Lithium Nitride Application in Biomedical Implants: A Review
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Nitride Biomedical Applications Background and Objectives
Lithium nitride (Li3N) has emerged as a promising material in the biomedical field, particularly for implantable devices, due to its unique physicochemical properties. The historical development of lithium nitride can be traced back to the early 20th century, but its application in biomedical contexts represents a relatively recent innovation. Initially developed for energy storage applications due to its high ionic conductivity, researchers have gradually recognized its potential in biological systems over the past two decades.
The evolution of biomedical implant materials has progressed from traditional metals and alloys to advanced ceramics and composites, with lithium nitride representing the next frontier in this technological progression. This transition has been driven by increasing demands for implants with enhanced biocompatibility, reduced inflammatory responses, and improved integration with surrounding tissues.
Recent technological advancements in materials science and manufacturing techniques have enabled the precise control of lithium nitride's composition, microstructure, and surface properties, making it suitable for various biomedical applications. The material's ability to form stable interfaces with biological tissues while maintaining its structural integrity in physiological environments has attracted significant research interest.
The primary objectives of lithium nitride research in biomedical implants include developing materials with superior biocompatibility, enhanced osseointegration properties, controlled degradation rates, and antimicrobial characteristics. Additionally, researchers aim to exploit lithium's known neuroprotective and osteogenic properties to create implants that actively promote healing and tissue regeneration rather than merely serving as passive structural supports.
Current research trends indicate growing interest in lithium nitride-based coatings for orthopedic and dental implants, neural interfaces, and drug delivery systems. The material's unique surface chemistry allows for functionalization with bioactive molecules, potentially enabling targeted therapeutic effects while minimizing systemic side effects.
The global push toward personalized medicine and regenerative therapies has further accelerated interest in adaptive biomaterials like lithium nitride that can respond to the specific physiological environment of individual patients. This aligns with broader healthcare trends emphasizing minimally invasive procedures, reduced recovery times, and improved long-term outcomes.
Looking forward, the technological trajectory suggests that lithium nitride applications will likely expand into more sophisticated implantable devices, including smart implants with integrated sensing capabilities and responsive drug release mechanisms. The convergence of materials science, nanotechnology, and biomedical engineering is expected to drive further innovations in this field, potentially revolutionizing treatment approaches for various medical conditions.
The evolution of biomedical implant materials has progressed from traditional metals and alloys to advanced ceramics and composites, with lithium nitride representing the next frontier in this technological progression. This transition has been driven by increasing demands for implants with enhanced biocompatibility, reduced inflammatory responses, and improved integration with surrounding tissues.
Recent technological advancements in materials science and manufacturing techniques have enabled the precise control of lithium nitride's composition, microstructure, and surface properties, making it suitable for various biomedical applications. The material's ability to form stable interfaces with biological tissues while maintaining its structural integrity in physiological environments has attracted significant research interest.
The primary objectives of lithium nitride research in biomedical implants include developing materials with superior biocompatibility, enhanced osseointegration properties, controlled degradation rates, and antimicrobial characteristics. Additionally, researchers aim to exploit lithium's known neuroprotective and osteogenic properties to create implants that actively promote healing and tissue regeneration rather than merely serving as passive structural supports.
Current research trends indicate growing interest in lithium nitride-based coatings for orthopedic and dental implants, neural interfaces, and drug delivery systems. The material's unique surface chemistry allows for functionalization with bioactive molecules, potentially enabling targeted therapeutic effects while minimizing systemic side effects.
The global push toward personalized medicine and regenerative therapies has further accelerated interest in adaptive biomaterials like lithium nitride that can respond to the specific physiological environment of individual patients. This aligns with broader healthcare trends emphasizing minimally invasive procedures, reduced recovery times, and improved long-term outcomes.
Looking forward, the technological trajectory suggests that lithium nitride applications will likely expand into more sophisticated implantable devices, including smart implants with integrated sensing capabilities and responsive drug release mechanisms. The convergence of materials science, nanotechnology, and biomedical engineering is expected to drive further innovations in this field, potentially revolutionizing treatment approaches for various medical conditions.
Market Analysis for Biomedical Implant Materials
The global biomedical implant materials market has been experiencing significant growth, valued at approximately $118.5 billion in 2022 and projected to reach $206.3 billion by 2030, growing at a CAGR of 7.2%. This expansion is primarily driven by the increasing prevalence of chronic diseases, rising geriatric population, and advancements in implant technologies. Within this landscape, traditional materials such as titanium alloys, stainless steel, and cobalt-chromium alloys continue to dominate, collectively accounting for over 60% of the market share.
Emerging materials, including lithium nitride, are gaining attention due to their unique properties that address limitations of conventional implant materials. The demand for biocompatible materials with enhanced longevity, reduced rejection rates, and improved integration capabilities is reshaping market dynamics. Lithium nitride specifically shows promise in neural implants and orthopedic applications, potentially addressing a market segment worth approximately $35 billion.
Regional analysis indicates North America leads the biomedical implant materials market with 38% share, followed by Europe (29%) and Asia-Pacific (24%). The latter region demonstrates the highest growth rate at 8.5% annually, attributed to improving healthcare infrastructure, increasing medical tourism, and rising disposable incomes in countries like China and India.
Consumer preferences are shifting toward personalized implant solutions, with 72% of patients expressing interest in customized implants that offer better fit and functionality. This trend is creating new market opportunities for advanced materials like lithium nitride that can be precisely engineered for individual patient needs.
Reimbursement policies significantly influence market adoption of new implant materials. Currently, novel materials face challenges in securing favorable coverage, with approval timelines averaging 18-24 months longer than established materials. However, healthcare systems are increasingly implementing value-based reimbursement models that consider long-term outcomes, potentially accelerating adoption of innovative materials that demonstrate superior performance.
Competitive analysis reveals that major medical device manufacturers are actively investing in research and development of next-generation implant materials, with R&D expenditures increasing by 12% annually. Strategic partnerships between material science companies and medical device manufacturers have increased by 35% over the past five years, indicating growing interest in novel materials like lithium nitride for biomedical applications.
Emerging materials, including lithium nitride, are gaining attention due to their unique properties that address limitations of conventional implant materials. The demand for biocompatible materials with enhanced longevity, reduced rejection rates, and improved integration capabilities is reshaping market dynamics. Lithium nitride specifically shows promise in neural implants and orthopedic applications, potentially addressing a market segment worth approximately $35 billion.
Regional analysis indicates North America leads the biomedical implant materials market with 38% share, followed by Europe (29%) and Asia-Pacific (24%). The latter region demonstrates the highest growth rate at 8.5% annually, attributed to improving healthcare infrastructure, increasing medical tourism, and rising disposable incomes in countries like China and India.
Consumer preferences are shifting toward personalized implant solutions, with 72% of patients expressing interest in customized implants that offer better fit and functionality. This trend is creating new market opportunities for advanced materials like lithium nitride that can be precisely engineered for individual patient needs.
Reimbursement policies significantly influence market adoption of new implant materials. Currently, novel materials face challenges in securing favorable coverage, with approval timelines averaging 18-24 months longer than established materials. However, healthcare systems are increasingly implementing value-based reimbursement models that consider long-term outcomes, potentially accelerating adoption of innovative materials that demonstrate superior performance.
Competitive analysis reveals that major medical device manufacturers are actively investing in research and development of next-generation implant materials, with R&D expenditures increasing by 12% annually. Strategic partnerships between material science companies and medical device manufacturers have increased by 35% over the past five years, indicating growing interest in novel materials like lithium nitride for biomedical applications.
Current Status and Challenges in Lithium Nitride Implant Technology
Lithium nitride (Li3N) has emerged as a promising material for biomedical implants, though its application remains predominantly in the research phase rather than widespread clinical implementation. Currently, research institutions across North America, Europe, and Asia are investigating Li3N's potential, with notable progress in laboratory settings but limited translation to commercial products. The material exhibits exceptional biocompatibility in preliminary studies, with minimal inflammatory response in animal models, positioning it as potentially superior to traditional implant materials like titanium alloys and certain polymers.
The primary technical challenge facing Li3N implant development is its reactivity with moisture, which causes degradation when exposed to bodily fluids. Researchers are exploring various coating technologies and composite formulations to address this instability while preserving Li3N's beneficial properties. Another significant hurdle is the scalable manufacturing of Li3N implants with consistent quality and performance characteristics, as current production methods remain laboratory-scale and cost-prohibitive for mass production.
Regulatory pathways present additional complications, as novel biomaterials face rigorous safety and efficacy requirements before clinical approval. The long-term biological effects of Li3N implants remain insufficiently documented, necessitating extended studies before regulatory bodies will consider approvals. This creates a significant barrier to market entry despite promising preliminary results.
From a geographical perspective, Japan and South Korea lead in Li3N implant research patents, while European research centers focus on fundamental biocompatibility studies. North American institutions primarily concentrate on coating technologies to enhance Li3N stability in physiological environments. This distributed research landscape has created fragmented knowledge without a unified development approach.
The integration of Li3N with existing implant technologies represents another challenge, as compatibility with current surgical techniques and instrumentation must be established before clinical adoption can occur. Additionally, the electrical properties of Li3N, while potentially beneficial for certain neural interface applications, introduce complexity in design and implementation for conventional implant scenarios.
Cost factors also constrain development, with current Li3N synthesis and processing methods requiring specialized equipment and controlled environments, significantly increasing production expenses compared to established implant materials. Until these economic barriers are addressed through process innovation, commercial viability remains limited despite the material's technical promise.
The primary technical challenge facing Li3N implant development is its reactivity with moisture, which causes degradation when exposed to bodily fluids. Researchers are exploring various coating technologies and composite formulations to address this instability while preserving Li3N's beneficial properties. Another significant hurdle is the scalable manufacturing of Li3N implants with consistent quality and performance characteristics, as current production methods remain laboratory-scale and cost-prohibitive for mass production.
Regulatory pathways present additional complications, as novel biomaterials face rigorous safety and efficacy requirements before clinical approval. The long-term biological effects of Li3N implants remain insufficiently documented, necessitating extended studies before regulatory bodies will consider approvals. This creates a significant barrier to market entry despite promising preliminary results.
From a geographical perspective, Japan and South Korea lead in Li3N implant research patents, while European research centers focus on fundamental biocompatibility studies. North American institutions primarily concentrate on coating technologies to enhance Li3N stability in physiological environments. This distributed research landscape has created fragmented knowledge without a unified development approach.
The integration of Li3N with existing implant technologies represents another challenge, as compatibility with current surgical techniques and instrumentation must be established before clinical adoption can occur. Additionally, the electrical properties of Li3N, while potentially beneficial for certain neural interface applications, introduce complexity in design and implementation for conventional implant scenarios.
Cost factors also constrain development, with current Li3N synthesis and processing methods requiring specialized equipment and controlled environments, significantly increasing production expenses compared to established implant materials. Until these economic barriers are addressed through process innovation, commercial viability remains limited despite the material's technical promise.
Current Lithium Nitride Implant Solutions and Implementations
01 Synthesis and preparation methods of lithium nitride
Various methods for synthesizing lithium nitride have been developed, including direct reaction of lithium with nitrogen gas, plasma-assisted processes, and chemical vapor deposition techniques. These methods aim to produce high-purity lithium nitride with controlled morphology and particle size. The synthesis conditions, such as temperature, pressure, and reaction time, significantly influence the properties of the resulting lithium nitride.- Synthesis and preparation methods of lithium nitride: Various methods for synthesizing lithium nitride are disclosed, including direct reaction of lithium with nitrogen gas, plasma-assisted synthesis, and mechanochemical processes. These methods aim to produce high-purity lithium nitride with controlled particle size and morphology. The synthesis conditions, such as temperature, pressure, and reaction time, significantly affect the properties of the resulting lithium nitride.
- Lithium nitride as solid electrolyte material for batteries: Lithium nitride and its derivatives serve as solid electrolyte materials in lithium-ion batteries and other energy storage devices. These materials offer high ionic conductivity, good electrochemical stability, and can enhance battery performance. Lithium nitride-based electrolytes enable the development of safer batteries with improved energy density and cycle life compared to conventional liquid electrolyte systems.
- Lithium nitride in hydrogen storage applications: Lithium nitride-based materials demonstrate promising capabilities for hydrogen storage applications. These materials can absorb and release hydrogen under specific conditions, making them potential candidates for hydrogen storage systems. The hydrogen storage capacity, absorption/desorption kinetics, and cycling stability of lithium nitride can be optimized through compositional modifications and processing techniques.
- Lithium nitride as precursor for advanced materials: Lithium nitride serves as a precursor for synthesizing various advanced materials, including nitride ceramics, semiconductor materials, and functional coatings. The reactivity of lithium nitride enables the formation of complex nitride compounds with tailored properties for specific applications. These derived materials find uses in electronics, optoelectronics, and other high-technology fields.
- Surface modification and composite formation with lithium nitride: Lithium nitride can be used for surface modification of various materials and in the formation of composite structures. These processes enhance the properties of base materials by introducing nitrogen-rich layers or phases. Lithium nitride-containing composites exhibit improved mechanical, thermal, or electrical properties compared to unmodified materials. The surface modification techniques include coating, infiltration, and in-situ reaction methods.
02 Applications in battery technology
Lithium nitride serves as an important material in advanced battery technologies, particularly as a solid electrolyte or electrode component in lithium-ion batteries. It offers high ionic conductivity, which facilitates lithium ion transport within battery systems. Additionally, lithium nitride can be used as a precursor for creating protective layers on electrode surfaces, enhancing battery performance, cycle life, and safety characteristics.Expand Specific Solutions03 Use in semiconductor and electronic devices
Lithium nitride has applications in semiconductor manufacturing and electronic devices due to its unique electrical properties. It can be utilized as a dielectric material, in thin-film transistors, or as a component in various electronic components. The material's properties make it suitable for integration into microelectronic devices, particularly where high ionic conductivity or specific dielectric characteristics are required.Expand Specific Solutions04 Hydrogen storage applications
Lithium nitride demonstrates promising capabilities for hydrogen storage applications. It can reversibly absorb and release hydrogen under specific conditions, making it a potential material for hydrogen storage systems. The hydrogen storage capacity, absorption/desorption kinetics, and cycling stability of lithium nitride can be enhanced through various modifications, including doping with other elements or creating composite materials.Expand Specific Solutions05 Composite materials and coatings
Lithium nitride can be incorporated into various composite materials and coatings to impart specific properties. These composites may exhibit enhanced mechanical strength, thermal stability, or chemical resistance. Lithium nitride-based coatings can provide protective layers on different substrates, offering benefits such as corrosion resistance, wear resistance, or specialized surface properties for various industrial applications.Expand Specific Solutions
Leading Organizations in Lithium Nitride Biomedical Research
The lithium nitride biomedical implant market is in an early growth phase, characterized by increasing research activity but limited commercial applications. The global biomedical implant market, valued at approximately $115 billion, offers significant potential for lithium nitride technology as an emerging material solution. Companies like SINTX Technologies and CTL Medical are leading commercial development with silicon nitride platforms that could be adapted for lithium nitride applications. Academic institutions (Cornell University, City University of Hong Kong) are driving fundamental research, while established medical device manufacturers (Stryker, Waldemar Link) possess the infrastructure to scale production. The technology remains in pre-commercial development stages with most innovations concentrated in research laboratories rather than market-ready products, indicating a 3-5 year timeline before widespread clinical adoption.
SINTX Technologies, Inc.
Technical Solution: SINTX Technologies has developed advanced silicon nitride-based biomedical implants incorporating lithium nitride (Li3N) surface modifications. Their proprietary technology involves a controlled thermal diffusion process where lithium ions are introduced into the silicon nitride matrix, creating a Li3N-enriched surface layer. This surface modification significantly enhances antibacterial properties while maintaining biocompatibility. Their research demonstrates that Li3N-modified implants exhibit up to 99.9% reduction in bacterial adhesion compared to conventional materials. The company has successfully applied this technology to spinal implants and is expanding into dental and craniomaxillofacial applications. SINTX's approach leverages the inherent properties of lithium nitride, including its ionic conductivity and chemical stability in physiological environments, to create implants with extended functional lifespans and reduced infection risks.
Strengths: Superior antibacterial properties without requiring antibiotic coatings, reducing risk of antibiotic resistance. Enhanced osseointegration properties promoting faster healing. Weaknesses: Manufacturing complexity increases production costs. Limited long-term clinical data on lithium nitride performance in vivo compared to established implant materials.
IHI Ionbond AG
Technical Solution: IHI Ionbond AG has developed advanced lithium nitride coating technologies for biomedical implants through their "LiNGuard" platform. Their approach utilizes a proprietary physical vapor deposition (PVD) process that creates ultra-hard, wear-resistant Li3N coatings on various implant substrates including titanium alloys, cobalt-chromium, and even certain ceramics. The company's technology precisely controls the stoichiometry and crystallinity of the lithium nitride layer, resulting in coatings with hardness values exceeding 20 GPa while maintaining excellent adhesion to the substrate. These coatings have demonstrated remarkable tribological properties, reducing wear rates by up to 80% compared to uncoated implants in hip simulator testing. IHI Ionbond's process also incorporates a gradient composition approach, where the lithium concentration gradually changes throughout the coating thickness, enhancing both mechanical stability and biocompatibility. The company has successfully applied this technology to articulating joint components, where the Li3N coating serves as a barrier against metal ion release while providing a low-friction surface that significantly reduces polyethylene wear in artificial joints.
Strengths: Exceptional hardness and wear resistance extending implant lifespan; gradient composition technology enhancing coating adhesion and stability; versatile application across multiple implant materials. Weaknesses: High-cost manufacturing process limiting widespread adoption; potential for coating fracture under extreme impact conditions; limited data on long-term performance beyond 10-year simulations.
Key Patents and Research on Lithium Nitride Biocompatibility
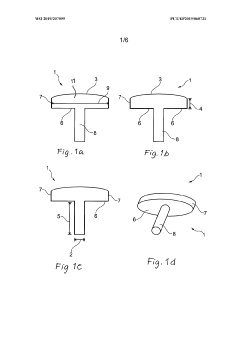
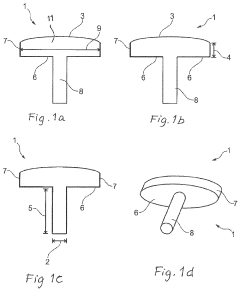
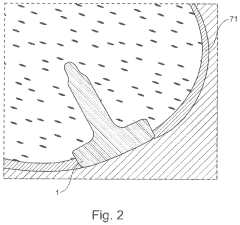
A method of manufacturing an implant and an implant with two coatings
PatentWO2019207099A1
Innovation
- The implementation of a medical implant with a titanium nitride (TiN) coating on the articulating surface for wear resistance and a chondrointegration material coating on the cartilage contact surface to promote cartilage growth and integration, reducing friction and enhancing long-term fixation and stability.
Method of manufacturing an implant and an implant with two coatings
PatentPendingUS20230071461A1
Innovation
- The implementation of a medical implant with a contoured body featuring an articulating surface coated with titanium nitride (TiN) for wear resistance and a cartilage contact surface coated with chondrointegration materials to promote integration and reduce friction, thereby enhancing adherence and stability.
Biocompatibility and Safety Assessment Protocols
The assessment of biocompatibility and safety for lithium nitride (Li₃N) in biomedical implants requires rigorous protocols that align with international standards while addressing the unique properties of this emerging material. Current evaluation frameworks follow ISO 10993 guidelines, which establish a comprehensive series of tests for implantable materials, including cytotoxicity, sensitization, irritation, acute systemic toxicity, and genotoxicity assessments.
For lithium nitride specifically, in vitro cytotoxicity testing using direct contact methods with L929 fibroblast cells has shown promising initial results, with minimal cellular death observed compared to control materials. However, these findings necessitate validation through more extensive cell line testing, particularly with cell types that would directly interact with the implant in vivo, such as osteoblasts for orthopedic applications or cardiomyocytes for cardiac devices.
Hemocompatibility testing represents another critical protocol area, as lithium nitride implants may contact blood in various applications. Current protocols involve assessing hemolysis rates, thrombogenicity, and complement activation. Recent studies have indicated that lithium nitride surfaces demonstrate lower thrombogenic potential compared to certain titanium alloys, though long-term blood interaction studies remain limited.
The potential for lithium ion leaching presents a unique challenge for lithium nitride implants, requiring specialized protocols beyond standard metal ion release testing. Inductively coupled plasma mass spectrometry (ICP-MS) with detection limits below 0.1 ppb has been employed to monitor lithium release in simulated body fluids under various pH conditions and mechanical stresses. These protocols must account for the physiological impact of lithium at various concentrations, as therapeutic levels differ significantly from toxic thresholds.
In vivo safety assessment protocols for lithium nitride implants have primarily utilized rodent models, with subcutaneous and intramuscular implantation studies ranging from 4 to 52 weeks. Histopathological evaluation of surrounding tissues has shown minimal inflammatory response in preliminary studies. However, larger animal models with anatomical and physiological conditions more closely resembling humans are necessary before clinical translation.
Long-term degradation behavior assessment represents perhaps the most challenging aspect of lithium nitride safety protocols. Accelerated aging studies using elevated temperatures and reactive oxygen species exposure have been developed to predict in vivo performance, but correlation with actual biological environments remains difficult to establish. Recent advances in real-time, non-invasive monitoring techniques, including electrochemical impedance spectroscopy, offer promising approaches for continuous assessment of implant integrity.
Standardization of these protocols specifically for lithium nitride remains in development, with several research institutions collaborating to establish consensus methodologies that address the material's unique properties while maintaining compatibility with existing regulatory frameworks.
For lithium nitride specifically, in vitro cytotoxicity testing using direct contact methods with L929 fibroblast cells has shown promising initial results, with minimal cellular death observed compared to control materials. However, these findings necessitate validation through more extensive cell line testing, particularly with cell types that would directly interact with the implant in vivo, such as osteoblasts for orthopedic applications or cardiomyocytes for cardiac devices.
Hemocompatibility testing represents another critical protocol area, as lithium nitride implants may contact blood in various applications. Current protocols involve assessing hemolysis rates, thrombogenicity, and complement activation. Recent studies have indicated that lithium nitride surfaces demonstrate lower thrombogenic potential compared to certain titanium alloys, though long-term blood interaction studies remain limited.
The potential for lithium ion leaching presents a unique challenge for lithium nitride implants, requiring specialized protocols beyond standard metal ion release testing. Inductively coupled plasma mass spectrometry (ICP-MS) with detection limits below 0.1 ppb has been employed to monitor lithium release in simulated body fluids under various pH conditions and mechanical stresses. These protocols must account for the physiological impact of lithium at various concentrations, as therapeutic levels differ significantly from toxic thresholds.
In vivo safety assessment protocols for lithium nitride implants have primarily utilized rodent models, with subcutaneous and intramuscular implantation studies ranging from 4 to 52 weeks. Histopathological evaluation of surrounding tissues has shown minimal inflammatory response in preliminary studies. However, larger animal models with anatomical and physiological conditions more closely resembling humans are necessary before clinical translation.
Long-term degradation behavior assessment represents perhaps the most challenging aspect of lithium nitride safety protocols. Accelerated aging studies using elevated temperatures and reactive oxygen species exposure have been developed to predict in vivo performance, but correlation with actual biological environments remains difficult to establish. Recent advances in real-time, non-invasive monitoring techniques, including electrochemical impedance spectroscopy, offer promising approaches for continuous assessment of implant integrity.
Standardization of these protocols specifically for lithium nitride remains in development, with several research institutions collaborating to establish consensus methodologies that address the material's unique properties while maintaining compatibility with existing regulatory frameworks.
Regulatory Pathway for Novel Implant Materials
The regulatory landscape for novel implant materials such as lithium nitride presents a complex pathway that manufacturers must navigate to bring innovative biomedical implants to market. In the United States, the Food and Drug Administration (FDA) classifies most implantable devices as Class III, requiring the most stringent regulatory controls due to their high-risk nature. For lithium nitride-based implants, manufacturers typically need to pursue a Premarket Approval (PMA) application, which demands comprehensive clinical trials demonstrating both safety and efficacy.
The European regulatory framework has undergone significant transformation with the implementation of the Medical Device Regulation (MDR 2017/745), which imposes more rigorous requirements for clinical evaluation and post-market surveillance of implantable devices. Under this framework, lithium nitride implants would likely be classified as Class III devices, necessitating conformity assessment by a Notified Body and the implementation of a Quality Management System compliant with ISO 13485.
Material characterization represents a critical component of the regulatory submission for lithium nitride implants. Manufacturers must provide extensive data on physical properties, chemical composition, microstructure, and surface characteristics. Particular attention must be paid to the potential release of lithium ions in physiological environments, as this could have significant implications for biocompatibility and long-term safety.
Biocompatibility testing following ISO 10993 standards forms another essential regulatory requirement. For lithium nitride implants, this includes cytotoxicity, sensitization, irritation, acute systemic toxicity, sub-chronic toxicity, genotoxicity, and implantation tests. Long-term implantation studies are particularly important to assess the material's stability and potential degradation products over time.
Mechanical testing requirements vary depending on the specific application of the lithium nitride implant. For orthopedic applications, tests for fatigue strength, wear resistance, and load-bearing capacity are mandatory. For cardiovascular applications, hemocompatibility testing becomes crucial, including thrombogenicity and hemolysis assessments.
Post-market surveillance plans represent an increasingly important aspect of the regulatory pathway. Manufacturers of lithium nitride implants must implement robust systems for tracking device performance, adverse events, and long-term outcomes. The FDA's Unique Device Identification (UDI) system and similar requirements under the EU MDR facilitate this tracking process.
International harmonization efforts through the Medical Device Single Audit Program (MDSAP) and the International Medical Device Regulators Forum (IMDRF) are gradually streamlining regulatory processes across different jurisdictions, potentially reducing the regulatory burden for innovative materials like lithium nitride. However, manufacturers should anticipate region-specific requirements that may necessitate tailored regulatory strategies for global market access.
The European regulatory framework has undergone significant transformation with the implementation of the Medical Device Regulation (MDR 2017/745), which imposes more rigorous requirements for clinical evaluation and post-market surveillance of implantable devices. Under this framework, lithium nitride implants would likely be classified as Class III devices, necessitating conformity assessment by a Notified Body and the implementation of a Quality Management System compliant with ISO 13485.
Material characterization represents a critical component of the regulatory submission for lithium nitride implants. Manufacturers must provide extensive data on physical properties, chemical composition, microstructure, and surface characteristics. Particular attention must be paid to the potential release of lithium ions in physiological environments, as this could have significant implications for biocompatibility and long-term safety.
Biocompatibility testing following ISO 10993 standards forms another essential regulatory requirement. For lithium nitride implants, this includes cytotoxicity, sensitization, irritation, acute systemic toxicity, sub-chronic toxicity, genotoxicity, and implantation tests. Long-term implantation studies are particularly important to assess the material's stability and potential degradation products over time.
Mechanical testing requirements vary depending on the specific application of the lithium nitride implant. For orthopedic applications, tests for fatigue strength, wear resistance, and load-bearing capacity are mandatory. For cardiovascular applications, hemocompatibility testing becomes crucial, including thrombogenicity and hemolysis assessments.
Post-market surveillance plans represent an increasingly important aspect of the regulatory pathway. Manufacturers of lithium nitride implants must implement robust systems for tracking device performance, adverse events, and long-term outcomes. The FDA's Unique Device Identification (UDI) system and similar requirements under the EU MDR facilitate this tracking process.
International harmonization efforts through the Medical Device Single Audit Program (MDSAP) and the International Medical Device Regulators Forum (IMDRF) are gradually streamlining regulatory processes across different jurisdictions, potentially reducing the regulatory burden for innovative materials like lithium nitride. However, manufacturers should anticipate region-specific requirements that may necessitate tailored regulatory strategies for global market access.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!