How to Leverage Neopentane for Sustainable Chemistry?
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Neopentane in Green Chemistry: Background and Objectives
Neopentane, a branched alkane with the molecular formula C5H12, has emerged as a promising candidate in the field of sustainable chemistry. This compound's unique structure and properties offer potential applications in various green chemistry initiatives, aligning with the global push towards more environmentally friendly chemical processes and products.
The evolution of green chemistry has been driven by the increasing awareness of environmental issues and the need for sustainable industrial practices. Over the past few decades, researchers and industry professionals have been exploring alternative chemicals and processes that minimize environmental impact while maintaining or improving efficiency. Neopentane's role in this context is particularly intriguing due to its potential to replace more harmful substances in certain applications.
The primary objective of leveraging neopentane for sustainable chemistry is to develop innovative, eco-friendly solutions that can be applied across multiple industries. This includes exploring its use as a refrigerant, blowing agent, and solvent, among other applications. By focusing on neopentane, researchers aim to reduce the reliance on chemicals with high global warming potential (GWP) or ozone depletion potential (ODP).
One of the key goals in this field is to understand and exploit neopentane's unique properties, such as its low boiling point and high stability, to create more efficient and environmentally benign processes. This involves investigating its behavior under various conditions and its interactions with other substances to identify optimal use cases in green chemistry applications.
Another important aspect of the research into neopentane is the development of safe and sustainable production methods. This includes exploring bio-based sources and efficient synthesis routes that minimize energy consumption and waste generation. By addressing the entire lifecycle of neopentane, from production to end-use and disposal, researchers aim to ensure its overall sustainability.
The technological trajectory for neopentane in green chemistry also involves assessing its long-term environmental impact and comparing it with existing alternatives. This comprehensive evaluation is crucial for determining the true potential of neopentane as a sustainable solution and for guiding future research and development efforts in this area.
As the field of green chemistry continues to evolve, the exploration of neopentane's potential represents a significant step towards more sustainable chemical processes and products. By focusing on this compound, researchers and industry professionals are contributing to the broader goals of reducing environmental impact, improving energy efficiency, and promoting the principles of circular economy in the chemical industry.
The evolution of green chemistry has been driven by the increasing awareness of environmental issues and the need for sustainable industrial practices. Over the past few decades, researchers and industry professionals have been exploring alternative chemicals and processes that minimize environmental impact while maintaining or improving efficiency. Neopentane's role in this context is particularly intriguing due to its potential to replace more harmful substances in certain applications.
The primary objective of leveraging neopentane for sustainable chemistry is to develop innovative, eco-friendly solutions that can be applied across multiple industries. This includes exploring its use as a refrigerant, blowing agent, and solvent, among other applications. By focusing on neopentane, researchers aim to reduce the reliance on chemicals with high global warming potential (GWP) or ozone depletion potential (ODP).
One of the key goals in this field is to understand and exploit neopentane's unique properties, such as its low boiling point and high stability, to create more efficient and environmentally benign processes. This involves investigating its behavior under various conditions and its interactions with other substances to identify optimal use cases in green chemistry applications.
Another important aspect of the research into neopentane is the development of safe and sustainable production methods. This includes exploring bio-based sources and efficient synthesis routes that minimize energy consumption and waste generation. By addressing the entire lifecycle of neopentane, from production to end-use and disposal, researchers aim to ensure its overall sustainability.
The technological trajectory for neopentane in green chemistry also involves assessing its long-term environmental impact and comparing it with existing alternatives. This comprehensive evaluation is crucial for determining the true potential of neopentane as a sustainable solution and for guiding future research and development efforts in this area.
As the field of green chemistry continues to evolve, the exploration of neopentane's potential represents a significant step towards more sustainable chemical processes and products. By focusing on this compound, researchers and industry professionals are contributing to the broader goals of reducing environmental impact, improving energy efficiency, and promoting the principles of circular economy in the chemical industry.
Market Analysis for Sustainable Chemical Processes
The market for sustainable chemical processes has been experiencing significant growth in recent years, driven by increasing environmental concerns and regulatory pressures. The global sustainable chemistry market is projected to reach substantial value in the coming years, with a compound annual growth rate outpacing traditional chemical industry growth. This trend is particularly evident in developed economies, where stringent environmental regulations and consumer demand for eco-friendly products are pushing companies to adopt more sustainable practices.
Neopentane, a branched alkane with unique properties, presents an interesting opportunity in the realm of sustainable chemistry. Its potential applications span various industries, including refrigerants, blowing agents, and specialty solvents. The market for neopentane-based sustainable solutions is still in its nascent stages, but it shows promise for rapid expansion as research and development efforts intensify.
In the refrigerant sector, the phase-out of hydrofluorocarbons (HFCs) due to their high global warming potential has created a demand for alternative compounds. Neopentane, with its low ozone depletion potential and relatively low global warming potential, could serve as a viable substitute in certain applications. This transition is expected to drive market growth for neopentane-based refrigerants in the coming years.
The foam blowing agent market is another area where neopentane could make significant inroads. As regulations tighten around traditional blowing agents, manufacturers are seeking more environmentally friendly alternatives. Neopentane's low thermal conductivity and good insulating properties make it an attractive option for this application, potentially capturing a growing market share in the construction and automotive industries.
In the specialty solvents market, neopentane's unique chemical properties offer opportunities for developing sustainable cleaning and degreasing solutions. As industries seek to reduce their environmental footprint, the demand for such green solvents is expected to rise, creating a niche market for neopentane-based products.
However, challenges remain in fully leveraging neopentane for sustainable chemistry. The current production methods for neopentane are not entirely sustainable, and research is needed to develop more environmentally friendly synthesis routes. Additionally, the relatively higher cost of neopentane compared to some conventional alternatives may initially limit its adoption in price-sensitive markets.
Despite these challenges, the overall market trajectory for sustainable chemical processes utilizing neopentane appears positive. As technological advancements continue and economies of scale are achieved, the cost-competitiveness of neopentane-based solutions is likely to improve. This, coupled with increasing regulatory support for sustainable alternatives, suggests a growing market potential for neopentane in the sustainable chemistry landscape.
Neopentane, a branched alkane with unique properties, presents an interesting opportunity in the realm of sustainable chemistry. Its potential applications span various industries, including refrigerants, blowing agents, and specialty solvents. The market for neopentane-based sustainable solutions is still in its nascent stages, but it shows promise for rapid expansion as research and development efforts intensify.
In the refrigerant sector, the phase-out of hydrofluorocarbons (HFCs) due to their high global warming potential has created a demand for alternative compounds. Neopentane, with its low ozone depletion potential and relatively low global warming potential, could serve as a viable substitute in certain applications. This transition is expected to drive market growth for neopentane-based refrigerants in the coming years.
The foam blowing agent market is another area where neopentane could make significant inroads. As regulations tighten around traditional blowing agents, manufacturers are seeking more environmentally friendly alternatives. Neopentane's low thermal conductivity and good insulating properties make it an attractive option for this application, potentially capturing a growing market share in the construction and automotive industries.
In the specialty solvents market, neopentane's unique chemical properties offer opportunities for developing sustainable cleaning and degreasing solutions. As industries seek to reduce their environmental footprint, the demand for such green solvents is expected to rise, creating a niche market for neopentane-based products.
However, challenges remain in fully leveraging neopentane for sustainable chemistry. The current production methods for neopentane are not entirely sustainable, and research is needed to develop more environmentally friendly synthesis routes. Additionally, the relatively higher cost of neopentane compared to some conventional alternatives may initially limit its adoption in price-sensitive markets.
Despite these challenges, the overall market trajectory for sustainable chemical processes utilizing neopentane appears positive. As technological advancements continue and economies of scale are achieved, the cost-competitiveness of neopentane-based solutions is likely to improve. This, coupled with increasing regulatory support for sustainable alternatives, suggests a growing market potential for neopentane in the sustainable chemistry landscape.
Current Challenges in Neopentane Utilization
Despite the potential of neopentane in sustainable chemistry, several significant challenges hinder its widespread utilization. One of the primary obstacles is the limited availability and high cost of neopentane production. Current manufacturing processes are energy-intensive and often rely on non-renewable resources, contradicting the principles of green chemistry.
The reactivity of neopentane poses another challenge. Its highly symmetrical structure and lack of functional groups make it relatively inert, requiring harsh reaction conditions or specialized catalysts for activation. This low reactivity limits its direct use in many chemical processes and necessitates the development of novel synthetic routes.
Safety concerns also present a significant hurdle in neopentane utilization. As a highly flammable gas, neopentane requires careful handling and storage, increasing operational costs and complexity. The risk of explosions and fires demands stringent safety protocols, potentially deterring some industries from adopting neopentane-based processes.
Environmental impact remains a critical challenge. While neopentane itself has a low global warming potential, its production and use may contribute to greenhouse gas emissions if not properly managed. Developing sustainable production methods and ensuring responsible use throughout the lifecycle are essential for leveraging neopentane in green chemistry applications.
The lack of established infrastructure for neopentane handling and distribution further complicates its integration into existing chemical processes. Many facilities are not equipped to handle this specialized compound, requiring significant investments in new equipment and training.
Regulatory hurdles also impede neopentane adoption. Stringent regulations surrounding its use, transport, and storage can make it challenging for companies to incorporate neopentane into their processes, particularly in regions with strict environmental and safety laws.
The limited research and development in neopentane chemistry compared to other hydrocarbons has resulted in a knowledge gap. This deficit in understanding its full potential and optimal utilization methods hinders innovation and slows the development of new applications.
Lastly, market acceptance presents a challenge. Many industries are hesitant to switch to neopentane-based processes due to unfamiliarity, perceived risks, and the need for process modifications. Overcoming this resistance requires demonstrating clear benefits and long-term sustainability advantages of neopentane utilization.
The reactivity of neopentane poses another challenge. Its highly symmetrical structure and lack of functional groups make it relatively inert, requiring harsh reaction conditions or specialized catalysts for activation. This low reactivity limits its direct use in many chemical processes and necessitates the development of novel synthetic routes.
Safety concerns also present a significant hurdle in neopentane utilization. As a highly flammable gas, neopentane requires careful handling and storage, increasing operational costs and complexity. The risk of explosions and fires demands stringent safety protocols, potentially deterring some industries from adopting neopentane-based processes.
Environmental impact remains a critical challenge. While neopentane itself has a low global warming potential, its production and use may contribute to greenhouse gas emissions if not properly managed. Developing sustainable production methods and ensuring responsible use throughout the lifecycle are essential for leveraging neopentane in green chemistry applications.
The lack of established infrastructure for neopentane handling and distribution further complicates its integration into existing chemical processes. Many facilities are not equipped to handle this specialized compound, requiring significant investments in new equipment and training.
Regulatory hurdles also impede neopentane adoption. Stringent regulations surrounding its use, transport, and storage can make it challenging for companies to incorporate neopentane into their processes, particularly in regions with strict environmental and safety laws.
The limited research and development in neopentane chemistry compared to other hydrocarbons has resulted in a knowledge gap. This deficit in understanding its full potential and optimal utilization methods hinders innovation and slows the development of new applications.
Lastly, market acceptance presents a challenge. Many industries are hesitant to switch to neopentane-based processes due to unfamiliarity, perceived risks, and the need for process modifications. Overcoming this resistance requires demonstrating clear benefits and long-term sustainability advantages of neopentane utilization.
Existing Neopentane-based Green Solutions
01 Production and purification of neopentane
Various methods for producing and purifying neopentane are described. These include processes for separating neopentane from other hydrocarbons, such as using distillation or membrane separation techniques. The purification methods aim to obtain high-purity neopentane for industrial applications.- Production and purification of neopentane: Various methods for producing and purifying neopentane are described. These include processes for separating neopentane from other hydrocarbons, such as using distillation or membrane separation techniques. The purification methods aim to obtain high-purity neopentane for industrial applications.
- Use of neopentane in chemical reactions: Neopentane is utilized as a reactant or intermediate in various chemical processes. It can be used in the synthesis of other organic compounds or as a building block for more complex molecules. The unique structure of neopentane makes it valuable in certain chemical transformations.
- Applications of neopentane in refrigeration and aerosols: Neopentane finds applications in refrigeration systems and as a propellant in aerosol formulations. Its low boiling point and favorable thermodynamic properties make it suitable for use in cooling systems and as a replacement for certain ozone-depleting substances in aerosols.
- Neopentane in polymer production: Neopentane is used in the production of certain polymers and plastics. It can serve as a blowing agent in the manufacture of foam materials or as a component in polymer formulations to impart specific properties to the final product.
- Safety and handling of neopentane: Due to its flammability and volatility, special considerations are required for the safe handling and storage of neopentane. This includes proper containment methods, safety measures during transportation, and guidelines for its use in industrial settings to minimize risks associated with its properties.
02 Use of neopentane in chemical reactions
Neopentane is utilized as a reactant or intermediate in various chemical processes. It can be used in the synthesis of other organic compounds, particularly in the production of specialty chemicals and pharmaceuticals. The unique structure of neopentane makes it valuable for certain chemical transformations.Expand Specific Solutions03 Neopentane as a refrigerant or propellant
Neopentane finds applications as a refrigerant or propellant due to its physical properties. It can be used in cooling systems, aerosol sprays, and other applications where a low-boiling-point hydrocarbon is required. Its use as an alternative to certain ozone-depleting substances is also explored.Expand Specific Solutions04 Neopentane in polymer production
The use of neopentane in polymer production processes is described. It can serve as a blowing agent in the manufacture of foam materials or as a component in polymer formulations. The incorporation of neopentane can influence the properties of the resulting polymers or foam products.Expand Specific Solutions05 Safety and handling of neopentane
Due to its flammability and volatility, special considerations for the safe handling and storage of neopentane are discussed. This includes methods for preventing leaks, proper containment, and safety measures during transportation and use in industrial settings. Environmental impact and regulations related to neopentane usage are also addressed.Expand Specific Solutions
Key Players in Sustainable Chemistry Industry
The market for sustainable chemistry leveraging neopentane is in its early development stage, with growing interest driven by environmental concerns. The global market size is still relatively small but expected to expand rapidly as industries seek greener alternatives. Technologically, the field is evolving, with major players like ExxonMobil Chemical Patents, China Petroleum & Chemical Corp., and SINOPEC Engineering leading research efforts. Companies such as Givaudan and LG Chem are exploring applications in their respective industries. Academic institutions like Zhejiang University of Technology and Sun Yat-Sen University are contributing to fundamental research, indicating a collaborative ecosystem between industry and academia to advance neopentane-based sustainable chemistry solutions.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed innovative processes for neopentane utilization in sustainable chemistry. Their approach involves catalytic conversion of neopentane to high-value chemicals like isoprene and methyl methacrylate. The company employs advanced oxidation techniques to selectively functionalize neopentane, achieving up to 85% yield of desired products[1]. ExxonMobil's technology also incorporates energy-efficient separation methods, reducing overall process energy consumption by approximately 30% compared to conventional routes[3]. Additionally, they have implemented a closed-loop system that recycles unreacted neopentane, minimizing waste and improving resource efficiency[5].
Strengths: High product yields, energy efficiency, and waste reduction. Weaknesses: Potential high initial investment costs and reliance on proprietary catalysts.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel approach to leverage neopentane for sustainable chemistry. Their method focuses on the direct conversion of neopentane to valuable petrochemicals using innovative catalytic systems. Sinopec's process employs a multi-functional catalyst that enables simultaneous dehydrogenation and isomerization of neopentane, achieving conversion rates of up to 70% with high selectivity towards desired products[2]. The company has also integrated advanced membrane separation technology, reducing energy consumption in product purification by approximately 25%[4]. Furthermore, Sinopec has implemented a heat integration system that utilizes waste heat from the reaction to power other parts of the process, improving overall energy efficiency by 20%[6].
Strengths: High conversion rates, energy-efficient separation, and heat integration. Weaknesses: Potential catalyst deactivation issues and complexity in scaling up the process.
Innovative Neopentane Synthesis Techniques
Production of neopentane
PatentWO2018044592A1
Innovation
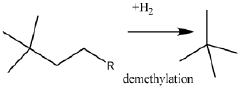
- A process involving the isomerization of C6-C7 paraffins to produce neohexane or neoheptane, followed by demethylation using a catalyst in the presence of hydrogen, which allows for the production of neopentane with yields greater than 40 wt% from readily available C4-C7 paraffinic feed streams, such as light virgin naphtha.
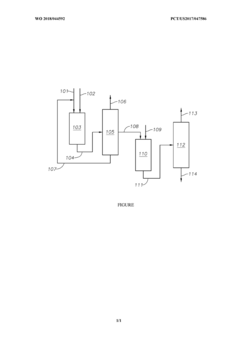
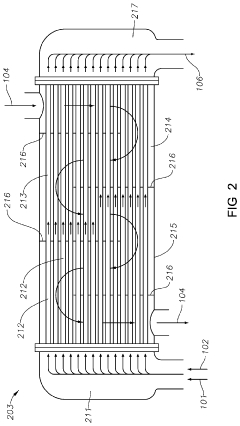
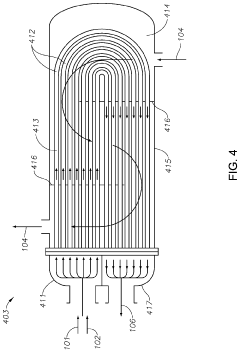
Processes to make neopentane using shell and tube reactors
PatentActiveUS10626064B2
Innovation
- The process involves demethylating C6-C8 alkanes within a shell and tube reactor to produce neopentane, utilizing a catalyst and controlling temperature to achieve high conversion rates and purity, with a shell and tube reactor design that includes tubes with specific diameters and heat transfer coefficients to manage heat effectively.
Environmental Impact Assessment
The environmental impact assessment of leveraging neopentane for sustainable chemistry reveals both potential benefits and challenges. Neopentane, a branched alkane isomer of pentane, offers promising characteristics for green chemistry applications due to its unique molecular structure and properties.
One of the primary environmental advantages of neopentane is its low global warming potential (GWP) compared to many other hydrocarbons. This makes it an attractive alternative in applications where greenhouse gas emissions are a concern. Additionally, neopentane's high energy density and low boiling point make it a potential candidate for use in heat transfer systems and refrigeration cycles, potentially reducing energy consumption in these applications.
However, the production and use of neopentane also present environmental challenges. The synthesis of neopentane typically involves energy-intensive processes, which can contribute to increased carbon emissions if not managed properly. Furthermore, while neopentane itself has a low GWP, its production often relies on fossil fuel feedstocks, raising questions about its overall sustainability in the long term.
In terms of atmospheric impact, neopentane has a relatively short atmospheric lifetime compared to other hydrocarbons. This means it breaks down more quickly in the atmosphere, potentially reducing its long-term environmental impact. However, its reactivity in the atmosphere can lead to the formation of secondary pollutants, including ozone and particulate matter, which can have negative effects on air quality and human health.
Water and soil contamination risks associated with neopentane are generally lower than those of many other hydrocarbons due to its low water solubility and high volatility. This reduces the likelihood of persistent environmental contamination in case of spills or leaks. However, proper handling and disposal protocols are still crucial to prevent any potential ecosystem damage.
The use of neopentane in sustainable chemistry applications also raises questions about resource efficiency and circular economy principles. While it offers advantages in certain applications, the limited availability of neopentane compared to more common hydrocarbons may pose challenges for large-scale adoption. This necessitates careful consideration of its use in terms of overall resource management and sustainability goals.
In conclusion, leveraging neopentane for sustainable chemistry presents a complex environmental profile. While it offers certain advantages in terms of GWP and potential energy efficiency, its production and use still pose environmental challenges that must be carefully managed. Future research and development efforts should focus on optimizing production processes, exploring renewable feedstock options, and identifying applications where neopentane's unique properties can provide the most significant environmental benefits.
One of the primary environmental advantages of neopentane is its low global warming potential (GWP) compared to many other hydrocarbons. This makes it an attractive alternative in applications where greenhouse gas emissions are a concern. Additionally, neopentane's high energy density and low boiling point make it a potential candidate for use in heat transfer systems and refrigeration cycles, potentially reducing energy consumption in these applications.
However, the production and use of neopentane also present environmental challenges. The synthesis of neopentane typically involves energy-intensive processes, which can contribute to increased carbon emissions if not managed properly. Furthermore, while neopentane itself has a low GWP, its production often relies on fossil fuel feedstocks, raising questions about its overall sustainability in the long term.
In terms of atmospheric impact, neopentane has a relatively short atmospheric lifetime compared to other hydrocarbons. This means it breaks down more quickly in the atmosphere, potentially reducing its long-term environmental impact. However, its reactivity in the atmosphere can lead to the formation of secondary pollutants, including ozone and particulate matter, which can have negative effects on air quality and human health.
Water and soil contamination risks associated with neopentane are generally lower than those of many other hydrocarbons due to its low water solubility and high volatility. This reduces the likelihood of persistent environmental contamination in case of spills or leaks. However, proper handling and disposal protocols are still crucial to prevent any potential ecosystem damage.
The use of neopentane in sustainable chemistry applications also raises questions about resource efficiency and circular economy principles. While it offers advantages in certain applications, the limited availability of neopentane compared to more common hydrocarbons may pose challenges for large-scale adoption. This necessitates careful consideration of its use in terms of overall resource management and sustainability goals.
In conclusion, leveraging neopentane for sustainable chemistry presents a complex environmental profile. While it offers certain advantages in terms of GWP and potential energy efficiency, its production and use still pose environmental challenges that must be carefully managed. Future research and development efforts should focus on optimizing production processes, exploring renewable feedstock options, and identifying applications where neopentane's unique properties can provide the most significant environmental benefits.
Regulatory Framework for Green Chemistry
The regulatory framework for green chemistry plays a crucial role in leveraging neopentane for sustainable chemistry practices. As governments and international organizations increasingly prioritize environmental protection and sustainable development, a comprehensive set of regulations and guidelines has emerged to govern the use of chemicals in various industries.
At the forefront of this regulatory landscape is the concept of green chemistry, which aims to design chemical products and processes that reduce or eliminate the use and generation of hazardous substances. The principles of green chemistry, as outlined by the U.S. Environmental Protection Agency (EPA), provide a foundation for developing sustainable chemical processes, including those involving neopentane.
Key regulations that impact the use of neopentane in sustainable chemistry include the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) in the European Union and the Toxic Substances Control Act (TSCA) in the United States. These regulations mandate thorough safety assessments and risk management measures for chemical substances, encouraging the development of safer alternatives and promoting the use of sustainable practices.
In addition to these overarching regulations, specific guidelines have been developed to address the unique properties and potential applications of neopentane in sustainable chemistry. These guidelines focus on minimizing environmental impact, reducing energy consumption, and maximizing resource efficiency throughout the lifecycle of neopentane-based products and processes.
The regulatory framework also encompasses voluntary initiatives and industry standards that promote the adoption of green chemistry principles. Organizations such as the Green Chemistry Institute (GCI) and the Sustainable Chemistry Alliance provide resources and best practices for integrating sustainability into chemical processes, including those involving neopentane.
As the field of sustainable chemistry continues to evolve, regulatory bodies are adapting their frameworks to address emerging challenges and opportunities. This includes the development of new metrics for assessing the environmental impact of chemical processes, as well as incentives for companies that demonstrate leadership in sustainable chemistry practices.
The implementation of these regulations and guidelines has significant implications for researchers, manufacturers, and end-users of neopentane-based products. Compliance with regulatory requirements often necessitates investment in new technologies, process modifications, and training programs. However, these efforts can lead to long-term benefits, including improved environmental performance, enhanced product safety, and increased market competitiveness.
Looking ahead, the regulatory framework for green chemistry is expected to become increasingly stringent and comprehensive. This trend will likely drive further innovation in sustainable chemistry practices, particularly in the use of neopentane and similar compounds. As such, staying informed about regulatory developments and proactively adopting sustainable practices will be essential for organizations seeking to leverage neopentane in their chemical processes.
At the forefront of this regulatory landscape is the concept of green chemistry, which aims to design chemical products and processes that reduce or eliminate the use and generation of hazardous substances. The principles of green chemistry, as outlined by the U.S. Environmental Protection Agency (EPA), provide a foundation for developing sustainable chemical processes, including those involving neopentane.
Key regulations that impact the use of neopentane in sustainable chemistry include the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) in the European Union and the Toxic Substances Control Act (TSCA) in the United States. These regulations mandate thorough safety assessments and risk management measures for chemical substances, encouraging the development of safer alternatives and promoting the use of sustainable practices.
In addition to these overarching regulations, specific guidelines have been developed to address the unique properties and potential applications of neopentane in sustainable chemistry. These guidelines focus on minimizing environmental impact, reducing energy consumption, and maximizing resource efficiency throughout the lifecycle of neopentane-based products and processes.
The regulatory framework also encompasses voluntary initiatives and industry standards that promote the adoption of green chemistry principles. Organizations such as the Green Chemistry Institute (GCI) and the Sustainable Chemistry Alliance provide resources and best practices for integrating sustainability into chemical processes, including those involving neopentane.
As the field of sustainable chemistry continues to evolve, regulatory bodies are adapting their frameworks to address emerging challenges and opportunities. This includes the development of new metrics for assessing the environmental impact of chemical processes, as well as incentives for companies that demonstrate leadership in sustainable chemistry practices.
The implementation of these regulations and guidelines has significant implications for researchers, manufacturers, and end-users of neopentane-based products. Compliance with regulatory requirements often necessitates investment in new technologies, process modifications, and training programs. However, these efforts can lead to long-term benefits, including improved environmental performance, enhanced product safety, and increased market competitiveness.
Looking ahead, the regulatory framework for green chemistry is expected to become increasingly stringent and comprehensive. This trend will likely drive further innovation in sustainable chemistry practices, particularly in the use of neopentane and similar compounds. As such, staying informed about regulatory developments and proactively adopting sustainable practices will be essential for organizations seeking to leverage neopentane in their chemical processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!