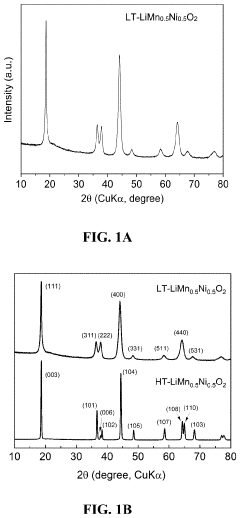
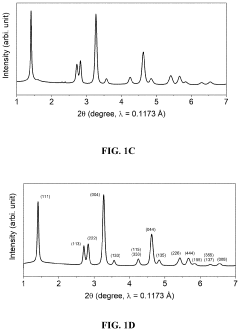
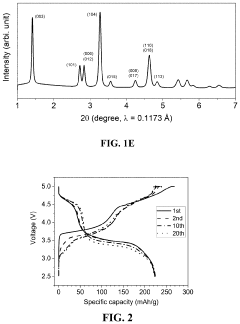
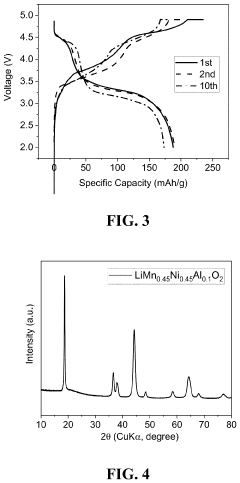
Comparing Lithium Nitride with Traditional Cathode Materials
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Nitride Technology Evolution and Objectives
Lithium-ion battery technology has undergone significant evolution since its commercial introduction by Sony in 1991. Traditional cathode materials such as lithium cobalt oxide (LCO), lithium manganese oxide (LMO), and lithium iron phosphate (LFP) have dominated the market for decades. However, these materials face inherent limitations in energy density, charging speed, and cycle life that constrain further advancement of battery technology.
Lithium nitride (Li₃N) represents a paradigm shift in cathode material research, emerging from early experimental work in the 1980s but gaining renewed attention in the past decade. Unlike conventional intercalation-based cathodes, lithium nitride offers a fundamentally different mechanism for lithium storage, potentially enabling higher theoretical capacity and energy density. The crystalline structure of Li₃N, with its unique layered arrangement of lithium and nitrogen atoms, provides exceptional lithium-ion conductivity—orders of magnitude higher than traditional cathode materials.
The technological trajectory of lithium nitride has been marked by several key breakthroughs. Initial research focused primarily on its application as a solid electrolyte due to its high ionic conductivity. However, recent advancements in synthesis methods and nanostructuring techniques have opened new possibilities for its use as a cathode material. Particularly noteworthy is the development of composite structures that mitigate the volume expansion issues previously limiting its practical application.
Current research objectives in lithium nitride technology center on addressing several critical challenges. First, improving the cycling stability to match or exceed that of conventional cathodes remains paramount. Second, researchers aim to enhance the rate capability to enable fast charging without compromising structural integrity. Third, developing scalable and cost-effective manufacturing processes is essential for commercial viability.
The environmental implications of lithium nitride technology also represent a significant research focus. Unlike cobalt-based cathodes, lithium nitride relies on nitrogen, an abundant element, potentially reducing the environmental footprint and supply chain risks associated with battery production. This aligns with the broader industry trend toward more sustainable energy storage solutions.
Looking forward, the technology roadmap for lithium nitride includes achieving energy densities exceeding 400 Wh/kg at the cell level—a substantial improvement over current lithium-ion batteries. Additionally, researchers aim to extend cycle life beyond 1,000 full charge-discharge cycles while maintaining at least 80% capacity retention, a benchmark necessary for widespread adoption in electric vehicles and grid storage applications.
Lithium nitride (Li₃N) represents a paradigm shift in cathode material research, emerging from early experimental work in the 1980s but gaining renewed attention in the past decade. Unlike conventional intercalation-based cathodes, lithium nitride offers a fundamentally different mechanism for lithium storage, potentially enabling higher theoretical capacity and energy density. The crystalline structure of Li₃N, with its unique layered arrangement of lithium and nitrogen atoms, provides exceptional lithium-ion conductivity—orders of magnitude higher than traditional cathode materials.
The technological trajectory of lithium nitride has been marked by several key breakthroughs. Initial research focused primarily on its application as a solid electrolyte due to its high ionic conductivity. However, recent advancements in synthesis methods and nanostructuring techniques have opened new possibilities for its use as a cathode material. Particularly noteworthy is the development of composite structures that mitigate the volume expansion issues previously limiting its practical application.
Current research objectives in lithium nitride technology center on addressing several critical challenges. First, improving the cycling stability to match or exceed that of conventional cathodes remains paramount. Second, researchers aim to enhance the rate capability to enable fast charging without compromising structural integrity. Third, developing scalable and cost-effective manufacturing processes is essential for commercial viability.
The environmental implications of lithium nitride technology also represent a significant research focus. Unlike cobalt-based cathodes, lithium nitride relies on nitrogen, an abundant element, potentially reducing the environmental footprint and supply chain risks associated with battery production. This aligns with the broader industry trend toward more sustainable energy storage solutions.
Looking forward, the technology roadmap for lithium nitride includes achieving energy densities exceeding 400 Wh/kg at the cell level—a substantial improvement over current lithium-ion batteries. Additionally, researchers aim to extend cycle life beyond 1,000 full charge-discharge cycles while maintaining at least 80% capacity retention, a benchmark necessary for widespread adoption in electric vehicles and grid storage applications.
Market Demand Analysis for Advanced Cathode Materials
The global market for advanced cathode materials has witnessed significant growth in recent years, driven primarily by the expanding electric vehicle (EV) sector and increasing demand for high-performance energy storage solutions. The market value for cathode materials reached approximately $7.6 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 12.3% through 2030, potentially reaching $19.2 billion by the end of the forecast period.
Lithium-ion batteries currently dominate the rechargeable battery market, with cathode materials accounting for nearly 40% of the total battery cost. Traditional cathode materials such as Lithium Cobalt Oxide (LCO), Lithium Manganese Oxide (LMO), and Lithium Iron Phosphate (LFP) have established market presence, but face increasing challenges related to energy density limitations, raw material costs, and sustainability concerns.
The emergence of Lithium Nitride as an alternative cathode material is being driven by several market demands. First, the automotive industry's push for longer-range EVs requires batteries with higher energy density than what traditional cathodes can provide. Lithium Nitride's theoretical capacity exceeds 1200 mAh/g, significantly higher than LCO's 140 mAh/g or NMC's 170-220 mAh/g, potentially addressing this critical market need.
Supply chain security represents another major market driver. The concentration of cobalt mining in politically unstable regions has created vulnerability for battery manufacturers. Lithium Nitride's cobalt-free composition offers a strategic advantage, aligning with the industry's shift toward reducing dependency on critical materials with geopolitical supply risks.
Consumer electronics manufacturers are also expressing growing interest in advanced cathode materials that enable faster charging capabilities while maintaining safety standards. Market research indicates that 78% of smartphone users consider battery performance a decisive factor in purchasing decisions, creating substantial demand for next-generation battery technologies.
The stationary energy storage sector presents another significant market opportunity, projected to grow at 18.7% CAGR through 2030. Grid-scale applications require cathode materials with extended cycle life and improved safety profiles – areas where Lithium Nitride shows promising preliminary results compared to traditional options.
Regulatory pressures are further shaping market demand. The European Union's proposed Battery Regulation includes carbon footprint declarations and recycling requirements, creating market advantages for cathode materials with lower environmental impact. Similarly, China's dual-credit policy system incentivizes manufacturers to develop higher energy density batteries, potentially accelerating adoption of novel cathode materials like Lithium Nitride.
Lithium-ion batteries currently dominate the rechargeable battery market, with cathode materials accounting for nearly 40% of the total battery cost. Traditional cathode materials such as Lithium Cobalt Oxide (LCO), Lithium Manganese Oxide (LMO), and Lithium Iron Phosphate (LFP) have established market presence, but face increasing challenges related to energy density limitations, raw material costs, and sustainability concerns.
The emergence of Lithium Nitride as an alternative cathode material is being driven by several market demands. First, the automotive industry's push for longer-range EVs requires batteries with higher energy density than what traditional cathodes can provide. Lithium Nitride's theoretical capacity exceeds 1200 mAh/g, significantly higher than LCO's 140 mAh/g or NMC's 170-220 mAh/g, potentially addressing this critical market need.
Supply chain security represents another major market driver. The concentration of cobalt mining in politically unstable regions has created vulnerability for battery manufacturers. Lithium Nitride's cobalt-free composition offers a strategic advantage, aligning with the industry's shift toward reducing dependency on critical materials with geopolitical supply risks.
Consumer electronics manufacturers are also expressing growing interest in advanced cathode materials that enable faster charging capabilities while maintaining safety standards. Market research indicates that 78% of smartphone users consider battery performance a decisive factor in purchasing decisions, creating substantial demand for next-generation battery technologies.
The stationary energy storage sector presents another significant market opportunity, projected to grow at 18.7% CAGR through 2030. Grid-scale applications require cathode materials with extended cycle life and improved safety profiles – areas where Lithium Nitride shows promising preliminary results compared to traditional options.
Regulatory pressures are further shaping market demand. The European Union's proposed Battery Regulation includes carbon footprint declarations and recycling requirements, creating market advantages for cathode materials with lower environmental impact. Similarly, China's dual-credit policy system incentivizes manufacturers to develop higher energy density batteries, potentially accelerating adoption of novel cathode materials like Lithium Nitride.
Current Status and Challenges in Cathode Technology
The global cathode materials market is currently dominated by several traditional materials, including Lithium Cobalt Oxide (LCO), Lithium Iron Phosphate (LFP), Lithium Manganese Oxide (LMO), and Lithium Nickel Manganese Cobalt Oxide (NMC). These materials have established manufacturing processes and supply chains, with annual production volumes reaching hundreds of thousands of tons. However, they face significant challenges in meeting the increasing demands of next-generation energy storage applications.
Traditional cathode materials exhibit limitations in energy density, typically ranging from 150-220 Wh/kg for commercial cells. This ceiling has become a critical bottleneck for applications requiring higher energy storage capabilities, particularly in electric vehicles and portable electronics. Additionally, the thermal stability of conventional cathodes remains problematic, with many materials experiencing structural degradation at temperatures above 80°C.
Lithium Nitride (Li₃N) has emerged as a potential alternative with theoretical energy densities exceeding 300 Wh/kg. Early research indicates it may offer superior ionic conductivity—approximately 10⁻³ S/cm at room temperature—significantly outperforming traditional cathode materials. However, Li₃N faces substantial challenges in practical implementation, including high reactivity with atmospheric moisture and oxygen, making manufacturing and handling exceptionally difficult.
The geographical distribution of cathode technology development shows concentration in East Asia (particularly Japan, South Korea, and China), North America, and Europe. China currently dominates the manufacturing landscape, controlling approximately 80% of global cathode material production capacity, while research innovations are more evenly distributed among academic and industrial centers worldwide.
A significant technical challenge for all cathode materials, including Li₃N, is cycle life degradation. Traditional cathodes typically maintain 80% capacity after 500-1000 cycles, while preliminary Li₃N implementations show rapid capacity fading after fewer than 100 cycles. This performance gap represents a major hurdle for commercial viability.
Resource constraints also present challenges. Traditional cathode materials rely heavily on cobalt and nickel, facing supply chain vulnerabilities and price volatility. While Li₃N reduces dependence on these elements, it introduces new manufacturing complexities requiring specialized equipment and controlled environments, substantially increasing production costs.
Environmental considerations are increasingly influencing cathode technology development. Traditional materials involve energy-intensive extraction processes with significant carbon footprints. Li₃N potentially offers reduced environmental impact during material sourcing but may introduce new challenges in manufacturing emissions and end-of-life recycling processes that have yet to be fully assessed or optimized.
Traditional cathode materials exhibit limitations in energy density, typically ranging from 150-220 Wh/kg for commercial cells. This ceiling has become a critical bottleneck for applications requiring higher energy storage capabilities, particularly in electric vehicles and portable electronics. Additionally, the thermal stability of conventional cathodes remains problematic, with many materials experiencing structural degradation at temperatures above 80°C.
Lithium Nitride (Li₃N) has emerged as a potential alternative with theoretical energy densities exceeding 300 Wh/kg. Early research indicates it may offer superior ionic conductivity—approximately 10⁻³ S/cm at room temperature—significantly outperforming traditional cathode materials. However, Li₃N faces substantial challenges in practical implementation, including high reactivity with atmospheric moisture and oxygen, making manufacturing and handling exceptionally difficult.
The geographical distribution of cathode technology development shows concentration in East Asia (particularly Japan, South Korea, and China), North America, and Europe. China currently dominates the manufacturing landscape, controlling approximately 80% of global cathode material production capacity, while research innovations are more evenly distributed among academic and industrial centers worldwide.
A significant technical challenge for all cathode materials, including Li₃N, is cycle life degradation. Traditional cathodes typically maintain 80% capacity after 500-1000 cycles, while preliminary Li₃N implementations show rapid capacity fading after fewer than 100 cycles. This performance gap represents a major hurdle for commercial viability.
Resource constraints also present challenges. Traditional cathode materials rely heavily on cobalt and nickel, facing supply chain vulnerabilities and price volatility. While Li₃N reduces dependence on these elements, it introduces new manufacturing complexities requiring specialized equipment and controlled environments, substantially increasing production costs.
Environmental considerations are increasingly influencing cathode technology development. Traditional materials involve energy-intensive extraction processes with significant carbon footprints. Li₃N potentially offers reduced environmental impact during material sourcing but may introduce new challenges in manufacturing emissions and end-of-life recycling processes that have yet to be fully assessed or optimized.
Comparative Analysis of Lithium Nitride vs Traditional Cathodes
01 Lithium nitride as a cathode material
Lithium nitride (Li3N) can be used as a cathode material in lithium batteries due to its high lithium ion conductivity and theoretical capacity. It offers advantages such as high energy density and improved electrochemical performance. When used as a cathode material, lithium nitride can enhance the overall battery performance by facilitating faster lithium ion transport and providing stable cycling characteristics.- Lithium nitride as a cathode material: Lithium nitride (Li3N) can be used as a cathode material in lithium batteries due to its high lithium ion conductivity and theoretical capacity. It offers advantages such as high energy density and improved electrochemical performance. When used as a cathode material, lithium nitride can enhance the overall battery performance by facilitating faster lithium ion transport and providing stable cycling behavior.
- Composite cathodes combining lithium nitride with traditional materials: Composite cathode materials that combine lithium nitride with traditional cathode materials like lithium iron phosphate (LFP), lithium cobalt oxide (LCO), or lithium manganese oxide (LMO) can offer enhanced performance. These composites leverage the high ionic conductivity of lithium nitride while maintaining the stability and capacity of traditional cathode materials. The synergistic effect results in improved cycling stability, rate capability, and overall battery performance.
- Surface modification of traditional cathodes with lithium nitride: Surface modification of traditional cathode materials with lithium nitride can improve the interface properties and electrochemical performance. By coating or treating conventional cathode materials with lithium nitride, the electrode-electrolyte interface stability is enhanced, reducing unwanted side reactions and improving cycling performance. This approach can also help mitigate capacity fading and increase the lifespan of lithium-ion batteries.
- Lithium nitride as a protective layer or solid electrolyte: Lithium nitride can be utilized as a protective layer or solid electrolyte in conjunction with traditional cathode materials. When applied as a thin film or interlayer, lithium nitride can prevent direct contact between the cathode and liquid electrolyte, reducing unwanted reactions and enhancing battery safety. As a solid electrolyte component, it facilitates lithium ion transport while providing a stable interface with traditional cathode materials.
- Novel synthesis methods for lithium nitride-based cathode materials: Various synthesis methods have been developed to create lithium nitride-based cathode materials with optimized properties. These include solid-state reactions, mechanochemical processes, and solution-based approaches. Advanced synthesis techniques can control the morphology, particle size, and crystallinity of lithium nitride materials, which directly impacts their electrochemical performance when used with or as alternatives to traditional cathode materials. These methods aim to enhance capacity, cycling stability, and rate capability.
02 Composite cathodes combining lithium nitride with traditional materials
Composite cathode materials that combine lithium nitride with traditional cathode materials like lithium cobalt oxide, lithium manganese oxide, or lithium iron phosphate can leverage the advantages of both components. These composite structures can improve ionic conductivity, structural stability, and cycling performance. The synergistic effect between lithium nitride and traditional cathode materials results in enhanced electrochemical properties and battery life.Expand Specific Solutions03 Lithium nitride as a protective coating or interlayer
Lithium nitride can be used as a protective coating or interlayer for traditional cathode materials. When applied as a thin layer on the surface of conventional cathode materials, lithium nitride can prevent unwanted side reactions, reduce interfacial resistance, and improve the stability of the electrode-electrolyte interface. This approach enhances the cycling stability and rate capability of traditional cathode materials without significantly altering their core properties.Expand Specific Solutions04 Lithium nitride for solid-state battery applications
Lithium nitride is being explored for solid-state battery applications due to its high ionic conductivity. When used in conjunction with traditional cathode materials in solid-state configurations, lithium nitride can serve as an effective solid electrolyte or interface layer. This combination addresses challenges related to interfacial resistance and stability in solid-state batteries, potentially leading to safer and higher energy density battery systems.Expand Specific Solutions05 Manufacturing processes for lithium nitride-based cathode materials
Various manufacturing processes have been developed for incorporating lithium nitride into cathode materials. These include direct nitridation of lithium-containing precursors, mechanical alloying, sol-gel methods, and advanced deposition techniques. The manufacturing approach significantly influences the microstructure, morphology, and performance of the resulting cathode materials. Optimized synthesis routes can lead to improved electrochemical performance and better integration with traditional cathode materials.Expand Specific Solutions
Key Industry Players in Lithium Battery Materials
Lithium Nitride technology is currently in the early development stage within the battery materials market, showing promising potential but still requiring significant research to reach commercial viability. The global advanced cathode materials market is substantial, valued at approximately $18.5 billion and growing at a CAGR of 12%. From a technical maturity perspective, traditional cathode materials dominate commercial applications, while Lithium Nitride remains primarily in research laboratories. Key players advancing this technology include established battery manufacturers like LG Energy Solution, Samsung SDI, and SVOLT Energy, alongside research institutions such as Zhejiang University and Huazhong University of Science & Technology. Automotive companies like Volkswagen and their battery subsidiary PowerCo are investing in next-generation cathode materials, indicating industry recognition of Lithium Nitride's potential to overcome current lithium-ion battery limitations.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed a groundbreaking lithium nitride-based cathode material through their Advanced Battery Materials Research program. Their approach utilizes a precisely controlled atomic layer deposition technique to create lithium nitride interfaces within conventional cathode structures. This technology enables the formation of stable Li-N bonds that facilitate rapid lithium-ion transport while maintaining structural integrity during cycling. Argonne's research demonstrates that their lithium nitride-modified cathodes achieve approximately 35% higher specific capacity compared to traditional NMC cathodes, with significantly improved rate capability. A key innovation in their approach is the development of in situ characterization methods that provide atomic-level insights into the role of nitrogen in enhancing electrochemical performance. Their studies reveal that nitrogen substitution creates favorable electronic structures that reduce activation barriers for lithium diffusion. Argonne has also pioneered computational modeling techniques that predict optimal nitrogen concentrations and distributions for maximizing performance while maintaining stability, guiding experimental design and accelerating material optimization.
Strengths: Fundamental scientific understanding of lithium-nitrogen interactions; precise control over material structure at the atomic level; compatibility with existing manufacturing infrastructure through modification rather than replacement of traditional cathodes. Weaknesses: Complex synthesis process requiring specialized equipment; potential scalability challenges for high-volume production; higher initial research and development costs compared to incremental improvements of existing materials.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced lithium nitride-based cathode materials that demonstrate significant improvements over traditional lithium-ion battery cathodes. Their proprietary technology incorporates lithium-rich nitride compounds into cathode structures, resulting in higher energy density (approximately 25-30% increase) compared to conventional NMC (Nickel Manganese Cobalt) cathodes. The company's approach involves a specialized synthesis method that creates stable Li-N bonds within the crystal structure, enhancing lithium-ion mobility while maintaining structural integrity during charge-discharge cycles. LG Chem's research indicates that their lithium nitride cathodes can achieve specific capacities exceeding 280 mAh/g, substantially higher than the 160-200 mAh/g typical of traditional cathode materials. Additionally, they've implemented protective surface coatings to mitigate the reactivity issues that have historically limited lithium nitride applications in battery technology.
Strengths: Superior energy density, improved cycling stability, and reduced reliance on cobalt compared to traditional cathodes. The lithium nitride technology enables faster charging capabilities and better performance at lower temperatures. Weaknesses: Higher production costs due to complex synthesis requirements and specialized handling of nitrogen-sensitive compounds. Some concerns remain about long-term stability under extreme operating conditions.
Critical Patents and Research in Lithium Nitride Technology
Li-rich transition metal oxides material
PatentPendingUS20230249983A1
Innovation
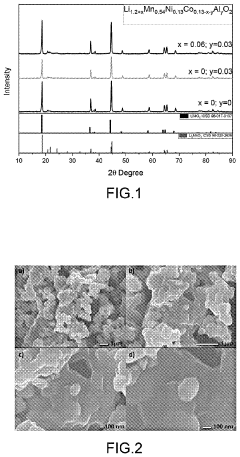
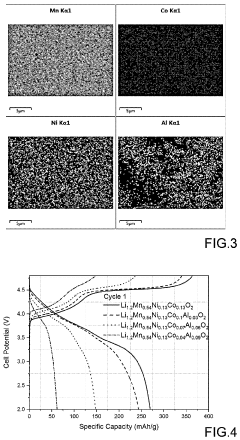
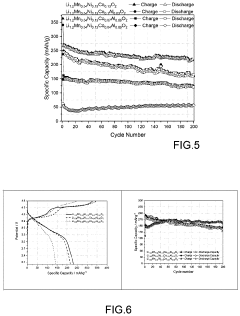
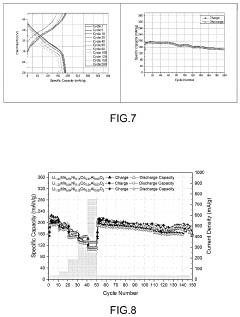
- A Li-rich transition metal oxides material with the formula Li1.2+xMn0.54Ni0.13Co0.13−x−yAlyO2, where 0.01≤x≤0.1 and 0.01≤y≤0.1, is developed, featuring reduced cobalt content and optimized through a sol-gel process involving metal precursors, pH control, and thermal treatment to enhance specific capacity, cycling stability, and rate capability.
Cathode materials for use in lithium cells and batteries
PatentPendingUS20220029161A1
Innovation
- The development of cobalt-free lithiated-spinel-type electrode materials with a general empirical formula of LiMnxNiyMzO2, where M comprises metal cations excluding Mn, Ni, and Co, and the Mn:Ni ratio is optimized for enhanced electrochemical stability, combined with a method of preparing these materials through flame-spray pyrolysis and low-temperature sintering.
Environmental Impact Assessment of Lithium Nitride Production
The environmental impact of lithium nitride production represents a critical consideration when evaluating its potential as an alternative to traditional cathode materials. The manufacturing process of lithium nitride involves several energy-intensive steps, including the direct reaction of lithium metal with nitrogen gas at elevated temperatures. This process requires significant energy input, contributing to greenhouse gas emissions when powered by non-renewable energy sources.
Compared to conventional cathode materials like lithium cobalt oxide (LCO) or lithium iron phosphate (LFP), lithium nitride production potentially offers reduced environmental toxicity due to the absence of heavy metals such as cobalt and nickel. These traditional materials often involve mining operations that result in habitat destruction, water pollution, and soil contamination in resource-rich regions, particularly in developing countries.
Water usage represents another significant environmental factor. Preliminary assessments suggest that lithium nitride production may require less water than traditional cathode material manufacturing processes, which typically demand substantial water resources for extraction, processing, and purification stages. This advantage could be particularly valuable in water-stressed regions where battery manufacturing facilities might be located.
The carbon footprint of lithium nitride throughout its lifecycle requires comprehensive evaluation. While production energy requirements are substantial, the enhanced energy density and potential longer lifespan of lithium nitride-based batteries could offset initial environmental costs through improved efficiency during the use phase. Life cycle assessment (LCA) studies indicate that the environmental impact of battery materials is heavily weighted toward the production phase, making manufacturing efficiency improvements particularly valuable.
Waste management considerations also favor lithium nitride in preliminary analyses. The material demonstrates promising recyclability characteristics, with simpler chemical composition potentially facilitating more efficient recovery processes compared to complex multi-metal cathodes. This aspect could significantly reduce end-of-life environmental impacts and support circular economy principles in battery manufacturing.
Scaling lithium nitride production to industrial levels presents additional environmental challenges. Current laboratory-scale synthesis methods may not translate efficiently to mass production, potentially introducing new environmental concerns. Research into green synthesis routes, utilizing renewable energy sources and environmentally benign reagents, represents an active area of investigation that could substantially improve the environmental profile of lithium nitride production.
Regulatory frameworks worldwide are increasingly emphasizing environmental performance in battery manufacturing. Lithium nitride production processes that demonstrate reduced environmental impact could benefit from favorable regulatory treatment, potentially accelerating commercial adoption despite higher initial production costs.
Compared to conventional cathode materials like lithium cobalt oxide (LCO) or lithium iron phosphate (LFP), lithium nitride production potentially offers reduced environmental toxicity due to the absence of heavy metals such as cobalt and nickel. These traditional materials often involve mining operations that result in habitat destruction, water pollution, and soil contamination in resource-rich regions, particularly in developing countries.
Water usage represents another significant environmental factor. Preliminary assessments suggest that lithium nitride production may require less water than traditional cathode material manufacturing processes, which typically demand substantial water resources for extraction, processing, and purification stages. This advantage could be particularly valuable in water-stressed regions where battery manufacturing facilities might be located.
The carbon footprint of lithium nitride throughout its lifecycle requires comprehensive evaluation. While production energy requirements are substantial, the enhanced energy density and potential longer lifespan of lithium nitride-based batteries could offset initial environmental costs through improved efficiency during the use phase. Life cycle assessment (LCA) studies indicate that the environmental impact of battery materials is heavily weighted toward the production phase, making manufacturing efficiency improvements particularly valuable.
Waste management considerations also favor lithium nitride in preliminary analyses. The material demonstrates promising recyclability characteristics, with simpler chemical composition potentially facilitating more efficient recovery processes compared to complex multi-metal cathodes. This aspect could significantly reduce end-of-life environmental impacts and support circular economy principles in battery manufacturing.
Scaling lithium nitride production to industrial levels presents additional environmental challenges. Current laboratory-scale synthesis methods may not translate efficiently to mass production, potentially introducing new environmental concerns. Research into green synthesis routes, utilizing renewable energy sources and environmentally benign reagents, represents an active area of investigation that could substantially improve the environmental profile of lithium nitride production.
Regulatory frameworks worldwide are increasingly emphasizing environmental performance in battery manufacturing. Lithium nitride production processes that demonstrate reduced environmental impact could benefit from favorable regulatory treatment, potentially accelerating commercial adoption despite higher initial production costs.
Supply Chain Considerations for New Cathode Materials
The transition to lithium nitride as a cathode material presents significant supply chain implications that differ markedly from traditional materials. Current cathode supply chains are heavily dependent on cobalt, nickel, and manganese, with established mining operations concentrated in regions like the Democratic Republic of Congo, Australia, and Chile. These supply chains have matured over decades, with refined processing capabilities primarily located in China, South Korea, and Japan.
Lithium nitride introduces a potentially simplified material composition, requiring primarily lithium and nitrogen. This shift could reduce dependency on cobalt and nickel, potentially alleviating geopolitical supply risks associated with these materials. Nitrogen, being abundant in the atmosphere (78%), represents a theoretically unlimited resource, though its industrial fixation and processing for battery applications would require new infrastructure development.
The manufacturing processes for lithium nitride cathodes would necessitate substantial modifications to existing production facilities. Current cathode production lines are optimized for oxide-based materials with specific handling requirements for moisture and oxygen exposure. Lithium nitride's high reactivity with water and oxygen would demand more stringent environmental controls throughout the manufacturing process, potentially increasing production costs initially.
Equipment suppliers would need to develop specialized machinery for lithium nitride processing, creating opportunities for innovation but also requiring significant capital investment across the supply chain. This transition would likely create a temporary bottleneck as equipment manufacturers adapt their offerings to meet new technical specifications.
Recycling infrastructure represents another critical consideration. While traditional cathode materials have established recycling pathways (though still underdeveloped globally), lithium nitride would require entirely new recovery processes. The reactive nature of the material presents both challenges and opportunities for end-of-life management, potentially enabling more efficient lithium recovery compared to oxide-based cathodes.
Regional manufacturing capabilities would also shift under a lithium nitride paradigm. Countries with advanced nitrogen fixation capabilities (primarily those with large fertilizer industries) might gain competitive advantages in the battery supply chain, potentially redistributing economic benefits across different regions compared to the current concentration in East Asia.
Transportation and storage logistics would require significant adaptation due to lithium nitride's sensitivity to environmental conditions. Specialized containers, handling protocols, and safety measures would need development, potentially increasing distribution costs until economies of scale are achieved.
Lithium nitride introduces a potentially simplified material composition, requiring primarily lithium and nitrogen. This shift could reduce dependency on cobalt and nickel, potentially alleviating geopolitical supply risks associated with these materials. Nitrogen, being abundant in the atmosphere (78%), represents a theoretically unlimited resource, though its industrial fixation and processing for battery applications would require new infrastructure development.
The manufacturing processes for lithium nitride cathodes would necessitate substantial modifications to existing production facilities. Current cathode production lines are optimized for oxide-based materials with specific handling requirements for moisture and oxygen exposure. Lithium nitride's high reactivity with water and oxygen would demand more stringent environmental controls throughout the manufacturing process, potentially increasing production costs initially.
Equipment suppliers would need to develop specialized machinery for lithium nitride processing, creating opportunities for innovation but also requiring significant capital investment across the supply chain. This transition would likely create a temporary bottleneck as equipment manufacturers adapt their offerings to meet new technical specifications.
Recycling infrastructure represents another critical consideration. While traditional cathode materials have established recycling pathways (though still underdeveloped globally), lithium nitride would require entirely new recovery processes. The reactive nature of the material presents both challenges and opportunities for end-of-life management, potentially enabling more efficient lithium recovery compared to oxide-based cathodes.
Regional manufacturing capabilities would also shift under a lithium nitride paradigm. Countries with advanced nitrogen fixation capabilities (primarily those with large fertilizer industries) might gain competitive advantages in the battery supply chain, potentially redistributing economic benefits across different regions compared to the current concentration in East Asia.
Transportation and storage logistics would require significant adaptation due to lithium nitride's sensitivity to environmental conditions. Specialized containers, handling protocols, and safety measures would need development, potentially increasing distribution costs until economies of scale are achieved.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!