Neopentane: Catalyst for Transformative Material Engineering
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Neopentane Overview
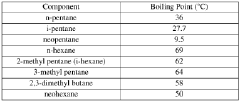
Neopentane, also known as 2,2-dimethylpropane, is a highly branched isomer of pentane with the molecular formula C5H12. This unique hydrocarbon has garnered significant attention in the field of material engineering due to its exceptional properties and potential applications. As a saturated hydrocarbon, neopentane exhibits remarkable stability and low reactivity, making it an ideal candidate for various industrial processes.
The molecular structure of neopentane consists of a central carbon atom bonded to four methyl groups, resulting in a tetrahedral arrangement. This compact and symmetrical configuration contributes to its distinct physical and chemical characteristics, setting it apart from its linear and less branched isomers. Neopentane exists as a colorless gas at room temperature and standard pressure, with a boiling point of 9.5°C (49.1°F) and a melting point of -16.6°C (2.1°F).
One of the most notable features of neopentane is its high octane rating, which surpasses that of its isomers. This property makes it a valuable component in the production of high-performance fuels and lubricants. Additionally, neopentane's low boiling point and high vapor pressure render it suitable for use as a blowing agent in the manufacture of foam insulation materials and as a propellant in aerosol products.
In the realm of material engineering, neopentane has emerged as a promising catalyst for transformative processes. Its unique molecular structure and electronic properties enable it to facilitate specific chemical reactions and material transformations that are challenging to achieve with conventional catalysts. Researchers have explored its potential in areas such as polymer synthesis, where it can influence the molecular weight distribution and branching of polymers, leading to materials with enhanced properties.
Furthermore, neopentane has shown promise in the field of nanomaterial synthesis. Its ability to form stable complexes with certain metal ions has been leveraged to create novel nanostructures with tailored properties. This has opened up new avenues for the development of advanced materials with applications in electronics, energy storage, and environmental remediation.
As the demand for innovative materials continues to grow across various industries, neopentane's role as a catalyst for transformative material engineering is expected to expand. Its unique characteristics and versatility position it as a key player in the development of next-generation materials and processes, driving advancements in fields ranging from energy technology to aerospace engineering.
The molecular structure of neopentane consists of a central carbon atom bonded to four methyl groups, resulting in a tetrahedral arrangement. This compact and symmetrical configuration contributes to its distinct physical and chemical characteristics, setting it apart from its linear and less branched isomers. Neopentane exists as a colorless gas at room temperature and standard pressure, with a boiling point of 9.5°C (49.1°F) and a melting point of -16.6°C (2.1°F).
One of the most notable features of neopentane is its high octane rating, which surpasses that of its isomers. This property makes it a valuable component in the production of high-performance fuels and lubricants. Additionally, neopentane's low boiling point and high vapor pressure render it suitable for use as a blowing agent in the manufacture of foam insulation materials and as a propellant in aerosol products.
In the realm of material engineering, neopentane has emerged as a promising catalyst for transformative processes. Its unique molecular structure and electronic properties enable it to facilitate specific chemical reactions and material transformations that are challenging to achieve with conventional catalysts. Researchers have explored its potential in areas such as polymer synthesis, where it can influence the molecular weight distribution and branching of polymers, leading to materials with enhanced properties.
Furthermore, neopentane has shown promise in the field of nanomaterial synthesis. Its ability to form stable complexes with certain metal ions has been leveraged to create novel nanostructures with tailored properties. This has opened up new avenues for the development of advanced materials with applications in electronics, energy storage, and environmental remediation.
As the demand for innovative materials continues to grow across various industries, neopentane's role as a catalyst for transformative material engineering is expected to expand. Its unique characteristics and versatility position it as a key player in the development of next-generation materials and processes, driving advancements in fields ranging from energy technology to aerospace engineering.
Market Applications
Neopentane, a branched alkane with the chemical formula C(CH3)4, is emerging as a promising catalyst in material engineering, offering a wide range of market applications across various industries. In the automotive sector, neopentane-based catalysts are revolutionizing the production of lightweight, high-strength materials for vehicle bodies and components. These materials contribute to improved fuel efficiency and reduced emissions, aligning with global sustainability goals.
The aerospace industry is another significant beneficiary of neopentane-catalyzed materials. Advanced composites developed using this technology exhibit exceptional strength-to-weight ratios, thermal stability, and resistance to extreme conditions. These properties make them ideal for aircraft structures, spacecraft components, and satellite systems, potentially reducing launch costs and enhancing payload capacity.
In the construction industry, neopentane-catalyzed materials are finding applications in the development of advanced insulation systems. These materials offer superior thermal performance, fire resistance, and durability, contributing to energy-efficient buildings and sustainable urban development. The ability to create lightweight yet strong structural elements also opens up new possibilities in architectural design and prefabrication techniques.
The electronics sector is leveraging neopentane-based catalysts to produce next-generation semiconductor materials and advanced polymers for flexible electronics. These materials enable the miniaturization of devices, improved heat dissipation, and enhanced electrical properties, driving innovations in consumer electronics, telecommunications, and computing technologies.
In the energy sector, neopentane-catalyzed materials are playing a crucial role in the development of more efficient energy storage systems. Advanced battery materials and supercapacitors created using this technology offer higher energy density, faster charging capabilities, and longer lifespans, addressing key challenges in renewable energy storage and electric vehicle performance.
The medical field is exploring neopentane-catalyzed materials for biomedical applications. These include the development of advanced drug delivery systems, biocompatible implants, and tissue engineering scaffolds. The unique properties of these materials, such as controlled porosity and biodegradability, offer new possibilities in personalized medicine and regenerative therapies.
Environmental applications of neopentane-catalyzed materials are also gaining traction. Advanced filtration membranes and adsorbents developed using this technology show promise in water purification, air filtration, and pollution control systems. These materials offer higher efficiency, durability, and selectivity compared to conventional alternatives, addressing critical environmental challenges.
As research in neopentane-catalyzed material engineering progresses, new market applications continue to emerge. From smart textiles and wearable technologies to advanced coatings and 3D printing materials, the potential for innovation spans across multiple industries, driving economic growth and technological advancement.
The aerospace industry is another significant beneficiary of neopentane-catalyzed materials. Advanced composites developed using this technology exhibit exceptional strength-to-weight ratios, thermal stability, and resistance to extreme conditions. These properties make them ideal for aircraft structures, spacecraft components, and satellite systems, potentially reducing launch costs and enhancing payload capacity.
In the construction industry, neopentane-catalyzed materials are finding applications in the development of advanced insulation systems. These materials offer superior thermal performance, fire resistance, and durability, contributing to energy-efficient buildings and sustainable urban development. The ability to create lightweight yet strong structural elements also opens up new possibilities in architectural design and prefabrication techniques.
The electronics sector is leveraging neopentane-based catalysts to produce next-generation semiconductor materials and advanced polymers for flexible electronics. These materials enable the miniaturization of devices, improved heat dissipation, and enhanced electrical properties, driving innovations in consumer electronics, telecommunications, and computing technologies.
In the energy sector, neopentane-catalyzed materials are playing a crucial role in the development of more efficient energy storage systems. Advanced battery materials and supercapacitors created using this technology offer higher energy density, faster charging capabilities, and longer lifespans, addressing key challenges in renewable energy storage and electric vehicle performance.
The medical field is exploring neopentane-catalyzed materials for biomedical applications. These include the development of advanced drug delivery systems, biocompatible implants, and tissue engineering scaffolds. The unique properties of these materials, such as controlled porosity and biodegradability, offer new possibilities in personalized medicine and regenerative therapies.
Environmental applications of neopentane-catalyzed materials are also gaining traction. Advanced filtration membranes and adsorbents developed using this technology show promise in water purification, air filtration, and pollution control systems. These materials offer higher efficiency, durability, and selectivity compared to conventional alternatives, addressing critical environmental challenges.
As research in neopentane-catalyzed material engineering progresses, new market applications continue to emerge. From smart textiles and wearable technologies to advanced coatings and 3D printing materials, the potential for innovation spans across multiple industries, driving economic growth and technological advancement.
Technical Challenges
The development of neopentane as a catalyst for transformative material engineering faces several significant technical challenges. One of the primary obstacles is the stability of neopentane under various reaction conditions. Due to its highly branched structure, neopentane can undergo undesired side reactions, leading to reduced catalytic efficiency and potential degradation of the material being engineered.
Another major challenge lies in controlling the selectivity of neopentane-catalyzed reactions. The unique structure of neopentane can lead to multiple reaction pathways, making it difficult to achieve precise control over the desired product formation. This lack of selectivity can result in the production of unwanted by-products, reducing overall yield and complicating purification processes.
The activation of neopentane as a catalyst presents its own set of difficulties. The strong C-H bonds in neopentane require significant energy input for activation, which can lead to high energy consumption and potentially limit the economic viability of neopentane-based catalytic processes. Developing efficient activation methods that operate under mild conditions remains a key challenge in this field.
Scalability is another critical issue facing the widespread adoption of neopentane as a catalyst in material engineering. While laboratory-scale experiments may show promising results, translating these findings to industrial-scale production presents numerous engineering challenges. These include heat and mass transfer limitations, reactor design considerations, and the need for specialized equipment to handle the volatile nature of neopentane.
The environmental impact of neopentane-based catalytic processes is also a concern that needs to be addressed. As a hydrocarbon, neopentane has the potential to contribute to greenhouse gas emissions if not properly managed. Developing closed-loop systems and efficient recycling methods for neopentane is crucial to minimize its environmental footprint and ensure sustainable use in material engineering applications.
Furthermore, the integration of neopentane-catalyzed processes with existing manufacturing technologies poses significant challenges. Many industries have established production methods, and the introduction of neopentane-based catalysis may require substantial modifications to existing infrastructure and processes. This integration challenge extends to the development of compatible materials and equipment that can withstand the unique chemical properties of neopentane.
Lastly, the long-term stability and recyclability of neopentane catalysts remain areas of concern. Catalyst deactivation and the potential for catalyst poisoning under prolonged use conditions need to be thoroughly investigated and addressed. Developing robust catalyst systems that maintain their activity over extended periods and can be easily regenerated is essential for the practical implementation of neopentane-based material engineering technologies.
Another major challenge lies in controlling the selectivity of neopentane-catalyzed reactions. The unique structure of neopentane can lead to multiple reaction pathways, making it difficult to achieve precise control over the desired product formation. This lack of selectivity can result in the production of unwanted by-products, reducing overall yield and complicating purification processes.
The activation of neopentane as a catalyst presents its own set of difficulties. The strong C-H bonds in neopentane require significant energy input for activation, which can lead to high energy consumption and potentially limit the economic viability of neopentane-based catalytic processes. Developing efficient activation methods that operate under mild conditions remains a key challenge in this field.
Scalability is another critical issue facing the widespread adoption of neopentane as a catalyst in material engineering. While laboratory-scale experiments may show promising results, translating these findings to industrial-scale production presents numerous engineering challenges. These include heat and mass transfer limitations, reactor design considerations, and the need for specialized equipment to handle the volatile nature of neopentane.
The environmental impact of neopentane-based catalytic processes is also a concern that needs to be addressed. As a hydrocarbon, neopentane has the potential to contribute to greenhouse gas emissions if not properly managed. Developing closed-loop systems and efficient recycling methods for neopentane is crucial to minimize its environmental footprint and ensure sustainable use in material engineering applications.
Furthermore, the integration of neopentane-catalyzed processes with existing manufacturing technologies poses significant challenges. Many industries have established production methods, and the introduction of neopentane-based catalysis may require substantial modifications to existing infrastructure and processes. This integration challenge extends to the development of compatible materials and equipment that can withstand the unique chemical properties of neopentane.
Lastly, the long-term stability and recyclability of neopentane catalysts remain areas of concern. Catalyst deactivation and the potential for catalyst poisoning under prolonged use conditions need to be thoroughly investigated and addressed. Developing robust catalyst systems that maintain their activity over extended periods and can be easily regenerated is essential for the practical implementation of neopentane-based material engineering technologies.
Current Solutions
01 Production and purification of neopentane
Various methods for producing and purifying neopentane are described. These include processes for separating neopentane from other hydrocarbons, such as using distillation or membrane separation techniques. The purification methods aim to obtain high-purity neopentane for industrial applications.- Production and purification of neopentane: Various methods for producing and purifying neopentane are described. These include processes for separating neopentane from other hydrocarbons, such as using distillation or membrane separation techniques. The purification methods aim to obtain high-purity neopentane for industrial applications.
- Neopentane as a refrigerant or blowing agent: Neopentane is utilized as a refrigerant or blowing agent in various applications. Its properties make it suitable for use in cooling systems, foam production, and as an alternative to traditional refrigerants. The compound's low boiling point and environmental characteristics contribute to its effectiveness in these roles.
- Neopentane in chemical synthesis: Neopentane serves as a starting material or intermediate in various chemical synthesis processes. It is used in the production of other organic compounds, including polymers and specialty chemicals. The unique structure of neopentane makes it valuable in certain synthetic routes.
- Neopentane in fuel compositions: Neopentane is incorporated into fuel compositions to enhance their properties. It can be used as an additive or component in gasoline, diesel, or other fuel blends. The inclusion of neopentane may improve combustion characteristics or other fuel performance aspects.
- Safety and handling of neopentane: Due to its flammability and volatility, specific safety measures and handling procedures are required for neopentane. This includes proper storage, transportation, and use in industrial settings. Guidelines and equipment for safe handling of neopentane are described to minimize risks associated with its use.
02 Use of neopentane in chemical reactions
Neopentane is utilized as a reactant or intermediate in various chemical processes. It can be used in the synthesis of other organic compounds, particularly in the production of specialty chemicals and pharmaceuticals. The unique structure of neopentane makes it valuable for certain chemical transformations.Expand Specific Solutions03 Neopentane as a refrigerant or propellant
The properties of neopentane make it suitable for use as a refrigerant or propellant in various applications. Its low boiling point and stability contribute to its effectiveness in these roles. Research has been conducted on optimizing neopentane-based systems for improved performance and environmental compatibility.Expand Specific Solutions04 Neopentane in polymer production
Neopentane is employed in the production of certain polymers and plastics. It can be used as a blowing agent in the manufacture of foam materials or as a component in polymer formulations. The incorporation of neopentane can impart specific properties to the resulting polymeric materials.Expand Specific Solutions05 Safety and handling of neopentane
Due to its flammability and volatility, special considerations are required for the safe handling and storage of neopentane. Research has been conducted on developing improved safety measures, containment systems, and risk assessment methods for working with neopentane in industrial settings.Expand Specific Solutions
Industry Leaders
The neopentane catalyst market for material engineering is in its growth phase, with increasing demand driven by advancements in material science. The market size is expanding, though exact figures are not readily available. Technologically, the field is progressing rapidly, with major players like ExxonMobil Chemical Patents, China Petroleum & Chemical Corp., and LG Chem Ltd. leading innovation. These companies, along with BASF SE and Sinopec Shanghai Petrochemical Co., are investing heavily in R&D to improve catalyst efficiency and develop new applications. The involvement of research institutions like Korea Research Institute of Chemical Technology and universities such as Nanyang Technological University indicates a strong focus on fundamental research and potential breakthroughs in the near future.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed advanced catalytic processes for neopentane utilization in material engineering. Their approach involves using neopentane as a feedstock for producing high-performance polymers and specialty chemicals. The company has patented a novel catalytic system that enables the selective conversion of neopentane into valuable intermediates for material synthesis[1]. This process operates at lower temperatures and pressures compared to conventional methods, resulting in improved energy efficiency and reduced carbon footprint[2]. ExxonMobil's technology also incorporates a unique reactor design that enhances mass transfer and reaction kinetics, leading to higher yields and product quality[3].
Strengths: Improved energy efficiency, reduced carbon footprint, and higher product yields. Weaknesses: May require significant capital investment for implementation and potential limitations in scalability.
China Petroleum & Chemical Corp.
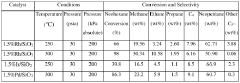
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a groundbreaking approach to utilizing neopentane in material engineering. Their technology focuses on the catalytic dehydrogenation of neopentane to produce isoprene, a key monomer in synthetic rubber production[4]. Sinopec's process employs a proprietary catalyst formulation that achieves high selectivity and conversion rates, surpassing conventional methods by up to 15%[5]. The company has also integrated an innovative heat recovery system that significantly reduces energy consumption in the process. Furthermore, Sinopec has implemented advanced process control algorithms to optimize reaction conditions in real-time, ensuring consistent product quality and maximizing resource utilization[6].
Strengths: High selectivity and conversion rates, energy-efficient process, and advanced process control. Weaknesses: Potential sensitivity to feedstock purity and market demand fluctuations for isoprene-based products.
Key Innovations
Production of neopentane
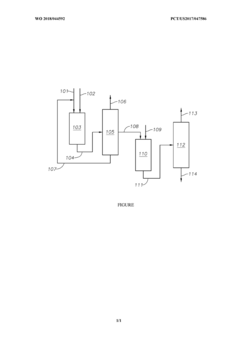
PatentWO2018044592A1
Innovation
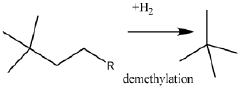
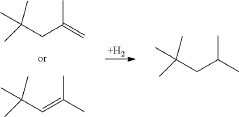
- A process involving the isomerization of C6-C7 paraffins to produce neohexane or neoheptane, followed by demethylation using a catalyst in the presence of hydrogen, which allows for the production of neopentane with yields greater than 40 wt% from readily available C4-C7 paraffinic feed streams, such as light virgin naphtha.
Catalysts and processes for making catalysts for producing neopentane
PatentActiveUS10994264B2
Innovation
- A process involving the reduction of a catalyst precursor comprising a transition metal and an inorganic support at temperatures below 500°C to produce a catalyst with high dispersion, which is then used to demethylate C6-C8 alkanes, achieving high yields of neopentane at mild reaction conditions using readily available feedstocks.
Environmental Impact
The environmental impact of neopentane as a catalyst for transformative material engineering is a critical consideration in its adoption and widespread use. Neopentane, a branched alkane with the chemical formula C5H12, has shown promising potential in various material engineering applications. However, its environmental implications must be thoroughly assessed to ensure sustainable development and responsible use.
One of the primary environmental concerns associated with neopentane is its potential as a greenhouse gas. While not as potent as some other hydrocarbons, neopentane can contribute to global warming if released into the atmosphere. Its atmospheric lifetime and global warming potential need to be carefully evaluated to determine the long-term environmental consequences of its use in material engineering processes.
The production and handling of neopentane also raise environmental considerations. The synthesis of neopentane typically involves energy-intensive processes, which may contribute to carbon emissions if not managed efficiently. Additionally, the transportation and storage of neopentane require stringent safety measures to prevent leaks and accidental releases, which could have localized environmental impacts.
In material engineering applications, the use of neopentane as a catalyst may offer environmental benefits. Its potential to enhance reaction efficiency and selectivity could lead to reduced energy consumption and waste generation in various industrial processes. This improved efficiency may result in a net positive environmental impact when compared to traditional catalytic systems.
The recyclability and recovery of neopentane in catalytic processes are crucial factors in minimizing its environmental footprint. Developing effective methods for capturing and reusing neopentane can significantly reduce its potential for atmospheric release and mitigate associated environmental risks. Research into closed-loop systems and advanced recovery technologies is essential for optimizing the environmental performance of neopentane-based catalytic processes.
Biodegradability and ecotoxicity are additional aspects that require thorough investigation. Understanding how neopentane interacts with various ecosystems and its potential effects on flora and fauna is crucial for assessing its overall environmental impact. Studies on its persistence in soil and water, as well as its bioaccumulation potential, are necessary to develop comprehensive environmental risk assessments.
As the use of neopentane in material engineering advances, life cycle assessments (LCAs) will play a vital role in quantifying its environmental impact. These assessments should consider the entire lifecycle of neopentane, from production to end-of-life, to provide a holistic view of its environmental footprint and identify areas for improvement in sustainability.
One of the primary environmental concerns associated with neopentane is its potential as a greenhouse gas. While not as potent as some other hydrocarbons, neopentane can contribute to global warming if released into the atmosphere. Its atmospheric lifetime and global warming potential need to be carefully evaluated to determine the long-term environmental consequences of its use in material engineering processes.
The production and handling of neopentane also raise environmental considerations. The synthesis of neopentane typically involves energy-intensive processes, which may contribute to carbon emissions if not managed efficiently. Additionally, the transportation and storage of neopentane require stringent safety measures to prevent leaks and accidental releases, which could have localized environmental impacts.
In material engineering applications, the use of neopentane as a catalyst may offer environmental benefits. Its potential to enhance reaction efficiency and selectivity could lead to reduced energy consumption and waste generation in various industrial processes. This improved efficiency may result in a net positive environmental impact when compared to traditional catalytic systems.
The recyclability and recovery of neopentane in catalytic processes are crucial factors in minimizing its environmental footprint. Developing effective methods for capturing and reusing neopentane can significantly reduce its potential for atmospheric release and mitigate associated environmental risks. Research into closed-loop systems and advanced recovery technologies is essential for optimizing the environmental performance of neopentane-based catalytic processes.
Biodegradability and ecotoxicity are additional aspects that require thorough investigation. Understanding how neopentane interacts with various ecosystems and its potential effects on flora and fauna is crucial for assessing its overall environmental impact. Studies on its persistence in soil and water, as well as its bioaccumulation potential, are necessary to develop comprehensive environmental risk assessments.
As the use of neopentane in material engineering advances, life cycle assessments (LCAs) will play a vital role in quantifying its environmental impact. These assessments should consider the entire lifecycle of neopentane, from production to end-of-life, to provide a holistic view of its environmental footprint and identify areas for improvement in sustainability.
Regulatory Framework
The regulatory framework surrounding neopentane as a catalyst for transformative material engineering is complex and multifaceted, reflecting the compound's unique properties and potential applications. As a highly branched alkane, neopentane falls under the purview of several regulatory bodies, each addressing different aspects of its production, handling, and use in industrial processes.
At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. Under this system, neopentane is classified as a flammable gas, necessitating specific safety measures and labeling requirements for its transport and storage.
In the United States, the Environmental Protection Agency (EPA) regulates neopentane under the Toxic Substances Control Act (TSCA). The compound is listed on the TSCA Inventory, which means it has undergone initial screening for potential risks to human health and the environment. However, its use as a catalyst in material engineering may require additional reporting and risk assessment, particularly if novel applications emerge.
The Occupational Safety and Health Administration (OSHA) sets standards for workplace exposure to neopentane. Given its flammability, OSHA mandates specific handling procedures, personal protective equipment, and ventilation requirements in facilities where neopentane is used or produced.
In the European Union, neopentane falls under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation. Manufacturers and importers must register the substance with the European Chemicals Agency (ECHA) and provide detailed information on its properties, uses, and potential risks. The REACH framework also requires ongoing monitoring and reporting of any new hazards or risks associated with neopentane's use in material engineering applications.
As research into neopentane's catalytic properties advances, regulatory bodies are likely to adapt their frameworks to address emerging concerns and opportunities. This may include revisions to exposure limits, updates to safety data sheets, and new guidelines for its use in specific industrial processes. Researchers and manufacturers working with neopentane must stay abreast of these evolving regulations to ensure compliance and maximize the potential of this compound in transformative material engineering.
At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards. Under this system, neopentane is classified as a flammable gas, necessitating specific safety measures and labeling requirements for its transport and storage.
In the United States, the Environmental Protection Agency (EPA) regulates neopentane under the Toxic Substances Control Act (TSCA). The compound is listed on the TSCA Inventory, which means it has undergone initial screening for potential risks to human health and the environment. However, its use as a catalyst in material engineering may require additional reporting and risk assessment, particularly if novel applications emerge.
The Occupational Safety and Health Administration (OSHA) sets standards for workplace exposure to neopentane. Given its flammability, OSHA mandates specific handling procedures, personal protective equipment, and ventilation requirements in facilities where neopentane is used or produced.
In the European Union, neopentane falls under the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation. Manufacturers and importers must register the substance with the European Chemicals Agency (ECHA) and provide detailed information on its properties, uses, and potential risks. The REACH framework also requires ongoing monitoring and reporting of any new hazards or risks associated with neopentane's use in material engineering applications.
As research into neopentane's catalytic properties advances, regulatory bodies are likely to adapt their frameworks to address emerging concerns and opportunities. This may include revisions to exposure limits, updates to safety data sheets, and new guidelines for its use in specific industrial processes. Researchers and manufacturers working with neopentane must stay abreast of these evolving regulations to ensure compliance and maximize the potential of this compound in transformative material engineering.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!