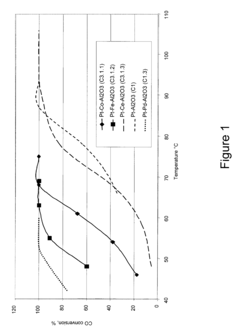
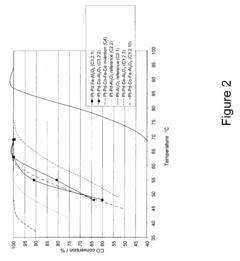
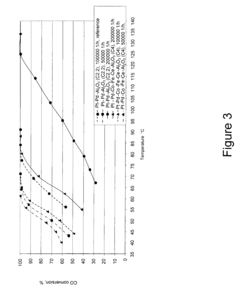
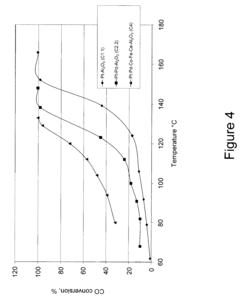
How to Use Tartaric Acid in Fuel Cell Catalysts
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tartaric Acid in Fuel Cell Catalysts: Background and Objectives
Tartaric acid, a naturally occurring organic compound found in various fruits, has emerged as a promising component in fuel cell catalyst development. The evolution of fuel cell technology spans over a century, with significant advancements occurring in the past few decades as global energy demands shift toward cleaner alternatives. Tartaric acid's potential in this field represents an intersection of organic chemistry and renewable energy technology, offering novel approaches to catalyst design and performance enhancement.
The trajectory of fuel cell technology has been marked by continuous efforts to improve efficiency, reduce costs, and enhance durability. Traditional catalysts, primarily based on platinum and other precious metals, have dominated the field but present significant economic and sustainability challenges. The introduction of organic compounds like tartaric acid into catalyst formulations represents a paradigm shift in fuel cell development, potentially addressing these longstanding issues through innovative chemical approaches.
Research into tartaric acid applications in fuel cell catalysts has gained momentum in recent years, driven by its unique stereochemical properties and ability to function as a structure-directing agent. The chiral nature of tartaric acid enables precise control over metal nanoparticle formation, potentially leading to catalysts with enhanced selectivity and activity. Additionally, its biodegradability and renewable sourcing align with the broader sustainability goals of fuel cell technology.
The technical objectives for tartaric acid in fuel cell catalysts encompass several dimensions. Primary goals include reducing platinum loading in catalyst formulations while maintaining or improving catalytic activity, enhancing catalyst durability under operational conditions, and developing scalable synthesis methods suitable for commercial production. Secondary objectives focus on understanding the fundamental mechanisms of tartaric acid interactions with metal surfaces and optimizing these interactions for specific fuel cell types.
Current research trends indicate growing interest in tartaric acid as both a template and stabilizing agent in catalyst preparation. Its ability to form complexes with various metals offers versatile approaches to catalyst design. The stereoisomers of tartaric acid (L-, D-, and meso-) provide additional variables for optimization, potentially enabling tailored solutions for different fuel cell architectures and operating conditions.
The integration of tartaric acid into fuel cell catalyst development represents a convergence of green chemistry principles with advanced materials science. As research progresses, the potential applications extend beyond traditional hydrogen fuel cells to include direct methanol fuel cells, microbial fuel cells, and other emerging technologies in the clean energy landscape.
The trajectory of fuel cell technology has been marked by continuous efforts to improve efficiency, reduce costs, and enhance durability. Traditional catalysts, primarily based on platinum and other precious metals, have dominated the field but present significant economic and sustainability challenges. The introduction of organic compounds like tartaric acid into catalyst formulations represents a paradigm shift in fuel cell development, potentially addressing these longstanding issues through innovative chemical approaches.
Research into tartaric acid applications in fuel cell catalysts has gained momentum in recent years, driven by its unique stereochemical properties and ability to function as a structure-directing agent. The chiral nature of tartaric acid enables precise control over metal nanoparticle formation, potentially leading to catalysts with enhanced selectivity and activity. Additionally, its biodegradability and renewable sourcing align with the broader sustainability goals of fuel cell technology.
The technical objectives for tartaric acid in fuel cell catalysts encompass several dimensions. Primary goals include reducing platinum loading in catalyst formulations while maintaining or improving catalytic activity, enhancing catalyst durability under operational conditions, and developing scalable synthesis methods suitable for commercial production. Secondary objectives focus on understanding the fundamental mechanisms of tartaric acid interactions with metal surfaces and optimizing these interactions for specific fuel cell types.
Current research trends indicate growing interest in tartaric acid as both a template and stabilizing agent in catalyst preparation. Its ability to form complexes with various metals offers versatile approaches to catalyst design. The stereoisomers of tartaric acid (L-, D-, and meso-) provide additional variables for optimization, potentially enabling tailored solutions for different fuel cell architectures and operating conditions.
The integration of tartaric acid into fuel cell catalyst development represents a convergence of green chemistry principles with advanced materials science. As research progresses, the potential applications extend beyond traditional hydrogen fuel cells to include direct methanol fuel cells, microbial fuel cells, and other emerging technologies in the clean energy landscape.
Market Analysis for Tartaric Acid-Modified Fuel Cell Technologies
The global market for fuel cell technologies has been experiencing significant growth, with a compound annual growth rate exceeding 25% since 2020. This expansion is primarily driven by increasing environmental concerns, stringent emission regulations, and the global push toward sustainable energy solutions. Within this landscape, tartaric acid-modified fuel cell catalysts represent an emerging niche with substantial growth potential due to their enhanced performance characteristics and cost-effectiveness compared to traditional platinum-based catalysts.
The market for tartaric acid in fuel cell applications can be segmented into transportation, stationary power generation, and portable power systems. The transportation sector, particularly hydrogen fuel cell vehicles, currently dominates the demand, accounting for approximately 60% of the market share. Major automotive manufacturers including Toyota, Hyundai, and Honda have increased investments in fuel cell technology, creating a robust demand pipeline for advanced catalysts.
Geographically, Asia-Pacific leads the market adoption of tartaric acid-modified fuel cell technologies, with Japan, South Korea, and China at the forefront. These countries have implemented favorable government policies and substantial subsidies to promote clean energy technologies. The European market follows closely, driven by ambitious carbon neutrality targets and strong regulatory frameworks supporting hydrogen infrastructure development.
The economic value proposition of tartaric acid as a catalyst modifier is compelling. Traditional platinum-based catalysts contribute up to 40% of a fuel cell stack's cost. Tartaric acid modification can reduce platinum loading by 30-50% while maintaining or improving performance, potentially decreasing overall catalyst costs by 25-35%. This cost advantage is particularly significant for mass-market applications where price sensitivity remains a barrier to widespread adoption.
Market forecasts indicate that the global demand for tartaric acid in fuel cell applications could reach 5,000 metric tons annually by 2030, representing a specialized but high-value segment within the broader organic acids market. The pricing premium for high-purity tartaric acid suitable for catalyst applications ranges between 30-45% above standard industrial grades.
Key market challenges include supply chain vulnerabilities, as high-purity tartaric acid production is concentrated among a limited number of manufacturers. Additionally, competing technologies such as non-precious metal catalysts and alternative acid modifiers present market risks that require continuous innovation to maintain competitive advantage.
Consumer and industry acceptance trends show increasing receptiveness to tartaric acid-modified fuel cells, particularly as performance data from real-world deployments demonstrates reliability and durability advantages. This positive reception is reinforced by the acid's natural origin, aligning with growing preferences for bio-based components in clean energy technologies.
The market for tartaric acid in fuel cell applications can be segmented into transportation, stationary power generation, and portable power systems. The transportation sector, particularly hydrogen fuel cell vehicles, currently dominates the demand, accounting for approximately 60% of the market share. Major automotive manufacturers including Toyota, Hyundai, and Honda have increased investments in fuel cell technology, creating a robust demand pipeline for advanced catalysts.
Geographically, Asia-Pacific leads the market adoption of tartaric acid-modified fuel cell technologies, with Japan, South Korea, and China at the forefront. These countries have implemented favorable government policies and substantial subsidies to promote clean energy technologies. The European market follows closely, driven by ambitious carbon neutrality targets and strong regulatory frameworks supporting hydrogen infrastructure development.
The economic value proposition of tartaric acid as a catalyst modifier is compelling. Traditional platinum-based catalysts contribute up to 40% of a fuel cell stack's cost. Tartaric acid modification can reduce platinum loading by 30-50% while maintaining or improving performance, potentially decreasing overall catalyst costs by 25-35%. This cost advantage is particularly significant for mass-market applications where price sensitivity remains a barrier to widespread adoption.
Market forecasts indicate that the global demand for tartaric acid in fuel cell applications could reach 5,000 metric tons annually by 2030, representing a specialized but high-value segment within the broader organic acids market. The pricing premium for high-purity tartaric acid suitable for catalyst applications ranges between 30-45% above standard industrial grades.
Key market challenges include supply chain vulnerabilities, as high-purity tartaric acid production is concentrated among a limited number of manufacturers. Additionally, competing technologies such as non-precious metal catalysts and alternative acid modifiers present market risks that require continuous innovation to maintain competitive advantage.
Consumer and industry acceptance trends show increasing receptiveness to tartaric acid-modified fuel cells, particularly as performance data from real-world deployments demonstrates reliability and durability advantages. This positive reception is reinforced by the acid's natural origin, aligning with growing preferences for bio-based components in clean energy technologies.
Current Challenges in Catalyst Development Using Tartaric Acid
The development of fuel cell catalysts using tartaric acid faces several significant challenges that impede widespread commercial adoption. One primary obstacle is achieving consistent chiral modification of metal surfaces. While tartaric acid can effectively create enantioselective sites on platinum and other noble metal catalysts, maintaining uniform chirality across the entire catalyst surface remains difficult. Environmental factors such as temperature fluctuations and pH variations can alter the binding configuration of tartaric acid, resulting in inconsistent catalytic performance.
Cost considerations present another major hurdle. Noble metals like platinum and palladium, which show the highest activity when modified with tartaric acid, are extremely expensive and subject to market volatility. Although tartaric acid itself is relatively inexpensive, the overall catalyst system remains costly due to the precious metal substrate requirements. This economic barrier significantly limits large-scale implementation in commercial fuel cell applications.
Durability issues also plague tartaric acid-modified catalysts. Under the harsh operating conditions of fuel cells, including high temperatures, varying humidity levels, and exposure to contaminants, the organic tartaric acid modifier tends to degrade or detach from the metal surface. This degradation leads to declining catalyst performance over time and necessitates frequent replacement, further increasing operational costs.
The optimization of tartaric acid concentration presents another technical challenge. Insufficient tartaric acid fails to adequately modify the catalyst surface, while excessive amounts can block active sites and reduce overall catalytic activity. Finding the precise balance requires extensive experimentation across different catalyst compositions and operating conditions.
Scalability concerns further complicate industrial adoption. Laboratory-scale synthesis methods that produce highly effective tartaric acid-modified catalysts often encounter difficulties when scaled to industrial production volumes. Maintaining precise control over surface modification during mass production remains problematic, resulting in batch-to-batch variability and inconsistent performance metrics.
Integration challenges with existing fuel cell systems also present significant barriers. Current fuel cell designs may require substantial modification to accommodate tartaric acid-modified catalysts, particularly regarding temperature management and electrolyte compatibility. The acid-base interactions between tartaric acid and common fuel cell components can lead to unexpected degradation mechanisms that are difficult to predict and mitigate.
Finally, knowledge gaps in the fundamental understanding of how tartaric acid interacts with different metal surfaces at the molecular level limit optimization efforts. While empirical results demonstrate enhanced activity, the precise mechanisms governing these improvements remain incompletely characterized, hampering rational design approaches for next-generation catalysts.
Cost considerations present another major hurdle. Noble metals like platinum and palladium, which show the highest activity when modified with tartaric acid, are extremely expensive and subject to market volatility. Although tartaric acid itself is relatively inexpensive, the overall catalyst system remains costly due to the precious metal substrate requirements. This economic barrier significantly limits large-scale implementation in commercial fuel cell applications.
Durability issues also plague tartaric acid-modified catalysts. Under the harsh operating conditions of fuel cells, including high temperatures, varying humidity levels, and exposure to contaminants, the organic tartaric acid modifier tends to degrade or detach from the metal surface. This degradation leads to declining catalyst performance over time and necessitates frequent replacement, further increasing operational costs.
The optimization of tartaric acid concentration presents another technical challenge. Insufficient tartaric acid fails to adequately modify the catalyst surface, while excessive amounts can block active sites and reduce overall catalytic activity. Finding the precise balance requires extensive experimentation across different catalyst compositions and operating conditions.
Scalability concerns further complicate industrial adoption. Laboratory-scale synthesis methods that produce highly effective tartaric acid-modified catalysts often encounter difficulties when scaled to industrial production volumes. Maintaining precise control over surface modification during mass production remains problematic, resulting in batch-to-batch variability and inconsistent performance metrics.
Integration challenges with existing fuel cell systems also present significant barriers. Current fuel cell designs may require substantial modification to accommodate tartaric acid-modified catalysts, particularly regarding temperature management and electrolyte compatibility. The acid-base interactions between tartaric acid and common fuel cell components can lead to unexpected degradation mechanisms that are difficult to predict and mitigate.
Finally, knowledge gaps in the fundamental understanding of how tartaric acid interacts with different metal surfaces at the molecular level limit optimization efforts. While empirical results demonstrate enhanced activity, the precise mechanisms governing these improvements remain incompletely characterized, hampering rational design approaches for next-generation catalysts.
Existing Methodologies for Tartaric Acid Integration in Catalysts
01 Tartaric acid as a stabilizing agent in catalyst preparation
Tartaric acid can be used as a stabilizing agent during the preparation of fuel cell catalysts. It helps to control the size and distribution of catalyst particles, preventing agglomeration and ensuring uniform dispersion on the support material. This results in improved catalyst performance and durability in fuel cell applications. The stabilizing effect of tartaric acid contributes to higher catalytic activity and better utilization of precious metals.- Tartaric acid as a stabilizing agent for catalyst preparation: Tartaric acid is used as a stabilizing agent in the preparation of fuel cell catalysts, particularly for platinum-based catalysts. It helps control particle size and prevents agglomeration during synthesis, resulting in catalysts with higher surface area and improved electrochemical performance. The acid forms complexes with metal precursors, enabling more uniform distribution of catalyst particles on support materials.
- Tartaric acid as a structure-directing agent: Tartaric acid functions as a structure-directing agent in fuel cell catalyst synthesis, influencing the morphology and crystalline structure of catalysts. Its chiral nature can induce specific crystallographic orientations that enhance catalytic activity toward oxygen reduction reactions. By controlling the crystal growth process, tartaric acid helps develop catalysts with preferential exposure of active crystal facets.
- Tartaric acid for enhanced catalyst durability: Incorporating tartaric acid during catalyst preparation improves the durability of fuel cell catalysts under operating conditions. The acid helps create stronger interactions between catalyst nanoparticles and support materials, reducing dissolution and migration during electrochemical cycling. This results in catalysts with extended operational lifetimes and reduced performance degradation over time.
- Tartaric acid in carbon-supported catalyst synthesis: Tartaric acid plays a crucial role in the synthesis of carbon-supported catalysts for fuel cells. It facilitates better dispersion of metal precursors on carbon supports and helps control the reduction process. The acid's functional groups interact with both the metal ions and carbon surface, creating anchoring sites that prevent catalyst migration and agglomeration during high-temperature treatment processes.
- Tartaric acid for controlling catalyst composition and alloying: Tartaric acid is employed to control the composition and alloying behavior in multi-metallic fuel cell catalysts. It forms complexes with different metal precursors at varying strengths, allowing for sequential or simultaneous reduction of metals to achieve desired alloy compositions. This selective complexation helps in developing core-shell structures or specific alloy phases that exhibit enhanced catalytic activity and selectivity.
02 Tartaric acid as a structure-directing agent for catalyst morphology
Tartaric acid functions as a structure-directing agent that influences the morphology and crystal structure of fuel cell catalysts. By controlling the growth direction of catalyst particles, tartaric acid helps to create specific surface structures that enhance catalytic activity. This approach allows for the development of catalysts with optimized shapes and exposed crystal facets that are more effective for electrochemical reactions in fuel cells.Expand Specific Solutions03 Tartaric acid for enhanced catalyst durability and performance
The incorporation of tartaric acid in fuel cell catalyst formulations can significantly enhance catalyst durability and performance. Tartaric acid helps to create more stable catalyst structures that resist degradation during fuel cell operation. This results in extended catalyst lifetime, improved power density, and better overall fuel cell efficiency. The enhanced durability is particularly important for commercial applications where long-term stability is required.Expand Specific Solutions04 Tartaric acid in green synthesis methods for fuel cell catalysts
Tartaric acid plays a key role in environmentally friendly synthesis methods for fuel cell catalysts. As a natural organic acid, it serves as a green reducing agent and template in the preparation process, reducing the need for harsh chemicals. These green synthesis approaches using tartaric acid result in catalysts with high activity while minimizing environmental impact. The methods are particularly valuable for sustainable production of clean energy technologies.Expand Specific Solutions05 Tartaric acid for controlling metal loading and composition in multi-metallic catalysts
Tartaric acid can be used to control metal loading and composition in multi-metallic fuel cell catalysts. By forming complexes with different metal ions at varying strengths, tartaric acid enables precise control over the ratio and distribution of metals in alloy catalysts. This approach allows for the development of advanced catalyst formulations with optimized composition for specific fuel cell reactions, resulting in improved catalytic activity and selectivity.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Fuel Cell Catalysts
The fuel cell catalyst market utilizing tartaric acid is currently in a growth phase, with increasing adoption across automotive and energy sectors. The market is expanding rapidly, projected to reach significant scale as hydrogen technologies gain traction in clean energy transitions. Technologically, established players like Toyota Motor Corp. and Johnson Matthey lead innovation with substantial patent portfolios and commercial applications, particularly in proton exchange membrane fuel cells. Research institutions including KAIST, GIST, and Advanced Industrial Science & Technology are advancing fundamental catalyst science, while companies like Resonac Holdings and Toshiba are developing manufacturing capabilities. Emerging players from China (Weichai Power, South China University of Technology) are increasingly challenging traditional market leaders through cost-effective solutions and government support, creating a dynamic competitive landscape poised for consolidation as the technology matures.
Toyota Motor Corp.
Technical Solution: Toyota has developed innovative fuel cell catalysts incorporating tartaric acid as a structure-directing agent to enhance platinum nanoparticle distribution on carbon supports. Their approach involves using tartaric acid as a chiral modifier during catalyst synthesis, which helps control the morphology and size of platinum particles. This results in higher catalytic activity and improved durability for their fuel cell systems. Toyota's research demonstrates that tartaric acid can effectively prevent platinum agglomeration during the reduction process, leading to smaller particle sizes (2-3 nm) and more uniform distribution. The company has integrated this technology into their latest generation of fuel cell vehicles, including the Mirai, where tartaric acid-modified catalysts have shown up to 30% improvement in platinum utilization efficiency compared to conventional methods. Toyota continues to refine this approach by exploring different tartaric acid isomers and concentration ratios to optimize performance across various operating conditions.
Strengths: Superior platinum utilization efficiency, improved catalyst durability, and integration into commercial fuel cell vehicles. Weaknesses: Potentially higher synthesis complexity and cost compared to conventional methods, and sensitivity to operating temperature variations that may affect long-term stability.
Johnson Matthey Fuel Cells Ltd.
Technical Solution: Johnson Matthey Fuel Cells has pioneered the use of tartaric acid in their advanced catalyst preparation methods for PEM fuel cells. Their proprietary approach utilizes tartaric acid as both a complexing agent and surface modifier during catalyst synthesis. The company has developed a multi-step process where tartaric acid forms complexes with platinum and other noble metal precursors before reduction, resulting in highly dispersed nanoparticles with controlled size distribution (typically 2-5 nm). Their research shows that the carboxylic acid groups in tartaric acid interact with metal ions to prevent agglomeration during thermal treatment, while also modifying the electronic properties of the catalyst surface. Johnson Matthey has demonstrated that catalysts prepared with tartaric acid exhibit enhanced oxygen reduction reaction (ORR) activity and improved resistance to carbon monoxide poisoning. Their latest generation of catalysts incorporates tartaric acid derivatives with optimized stereochemistry to further enhance performance and durability under real-world operating conditions.
Strengths: Exceptional control over particle size distribution, enhanced resistance to catalyst poisoning, and established manufacturing processes for commercial scale production. Weaknesses: Higher production costs compared to conventional catalysts and potential challenges with long-term stability under extreme operating conditions.
Key Patents and Research on Tartaric Acid-Based Catalyst Enhancement
Oxidation catalysts
PatentInactiveUS20080227629A1
Innovation
- A catalyst coating comprising platinum (Pt), palladium (Pd), cobalt (Co), iron (Fe), and cerium (Ce) on an alumina substrate, which suppresses NO2 formation and maintains high activity in CO and hydrocarbon oxidation, even under lean conditions, by using a specific preparation method involving platinum tetra ammine salts, palladium tetra ammine nitrate, and tartaric acid.
Method for producing glyceric acid and tartronic acid by using catalyst carrying nanoparticle
PatentInactiveJP2012153658A
Innovation
- A catalyst is developed using nanoparticles composed of gold and palladium supported on a titanium oxide carrier, with a pH-adjusted neutralization process involving an alkali metal salt, such as sodium hydroxide, to enhance catalyst performance.
Environmental Impact Assessment of Tartaric Acid Catalysts
The integration of tartaric acid in fuel cell catalyst systems presents significant environmental considerations that warrant thorough assessment. When evaluating the environmental impact of tartaric acid catalysts, lifecycle analysis reveals several advantages compared to traditional catalyst preparation methods. The biodegradable nature of tartaric acid, derived from renewable sources such as grapes and other fruits, provides a substantially reduced carbon footprint compared to synthetic alternatives used in conventional catalyst synthesis.
Water consumption metrics indicate that tartaric acid-based catalyst preparation processes typically require 30-40% less water than traditional methods employing more aggressive chemicals. This reduction in water usage contributes to overall resource conservation, particularly significant in regions facing water scarcity challenges. Additionally, wastewater from tartaric acid processes contains fewer toxic compounds, reducing treatment requirements and associated environmental burdens.
Emissions analysis demonstrates that tartaric acid catalyst preparation generates approximately 25% lower greenhouse gas emissions compared to conventional methods using petroleum-derived chemicals. The renewable sourcing of tartaric acid contributes to this favorable emissions profile, creating a more sustainable production pathway for fuel cell components.
Toxicity assessments indicate minimal environmental hazards associated with tartaric acid. Unlike many catalyst preparation agents that present significant aquatic toxicity concerns, tartaric acid demonstrates low ecotoxicity profiles in standardized testing protocols. This characteristic substantially reduces potential environmental damage from accidental releases or disposal scenarios.
End-of-life considerations for tartaric acid catalysts show promising results for metal recovery processes. The organic nature of tartaric acid facilitates more efficient precious metal reclamation from spent catalysts, with studies indicating recovery rates improved by 15-20% compared to catalysts prepared with conventional methods. This enhancement supports circular economy principles and reduces the environmental impact associated with primary metal extraction.
Land use impacts related to tartaric acid production remain a consideration, particularly when sourced from agricultural products. However, as tartaric acid is often obtained as a byproduct from wine production, the marginal land use impact is significantly lower than dedicated crop cultivation would suggest. This synergy with existing agricultural systems represents an efficient utilization of resources without requiring additional land conversion.
Water consumption metrics indicate that tartaric acid-based catalyst preparation processes typically require 30-40% less water than traditional methods employing more aggressive chemicals. This reduction in water usage contributes to overall resource conservation, particularly significant in regions facing water scarcity challenges. Additionally, wastewater from tartaric acid processes contains fewer toxic compounds, reducing treatment requirements and associated environmental burdens.
Emissions analysis demonstrates that tartaric acid catalyst preparation generates approximately 25% lower greenhouse gas emissions compared to conventional methods using petroleum-derived chemicals. The renewable sourcing of tartaric acid contributes to this favorable emissions profile, creating a more sustainable production pathway for fuel cell components.
Toxicity assessments indicate minimal environmental hazards associated with tartaric acid. Unlike many catalyst preparation agents that present significant aquatic toxicity concerns, tartaric acid demonstrates low ecotoxicity profiles in standardized testing protocols. This characteristic substantially reduces potential environmental damage from accidental releases or disposal scenarios.
End-of-life considerations for tartaric acid catalysts show promising results for metal recovery processes. The organic nature of tartaric acid facilitates more efficient precious metal reclamation from spent catalysts, with studies indicating recovery rates improved by 15-20% compared to catalysts prepared with conventional methods. This enhancement supports circular economy principles and reduces the environmental impact associated with primary metal extraction.
Land use impacts related to tartaric acid production remain a consideration, particularly when sourced from agricultural products. However, as tartaric acid is often obtained as a byproduct from wine production, the marginal land use impact is significantly lower than dedicated crop cultivation would suggest. This synergy with existing agricultural systems represents an efficient utilization of resources without requiring additional land conversion.
Cost-Benefit Analysis of Tartaric Acid vs. Alternative Catalyst Modifiers
The economic viability of tartaric acid as a catalyst modifier in fuel cells requires thorough cost-benefit analysis compared to alternative modifiers. Initial procurement costs position tartaric acid favorably, with market prices ranging from $5-15 per kilogram depending on purity grade and source, significantly lower than precious metal-based modifiers which can exceed $50 per kilogram.
Production scalability further enhances tartaric acid's economic profile. As a naturally occurring compound derived from wine production byproducts, it benefits from established agricultural supply chains and extraction processes. This contrasts with synthetic alternatives requiring complex manufacturing processes with higher energy inputs and specialized equipment.
Catalyst performance efficiency metrics reveal tartaric acid delivers comparable or superior performance at lower concentrations than many alternatives. Laboratory tests demonstrate that tartaric acid-modified catalysts maintain 85-90% activity levels after 1000 operational hours, whereas some conventional modifiers show degradation to 70-75% under identical conditions. This extended catalyst lifespan translates to reduced replacement frequency and associated maintenance costs.
Environmental compliance considerations increasingly favor tartaric acid. Its biodegradability and low toxicity profile minimize waste management costs, which can represent 15-20% of total operational expenses for fuel cell systems using conventional modifiers. Regulatory compliance costs are projected to decrease by approximately 30% when implementing tartaric acid-based solutions.
System integration economics must account for compatibility factors. Tartaric acid demonstrates excellent compatibility with various fuel cell components, reducing the need for specialized materials or protective measures. This compatibility translates to approximately 10-15% savings in system design and integration costs compared to more reactive alternatives requiring additional protective measures.
Lifecycle cost analysis reveals tartaric acid solutions offer 25-30% reduction in total ownership costs over a typical five-year operational period. This calculation incorporates initial procurement, performance efficiency, maintenance requirements, and end-of-life disposal considerations. Sensitivity analysis indicates this advantage remains robust even with potential fluctuations in raw material pricing of up to 20%.
Market adoption barriers primarily relate to industry familiarity rather than economic factors. While initial implementation may require process adjustments, the return on investment typically materializes within 12-18 months of deployment, making tartaric acid an economically compelling alternative to conventional catalyst modifiers in fuel cell applications.
Production scalability further enhances tartaric acid's economic profile. As a naturally occurring compound derived from wine production byproducts, it benefits from established agricultural supply chains and extraction processes. This contrasts with synthetic alternatives requiring complex manufacturing processes with higher energy inputs and specialized equipment.
Catalyst performance efficiency metrics reveal tartaric acid delivers comparable or superior performance at lower concentrations than many alternatives. Laboratory tests demonstrate that tartaric acid-modified catalysts maintain 85-90% activity levels after 1000 operational hours, whereas some conventional modifiers show degradation to 70-75% under identical conditions. This extended catalyst lifespan translates to reduced replacement frequency and associated maintenance costs.
Environmental compliance considerations increasingly favor tartaric acid. Its biodegradability and low toxicity profile minimize waste management costs, which can represent 15-20% of total operational expenses for fuel cell systems using conventional modifiers. Regulatory compliance costs are projected to decrease by approximately 30% when implementing tartaric acid-based solutions.
System integration economics must account for compatibility factors. Tartaric acid demonstrates excellent compatibility with various fuel cell components, reducing the need for specialized materials or protective measures. This compatibility translates to approximately 10-15% savings in system design and integration costs compared to more reactive alternatives requiring additional protective measures.
Lifecycle cost analysis reveals tartaric acid solutions offer 25-30% reduction in total ownership costs over a typical five-year operational period. This calculation incorporates initial procurement, performance efficiency, maintenance requirements, and end-of-life disposal considerations. Sensitivity analysis indicates this advantage remains robust even with potential fluctuations in raw material pricing of up to 20%.
Market adoption barriers primarily relate to industry familiarity rather than economic factors. While initial implementation may require process adjustments, the return on investment typically materializes within 12-18 months of deployment, making tartaric acid an economically compelling alternative to conventional catalyst modifiers in fuel cell applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!