temperature synthesis process adds another layer to the cost structure. The nitridation reaction requires sustained heating at 600-800°C, consuming approximately 3.5-4.2 kWh of energy per kilogram of Li₃N produced. This energy requirement translates to roughly $0.35-0.50 per kilogram in additional production costs, depending on regional energy prices.
Recent advancements in manufacturing techniques show promise for improving scalability. Mechanochemical synthesis methods, which utilize high-energy ball milling to facilitate the reaction between lithium and nitrogen at near-ambient temperatures, could potentially reduce energy costs by 60-70%. Similarly, solution-based processing routes are under investigation, potentially offering more controlled morphology and improved integration with existing battery manufacturing infrastructure.
Economic modeling suggests that with current technologies, Li₃N electrolytes add approximately $75-90 per kWh to battery costs. However, technology learning curves indicate that costs could decrease by 8-12% annually as production volumes increase. Achieving price parity with conventional liquid electrolytes ($15-20 per kWh) would require significant technological breakthroughs in synthesis methods and substantial economies of scale, potentially achievable within 7-10 years according to industry projections.
Industrial-scale manufacturing of Li₃N electrolytes faces several technical hurdles that impact cost structures. The requirement for strictly controlled inert atmospheres throughout the production process necessitates specialized equipment and facilities, substantially increasing capital expenditure. Preliminary cost analyses indicate that manufacturing infrastructure for Li₃N electrolytes requires approximately 30-40% higher investment compared to conventional liquid electrolyte production lines.
Raw material costs constitute another significant factor in the economic equation. While nitrogen is abundant and inexpensive, high-purity lithium metal remains costly at $80-100/kg. The synthesis process currently achieves only moderate yields (70-85%), resulting in material utilization inefficiencies that further elevate production costs. Calculations suggest that material costs alone contribute approximately $45-60 per kWh of battery capacity when using Li₃N as the primary electrolyte.
Energy consumption during the high-temperature synthesis process adds another layer to the cost structure. The nitridation reaction requires sustained heating at 600-800°C, consuming approximately 3.5-4.2 kWh of energy per kilogram of Li₃N produced. This energy requirement translates to roughly $0.35-0.50 per kilogram in additional production costs, depending on regional energy prices.
Recent advancements in manufacturing techniques show promise for improving scalability. Mechanochemical synthesis methods, which utilize high-energy ball milling to facilitate the reaction between lithium and nitrogen at near-ambient temperatures, could potentially reduce energy costs by 60-70%. Similarly, solution-based processing routes are under investigation, potentially offering more controlled morphology and improved integration with existing battery manufacturing infrastructure.
Economic modeling suggests that with current technologies, Li₃N electrolytes add approximately $75-90 per kWh to battery costs. However, technology learning curves indicate that costs could decrease by 8-12% annually as production volumes increase. Achieving price parity with conventional liquid electrolytes ($15-20 per kWh) would require significant technological breakthroughs in synthesis methods and substantial economies of scale, potentially achievable within 7-10 years according to industry projections.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!